Abstract
The Golgi complex is a central processing compartment in the secretory pathway of eukaryotic cells. This essential compartment processes more than 30% of the proteins encoded by the human genome, yet we still do not fully understand how the Golgi is assembled and how proteins pass through it. Recent advances in our understanding of the molecular basis for protein transport through the Golgi and within the endocytic pathway provide clues to how this complex organelle may function and how proteins may be transported through it. Described here is a possible model for transport of cargo through a tightly stacked Golgi that involves continual fusion and fission of stable, “like” subcompartments and provides a mechanism to grow the Golgi complex from a stable progenitor, in an ordered manner.
Keywords: Rab GTPases, Rab cascade
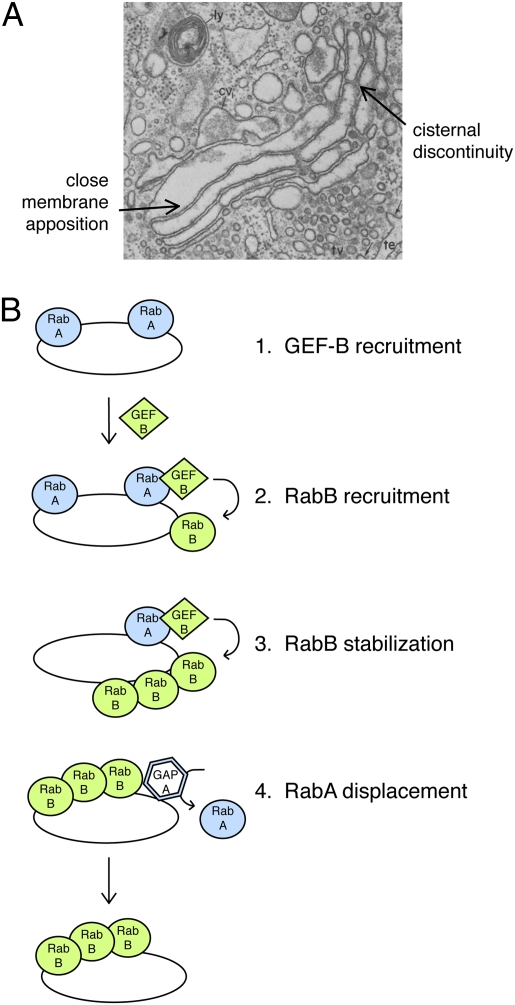
The Golgi comprises at least three subcompartments: the so-called early (cis), middle (medial), and late (trans) compartments. Each of these houses a distinct set of glycosyltransferases (along with other enzymes) that process glycoproteins in a reaction order that matches their relative locations: early, middle, or late. Proteins enter the Golgi at the cis compartment and exit at the trans compartment. In baker's yeast, Golgi subcompartments seem to be discrete structures. In contrast, in mammalian cells, the subcompartments are stacked very tightly together to form an elongated Golgi ribbon (Fig. 1A).
Fig. 1.
(A) Electron micrograph of the Golgi complex of a guinea pig exocrine pancreas cell. Note the tight stacking of individual cisternae and discontinuities within a given cisterna. © Rockefeller University Press, 1981. Originally published in J. Cell Biol. 91:77s. (B) Rab GTPases specify their successor to provide directionality in membrane traffic events. In a so-called “Rab cascade,” RabA can recruit a guanine nucleotide exchange factor that will activate the subsequent acting RabB. Activated Rabs are stabilized on membranes by binding their cognate effector proteins. RabB can also recruit a GAP that inactivates nearby RabA, to create a separate membrane microdomain.
Two prevailing models are discussed regarding how proteins traverse the Golgi (1–3). According to the cisternal maturation (or progression) model, cargo remains in a given compartment and different enzymes arrive there, to convert a cis cisterna into a medial one, or a medial cisterna into a trans cisterna. Alternatively, cargo moves from one Golgi compartment to the next, encountering different enzymes in each subsequent compartment, until it reaches the trans cisterna, where it is then sorted into carriers bound for post-Golgi destinations. This second model could use vesicles to transport cargo from one compartment to the next, and/or compartment-connecting tubules through which cargo could pass. Indeed, several thousand vesicles, some of which carry proteins back to the endoplasmic reticulum, surround the Golgi. Membrane tubules have also been detected between Golgi cisternae, under conditions of active secretion (4, 5); this scenario would permit cargo movement from one side of the stack to the next, without maturation or vesicle transfer.
