Abstract
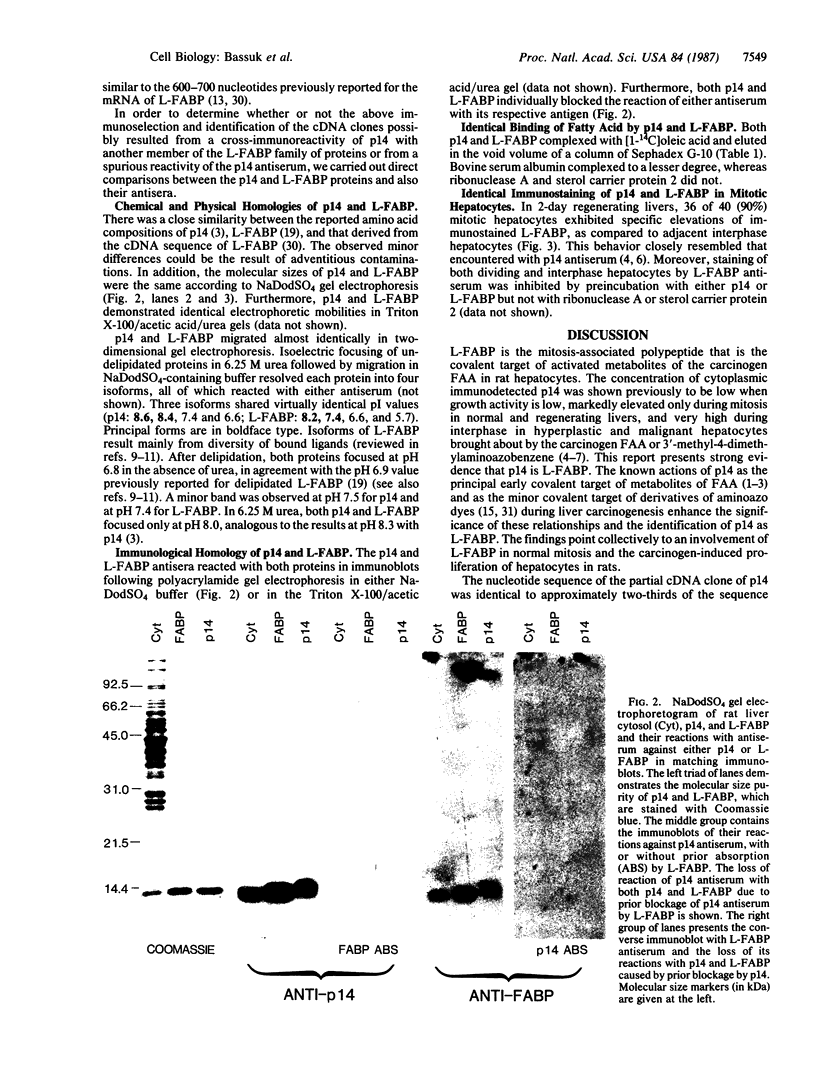
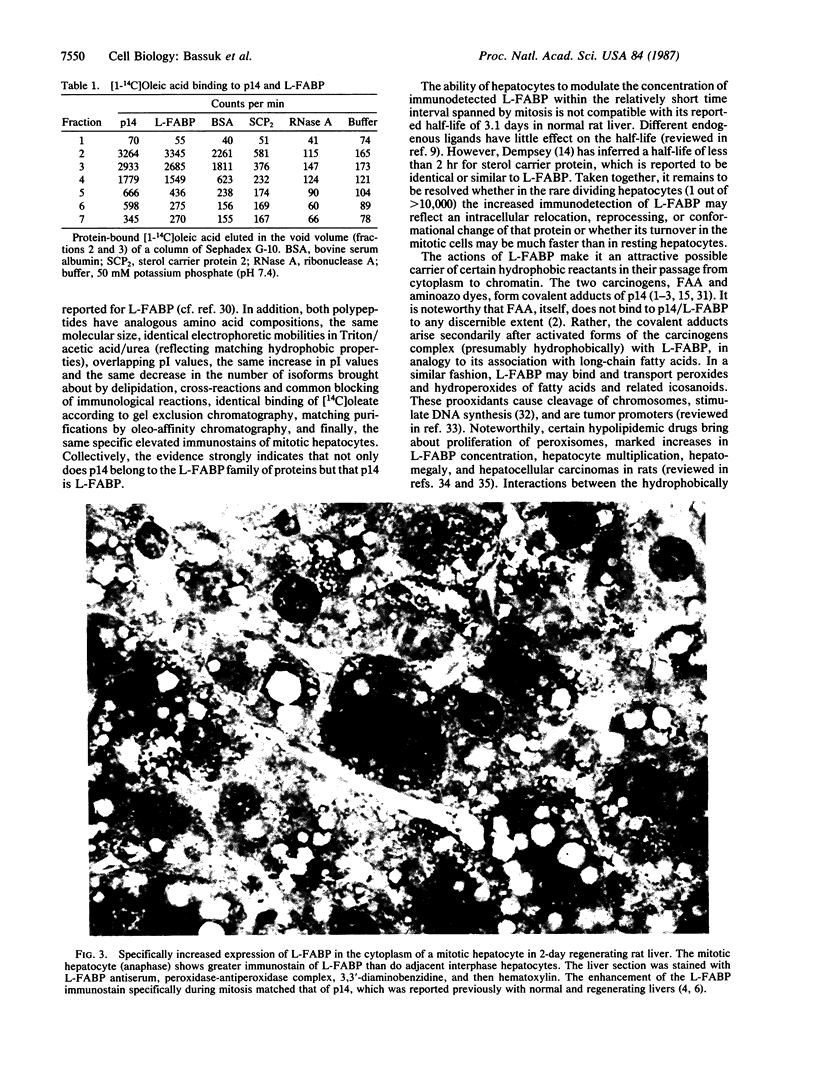
Hepatocytes in normal rat liver were found previously to contain a cytoplasmic 14,000-dalton polypeptide (p14) that is associated with mitosis and is the principal early covalent target of activated metabolites of the carcinogen N-2-fluorenylacetamide (2-acetylaminofluorene). The level of immunohistochemically detected p14 was low when growth activity of hepatocytes was low, was markedly elevated during mitosis in normal and regenerating livers, but was very high throughout interphase during proliferation of hyperplastic and malignant hepatocytes induced in rat liver by a carcinogen (N-2-fluorenylacetamide or 3'-methyl-4-dimethylaminoazobenzene). We report here that p14 is the liver fatty acid binding protein. The nucleotide sequence of p14 cDNA clones, isolated by screening a rat liver cDNA library in bacteriophage lambda gt11 using p14 antiserum, was completely identical to part of the sequence reported for liver fatty acid binding protein. Furthermore, the two proteins shared the following properties: size of mRNA, amino acid composition, molecular size according to NaDodSO4 gel electrophoresis, and electrophoretic mobilities in a Triton X-100/acetic acid/urea gel. Their pI values overlapped in 2-dimensional isoelectric focusing/NaDodSO4 gel electrophoresis and showed the same response to delipidation. Either polypeptide reacted with and blocked the antiserum raised against the other polypeptide. The two polypeptides bound oleic acid similarly. Finally, identical elevations of cytoplasmic immunostain were detected specifically in mitotic hepatocytes with either antiserum. The collected findings are suggestive that liver fatty acid binding protein may carry ligands that promote hepatocyte division and may transport certain activated chemical carcinogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M. Function and regulation of hepatic and intestinal fatty acid binding proteins. Chem Phys Lipids. 1985 Aug 30;38(1-2):95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Gaylor J. L. Cytosolic modulators of activities of microsomal enzyme of cholesterol biosynthesis. Role of a cytosolic protein with properties similar to Z-protein (fatty acid-binding protein). J Biol Chem. 1980 Sep 10;255(17):8128–8135. [PubMed] [Google Scholar]
- Blackburn G. R., Andrews J. P., Custer R. P., Sorof S. Early events during liver carcinogenesis involving two carcinogen:protein complexes. Cancer Res. 1981 Oct;41(10):4039–4049. [PubMed] [Google Scholar]