What looks like cisternal maturation has been visualized directly in yeast: three groups have detected the apparent conversion of one Golgi compartment into another by high-resolution, live-cell video microscopy (6–8). A minor limitation of those studies is that some (but not all) of the compartment markers monitored are capable of reversibly binding to and releasing from the Golgi surface. Additionally, it has not yet been possible to visualize cargo simultaneously. Nevertheless, the data are very compelling.
In mammalian cells, Golgi cisternae are stacked tightly together. Close examination of electron micrographs reveals a zone of exclusion between cisternae and continuous and tight apposition of adjacent membranes along the entire cisternal length (Fig. 1A). Stacking is so stable that the polarity of the stack seems to be maintained even during mitosis, during which the Golgi ribbon structure totally disassembles (9). Stacks are maintained by a meshwork of proteins that constitute a so-called Golgi matrix (10). The apparent stability of the stack does not interfere with large cargo, such as procollagen, traversing the entire Golgi without ever leaving a cisterna (11). The high level of evolutionary conservation from yeast to humans suggests strongly that Golgi transport will occur by relatively homologous mechanisms, despite these morphological differences.
I propose here an alternative, “cisternal progenitor” model that involves stable compartments that possess the capacity to generate subsequent compartments of the Golgi complex. This is distinct from a conversion model whereby one compartment is turned into another, because in this model the original compartment is maintained. The cisternal progenitor model is based on our current understanding of compartment maturation in the endocytic pathway, coupled with widely accepted but broadly ignored properties of Golgi complexes in cells. Experts in the field will note that this model represents a distinct synthesis of previously discussed concepts and proposes roles for specific molecular players in this process.
Premise 1: Lateral Fusion Within the Golgi
The first premise of this model is the widely accepted observation that the Golgi ribbon undergoes continual, lateral fission and fusion and is actually a highly metastable structure. In interphase cells treated with the drug nocodazole to depolymerize microtubules, the Golgi readily fragments into ministacks that disperse throughout the cytosol (12). Simple drug washout leads to reformation of an intact, perinuclear Golgi ribbon. This indicates that the Golgi is capable of fission as soon as microtubules are lost—and fusion with itself as soon as microtubules repolymerize. The requirement for intact microtubules is most easily explained by microtubule-based motors using the tracks to enhance collision between Golgi ministacks. Elongated ribbon reformation demonstrates the capacity of Golgi ministacks to fuse laterally with one another during interphase. Indeed, cells lacking cytoplasmic dynein show dispersed Golgi ministacks (13). A certain degree of fission activity must be present in the system as it loses microtubules, because the fragmented Golgi complexes are all of a similar diameter; the molecular basis for Golgi fission is not yet established.
Significant additional evidence supports the notion that the Golgi ribbon is less stable than static images imply. The Golgi is decorated with so-called Golgin proteins that stabilize the ribbon and tether transport vesicles that dock there. Simple siRNA depletion of any one of these proteins leads to the formation of ministacks (14–19). “GRIP” domain Golgins localize to the exit face of the Golgi, are predicted to be extended, coiled-coil structures, and they each contain multiple binding sites for Golgi Rab GTPases across their entire lengths (20, 21). Yet cells seem to need all of them to maintain an intact ribbon. The tethers have been postulated to act as a sort of “Velcro” to catch vesicles and keep them near the Golgi to enhance their eventual fusion (22). However, these proteins also seem to reach across the stack and encourage ribbon stability (cf. 18). Because individual depletion of at least 10 different proteins leads to ministack formation, it seems quite reasonable to conclude that the elongated, stacked ribbon is a metastable structure that is likely subject to continual fission and fusion.
If the Golgi does undergo continual fusion and fission, some of that may occur within the elongated ribbon, such as that seen as individual cisternal interruptions in Fig. 1A. These discontinuities are also seen in the ministacks generated upon nocodazole treatment. An important part of the present model is the premise that lateral fission and fusion take place even within ministacks.
Initial glimpses of interstack fusion may have been detected in cell fusion experiments pioneered by Rothman many years ago (23). In those experiments, two populations of cells were fused with one another to introduce biochemically distinct Golgi complexes into common cytosol. Cargo from one Golgi was then seen to gain access to the glycosyltransferases present in the other Golgi complex. This could have involved transport vesicles budding from one Golgi stack and transferring to another with Golgi tethers. Yet it is also possible that transfer included interstack fusion events. Distinguishing between these possibilities may now be facilitated by the availability of fluorescently labeled Golgi complexes and higher-resolution, live-cell video microscopy.
Premise 2: Rab GTPases Define Compartments and Direct Vectoriality
Rab GTPases decorate the surfaces of almost all membrane compartments in the cytosol, and they function to catalyze the formation of function-specifying membrane microdomains. For example, Rabs organize membrane domains that contain specific, so-called tethering factors and the SNARE proteins that mediate membrane docking and fusion events, respectively (24, 25); Rabs also link to motor proteins to permit organelle motility. A given cell may contain more than 40 different Rab proteins (26), and each Rab will have distinct sets of binding partners that drive cargo collection into transport vesicles, link to molecular motors, and/or facilitate docking and fusion.
In the endocytic pathway, internalized material is first detected in early endosomes and then moves to late endosomes. The Rab5 GTPase directs entry of cargo into early endosomes and also drives the fusion of early endosomes with one another. This latter type of fusion is called homotypic because it utilizes identical tethering proteins and identical SNARE complexes on each membrane surface. Zerial and coworkers (27) have shown that delivery to late endosomes involves the loss of Rab5 GTPase and the acquisition of Rab7 GTPase. This transition is accompanied by the recruitment, by Rab5, of a guanine nucleotide exchange factor (GEF) that activates the Rab GTPase predominant on late endosomes, Rab7 (27). Active, GTP-bound Rabs are then stabilized on membranes by binding to cognate effector proteins (28).
The ordered recruitment of sequentially acting Rab GTPases has been termed a Rab cascade (29); they were first discovered in the yeast Golgi complex, where one Rab GTPase was needed to recruit the GEF for the subsequent acting Rab in the pathway. More recently, Novick has found additional evidence in support of Rab GTPases providing vectoriality in transport events. A late Golgi Rab has been shown to recruit the GTPase-activating protein (GAP) that inactivates a prior acting Rab GTPase (8). The sequential regulation of Rabs provides a means to order their action and likely provides the basis for the vectoriality of transport in the secretory and endocytic pathways (Fig. 1B).
The discovery of Rab cascades has important implications for our thinking about Golgi function. Live-cell video microscopy detected Rab conversion at the yeast Golgi: compartments containing the early Golgi Rab, Ypt1p, seemed to convert into a compartment containing the late Golgi Rab, Ypt32p (8). The data provide a direct molecular mechanism for morphological compartment interconversion at the Golgi, reminiscent of endosomal maturation (27) in both appearance and molecular mechanism. Importantly, the authors wrote that in addition to compartment conversion, “…close examination suggests that other processes may contribute as well. Golgi compartments were seen to be dynamic, undergoing a certain amount of fission and fusion. In some cases (30%), a Ypt32p compartment appeared to fuse to a Ypt1p compartment to yield a mixed compartment or a mixed compartment appeared to undergo segregation and fission to yield separate Ypt1p and Ypt32p compartments.” What this implies overall is that yeast Golgi compartments undergo apparent maturation by Rab conversion, and as described below, cargo may get the “fast track” from one compartment to the next, by intermittent, cisternal fusion and fission events.
Combining Premises 1 and 2
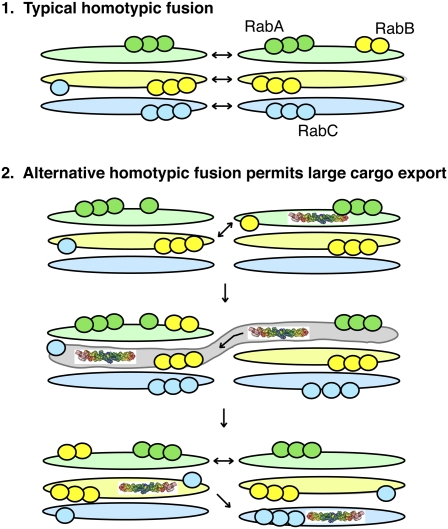
In the yeast, Saccharomyces cerevisiae, the Golgi is unstacked (as in Fig. 1B). Imagine instead a Golgi stack in which each of the three cisternae is marked by RabA, RabB, or RabC (Figs. 2 and 3). The cisternae are held together tightly, and stably, by stacking proteins. RabA would represent a domain containing the machinery to permit RabA-containing compartments to fuse with other RabA compartments; RabB would organize a domain with the capacity to fuse with other RabB compartments. This arrangement would permit two ministacks to fuse laterally (Fig. 3, Top). Because this elongated ribbon reformation process is readily detected, it suggests that SNARE complexes and docking factors are normally available and poised to receive homotypic membrane fusion partners.