- Blackburn G. R., Andrews J. P., Rao K. V., Sorof S. An early event associated with liver carcinogenesis involving loss of a polypeptide that binds carcinogen. Cancer Res. 1980 Dec;40(12):4688–4693. [PubMed] [Google Scholar]
- Blackburn G. R., Schnabel S. J., Danley J. M., Hogue-Angeletti R. A., Sorof S. Principal polypeptide target of carcinogen at the beginning of liver carcinogenesis by three carcinogens. Cancer Res. 1982 Nov;42(11):4664–4672. [PubMed] [Google Scholar]
- Bull A. W., Nigro N. D., Golembieski W. A., Crissman J. D., Marnett L. J. In vivo stimulation of DNA synthesis and induction of ornithine decarboxylase in rat colon by fatty acid hydroperoxides, autoxidation products of unsaturated fatty acids. Cancer Res. 1984 Nov;44(11):4924–4928. [PubMed] [Google Scholar]
- Burn P., Burger M. M. The cytoskeletal protein vinculin contains transformation-sensitive, covalently bound lipid. Science. 1987 Jan 23;235(4787):476–479. doi: 10.1126/science.3099391. [DOI] [PubMed] [Google Scholar]
- Burn P., Rotman A., Meyer R. K., Burger M. M. Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature. 1985 Apr 4;314(6010):469–472. doi: 10.1038/314469a0. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Sorof S. Mitosis in hepatocytes is generally associated with elevated levels of the target polypeptide of a liver carcinogen. Differentiation. 1985;30(2):176–181. doi: 10.1111/j.1432-0436.1985.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Sorof S. Target polypeptide of a carcinogen is associated with normal mitosis and carcinogen-induced hyperplasias in adult hepatocytes. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6738–6742. doi: 10.1073/pnas.81.21.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer L. A., Birkenmeier E. H., Sweetser D. A., Levin M. S., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The cellular retinol binding protein II gene. Sequence analysis of the rat gene, chromosomal localization in mice and humans, and documentation of its close linkage to the cellular retinol binding protein gene. J Biol Chem. 1987 Feb 25;262(6):2458–2467. [PubMed] [Google Scholar]
- Dempsey M. E. Regulation of lipid metabolism by a lipid-carrying protein. Curr Top Cell Regul. 1984;24:63–86. doi: 10.1016/b978-0-12-152824-9.50014-9. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., Veerkamp J. H. Intracellular fatty acid-binding proteins. Int J Biochem. 1985;17(1):13–22. doi: 10.1016/0020-711x(85)90080-1. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Alpers D. H., Ockner R. K., Strauss A. W. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J Biol Chem. 1983 Mar 10;258(5):3356–3363. [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Hackney J. F., Beale D. A low-molecular-weight protein from rat liver that resembles ligandin in its binding properties. Biochem J. 1976 Jun 1;155(3):511–521. doi: 10.1042/bj1550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Blumberg P. M. Modulation of protein kinase C activity and [3H]phorbol 12,13-dibutyrate binding by various tumor promoters in mouse brain cytosol. Cancer Res. 1985 May;45(5):1958–1963. [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Moonen P., Haagsman H. P., Van Deenen L. L., Wirtz K. W. Determination of the hydrophobic binding site of phosphatidylcholine exchange protein with photosensitive phosphatidylcholine. Eur J Biochem. 1979 Sep;99(3):439–445. doi: 10.1111/j.1432-1033.1979.tb13274.x. [DOI] [PubMed] [Google Scholar]
- Noland B. J., Arebalo R. E., Hansbury E., Scallen T. J. Purification and properties of sterol carrier protein2. J Biol Chem. 1980 May 10;255(9):4282–4289. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Kane J. P. Fatty acid binding protein. Isolation from rat liver, characterization, and immunochemical quantification. J Biol Chem. 1982 Jul 10;257(13):7872–7878. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sorof S., Custer R. P. Elevated expression and cell cycle deregulation of a mitosis-associated target polypeptide of a carcinogen in hyperplastic and malignant rat hepatocytes. Cancer Res. 1987 Jan 1;47(1):210–220. [PubMed] [Google Scholar]
- Sorof S., Young E. M., McBride R. A., Coffey C. B. On protein targets of chemical carcinogens: dissimilar molecular sizes of the principal protein conjugates. Cancer Res. 1970 Jul;30(7):2029–2034. [PubMed] [Google Scholar]
- Sweetser D. A., Lowe J. B., Gordon J. I. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem. 1986 Apr 25;261(12):5553–5561. [PubMed] [Google Scholar]
- Vincent S. H., Muller-Eberhard U. A protein of the Z class of liver cytosolic proteins in the rat that preferentially binds heme. J Biol Chem. 1985 Nov 25;260(27):14521–14528. [PubMed] [Google Scholar]
- Vinores S. A., Churey J. J., Haller J. M., Schnabel S. J., Custer R. P., Sorof S. Normal liver chromatin contains a firmly bound and larger protein related to the principal cytosolic target polypeptide of a hepatic carcinogen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2092–2096. doi: 10.1073/pnas.81.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]