Fig. 2.
Transport through the Golgi and Golgi stack creation in a cisternal progenitor model. (A) Consider a stably stacked Golgi where each cisterna is marked by a different Rab protein. The stack can grow if a Rab cascade builds sequential domains that can fuse with like domains (RabB regions with other RabB regions). RabA will create an adjacent RabB domain that may segregate by fission within the stack (B). The RabB bleb would fuse with the stable RabB cisterna, thereby growing. This process can include cargo. Alternatively (or simultaneously), vesicles may carry cargo from a RabA compartment to a RabB compartment by “heterotypic fusion.” Importantly, the RabA compartment is stable and the progenitor of the RabB compartment. The RabB compartment has the capacity to remove RabA for redelivery to the cis Golgi (C). (D) Loss of cisternal morphology in cells lacking p37 (reprinted from Developmental Cell, 11 /6, Keiji Uchiyama, Go Totsukawa, Maija Puhka, Yayoi Kaneko, Eija Jokitalo, Ingrid Dreveny, Fabienne Beuron, Xiaodong Zhang, Paul Freemont, Hisao Kondo, p37 Is a p97 Adaptor Required for Golgi and ER Biogenesis in Interphase and at the End of Mitosis, p 14, Copyright (2006), with permission from Elsevier. http://www.cell.com/developmental-cell/). Premise 1 states that Golgi stacks undergo continuous, reversible fission and fusion.
Fig. 3.
Large cargoes can move across the stack by “alternative” homotypic fusion. Here, Rab cascades create multidomain cisternae that can provide homotypic fusion capacity between distinct cisternal compartments. This would allow a large cargo such as collagen (shown) to access all Golgi compartments without leaving the stack or requiring cisternal progression.
Overlay on top of this scenario a Rab cascade in which RabA can recruit the GEF for RabB. A RabA domain could begin to form an adjacent RabB domain (Fig. 2A). This domain could separate from the RabA domain by fission as part of the steady-state fission and fusion that would be taking place in the Golgi, and it could segregate from the RabA domain by RabB recruitment of a RabA-specific GAP (Fig. 2A). Under physiological conditions in which secretion is activated, it may be important for the Golgi to expand to accommodate the increased volume of cargo. This could involve increased production of RabB and RabC domains to build a larger Golgi. Under these conditions, one might detect cisternal expansion at the edges of the stack. A RabB domain present there would have the capability of fusing with another RabB domain. This would lead to connections between cisternae at different levels of the stack that would then be resolved by fission events. Marsh et al. have reported precisely this morphology in cells stimulated to hypersecrete insulin (5).
This cisternal progenitor model involves stable compartments that form sequentially and retain their stacked nature. Satisfyingly, this model provides a ready means for cargoes that are too large to be accommodated by transport vesicles to traverse the Golgi stack, without moving the glycosyltransferase enzymes in transport vesicles. For example, collagen present in an early, “RabA” cisterna could move to the medial Golgi as soon as a little bit of the RabA compartment acquires RabB (Fig. 3). RabB would recruit the machinery to permit that cisterna to fuse with a RabB medial compartment. RabA would be removed from that compartment by a RabB-recruited, RabA-specific GAP. Now in a RabB compartment, acquisition of RabC would confer the ability of the medial Golgi cisterna to fuse with a later RabC compartment. At this stage, collagen would have traversed the stack and would have gained access to all of the glycosyltransferases that reside at each level of the stack. If this interpretation were correct, homotypic fusion would represent the predominant form of fusion used for large cargo transport through the Golgi. It is important to note that two stacks are not needed to accomplish such transport: because even ministack cisternae are discontinuous, such a fusion event could occur between cisternae within a ministack. In addition, this class of fusion may explain the tubules that have been detected in some laboratories to connect Golgi cisternae (4, 5, 30).
Rothman and coworkers, monitoring transport of an engineered, large cargo through the Golgi by electron microscopy, identified “megavesicles” as the cargo carriers (31). The present model is entirely consistent with megavesicle intermediates but suggests that their formation arises from continual fission and their consumption involves homotypic fusion to an adjacent compartment. This is molecularly distinct from heterotypic transport by a conventional transport vesicle mechanism.
The concentration of large algal scales across the Golgi complex and their presence in the lumen of every cisterna (32) has been used to argue in favor of cisternal maturation. Such images could equally well be the result of a cisternal progenitor model—a static picture does not provide clues to molecular mechanism.
I propose that glycosyltransferases are held in compartments by retention in specific Rab domains. Rabs may have as many as 30 different effectors, so some RabA molecules would participate in cis-Golgi enzyme retention while other RabA molecules organized homotypic fusion proteins (Fig. 2). Thus, a prediction of this model is that glycosyltransferases would not be localized to a single cisterna but might be detected in two adjacent Golgi regions. This is indeed the case for essentially all of the glycosyltransferases that have been localized by immunogold labeling electron microscopy: they are usually seen in cis and medial or medial and trans compartments. Glycosyltransferase spread could be the consequence of the presence of some RabB on a RabA compartment or some RabB on a RabC compartment.
A satisfying aspect of the model is that it incorporates many of the previously discrepant experimental observations reported by workers in yeast, plant cells, and human cells. It explains why Golgi compartments appear to mature in live-cell video micrographs and why the kinetics of Golgi export may not match that predicted by a pure maturation model (33). Compartments have the capacity to “grow” the next compartment in a templated, Rab-dependent fashion, but they are also fusing homotypically with one another. Thus, each cisterna can serve as a progenitor of the next. Glycosyltransferases are mostly excluded from the rims where cargo is present and would be predicted to be retained in a given compartment organized by Rab GTPases. And any enzymes seen at the rims may be the product of an intercisternal fusion event.
Tests of the Model.
The most important distinction between the cisternal progression model and a pure maturation model is the fact that each compartment is stable and can generate a subsequent compartment. Because a Rab cascade can accomplish this, proof of a Rab cascade in the Golgi will add strong support for the cisternal progenitor model. In yeast cells, the proof is accumulating (8). In mammalian cells, this will require identification of the specific Rab GTPases and their GEFs and GAPs at each level of the Golgi.
The model predicts that there will be three classes of Rab GTPases that define early, middle, and late Golgi. At present, Rab 1, Rab33b, and Rab6 could play these roles, although there are many additional Rabs on the Golgi complex (e.g., 2, 9B, 30, 32, 33a, 37, and 38), and it could be that particular Rab–effector protein pairs actually demarcate the different Golgi compartments, permitting one Rab to mark more than one compartment. It will be important to identify the Rabs, GEFs, and GAPs that act within the Golgi complex to provide the directionality and polarity of sequential Rab action. Once identified, such GEFs and GAPs should define the orientation of the stack and the localization of Golgi enzymes. Altering their localizations will also provide important information about how the Golgi is formed and how Rabs participate in that process.
Another prediction of the cisternal progenitor model is that conditions that enhance homotypic fusion might enhance large cargo export. Large cargoes may be more sensitive to nocodazole-induced ministack formation—if stacks could not fuse, larger cargoes would have a harder time accomplishing secretion from cells. Secretion of smaller cargoes is slowed approximately 50% in nocodazole-treated cells, but this may be due to motor-driven, post-Golgi export steps. The precise rate of collagen secretion should be measured in control and nocodazole treated cells; it should be compared with the secretion rate of a smaller cargo such as albumin.
Some may argue that nocodazole ministacks are still relatively functional, and nocodazole would not block the homotypic fusion between intracisternal discontinuities that the model proposes to take place (Fig. 1A). Results from a more rigorous test may already be in hand. Indeed, the importance of homotypic fusion in normal Golgi structure and protein secretion is highlighted by a recent study of a Golgi protein named p37 (34). p37 forms a tight complex with the homotypic fusion-mediating p97 ATPase in interphase cells, and p37 siRNA or anti-p37 antibody injection leads to breakdown of the Golgi into small vesicles and tubules (34) (Fig. 2D). Depletion of p37 strongly inhibits the transport of vesicular stomatitis virus-G protein transport from the Golgi to the cell surface. These findings strongly suggest that at steady state, the Golgi participates in frequent vesicle and tubular fusion and that this fusion is needed for efficient secretion. The ability of the Golgi to undergo continuous, homotypic fusion can nevertheless be monitored by live-cell video microscopy after microinjection or fusion of two cell types harboring different colored Golgi stacks. Indeed, exogenously added, wild-type Golgi complexes could receive cargo from the Golgi complexes of mutant, semiintact CHO cells (35).
Which SNARE proteins mediate the homotypic cisternal fusion postulated to be important for secretion? GS15 is important for p37/p97-mediated cisterna formation (34) and is found at all levels of the Golgi stack. GS15 acts as the v-SNARE in a fusion-capable complex containing Syntaxin 5, Gos28, and Ykt6 proteins (36); these proteins are needed for intra-Golgi transport (37). The presence of GS15 on all cisternae permits this SNARE complex to mediate homotypic membrane fusion. Interestingly, although the SNAREs needed for endoplasmic reticulum to Golgi transport are localized exclusively at the rims of Golgi cisternae, GS15 and Syntaxin 5 are only 2-fold enriched at that location and are also seen in the middle of cisternae (38). Thus, the SNAREs proposed to mediate homotypic fusion are found precisely where they may be needed for more local fusion events. The importance of homotypic fusion in large cargo secretion may be testable in cells in which the localization of GS15 or Syntaxin 5 are altered artificially. Such relocation would be predicted to alter the structure of the Golgi stack and the efficiency of large cargo secretion. Alternatively, exogenous p37 expression may enhance secretion rates.
If compartments can fuse with one another, what keeps Golgi compartments from mixing entirely? As long as Rab-demarcated fission events are relatively frequent and Rab domains remain assembled, compartments will be maintained. Presumably, specific Rab interactions with tethering factors will distinguish homotypic fusion events at each level of the Golgi stack; and forward flow will be driven by the arrival of membrane from the endoplasmic reticulum with which Golgi cisternae cannot fuse.
Finally, and importantly, the cisternal progenitor model does not in any way preclude the use of transport vesicles for movement of cargo from the cis to medial to trans cisternae; the question becomes one of whether the predominant means of fusion is homotypic or heterotypic in nature. Many of the predictions of the cisternal progenitor model should be testable in the near future and will provide important resolution to a long-standing controversy in our understanding of how proteins traverse the Golgi complex.
Acknowledgments
This work was supported by National Institutes of Health Grants DK37332 and GM79322.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Emr S, et al. Journeys through the Golgi—taking stock in a new era. J Cell Biol. 2009;187:449–453. doi: 10.1083/jcb.200909011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelham HR, Rothman JE. The debate about transport in the Golgi—two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 4.Trucco A, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 5.Marsh BJ, Volkmann N, McIntosh JR, Howell KE. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet beta cells. Proc Natl Acad Sci USA. 2004;101:5565–5570. doi: 10.1073/pnas.0401242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losev E, et al. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 8.Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 11.Bonfanti L, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: Evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- 12.Thyberg J, Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985;159:1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- 13.Harada A, et al. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao A, Rahman D, Pappin DJ, Lucocq J, Lowe M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol. 2003;160:201–212. doi: 10.1083/jcb.200207045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridmann-Sirkis Y, Siniossoglou S, Pelham HR. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 2004;5:18–22. doi: 10.1186/1471-2121-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ríos RM, Sanchís A, Tassin AM, Fedriani C, Bornens M. GMAP-210 recruits γ-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 2004;118:323–335. doi: 10.1016/j.cell.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy JV, et al. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17:4353–4363. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8:238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 20.Hayes GL, et al. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20:209–217. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- 23.Rothman JE, Miller RL, Urbani LJ. Intercompartmental transport in the Golgi complex is a dissociative process: facile transfer of membrane protein between two Golgi populations. J Cell Biol. 1984;99:260–271. doi: 10.1083/jcb.99.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen UT, et al. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol. 2009;5:227–235. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- 27.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Aivazian D, Serrano RL, Pfeffer SR. TIP47 is a key effector for Rab9 localization. J Cell Biol. 2006;173:917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Pietro E, et al. Group IV phospholipase A(2)alpha controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volchuk A, et al. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell. 2000;102:335–348. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 32.Melkonian M, Becker B, Becker D. Scale formation in algae. J Electron Microsc Tech. 1991;17:165–178. doi: 10.1002/jemt.1060170205. [DOI] [PubMed] [Google Scholar]
- 33.Patterson GH, et al. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiyama K, et al. p37 is a p97 adaptor required for Golgi and ER biogenesis in interphase and at the end of mitosis. Dev Cell. 2006;11:803–816. doi: 10.1016/j.devcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Corthésy-Theulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parlati F, et al. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc Natl Acad Sci USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Martin S, James DE, Hong W. GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol Biol Cell. 2002;13:3493–3507. doi: 10.1091/mbc.E02-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosson P, et al. Dynamic transport of SNARE proteins in the Golgi apparatus. Proc Natl Acad Sci USA. 2005;102:14647–14652. doi: 10.1073/pnas.0507394102. [DOI] [PMC free article] [PubMed] [Google Scholar]