Abstract
Class I PI3-kinases signal downstream of receptor tyrosine kinases and G protein-coupled receptors and have been implicated in tumorigenesis. Although the oncogenic potential of the PI3-kinase subunit p110α requires its mutational activation, other p110 isoforms can induce transformation when overexpressed in the wild-type state. In wild-type p110α, N345 in the C2 domain forms hydrogen bonds with D560 and N564 in the inter-SH2 (iSH2) domain of p85, and mutations of p110α or p85 that disrupt this interface lead to increased basal activity and transformation. Sequence analysis reveals that N345 in p110α aligns with K342 in p110β. This difference makes wild-type p110β analogous to a previously described oncogenic mutant, p110α-N345K. We now show that p110β is inhibited by p85 to a lesser extent than p110α and is not differentially inhibited by wild-type p85 versus p85 mutants that disrupt the C2–iSH2 domain interface. Similar results were seen in soft agar and focus-formation assays, where p110β was similar to p110α-N345K in transforming potential. Inhibition of p110β by p85 was enhanced by a K342N mutation in p110β, which led to decreased activity in vitro, decreased basal Akt and ribosomal protein S6 kinase (S6K1) activation, and decreased transformation in NIH 3T3 cells. Moreover, unlike wild-type p110β, p110β-K342N was differentially regulated by wild-type and mutant p85, suggesting that the inhibitory C2–iSH2 interface is functional in this mutant. This study shows that the enhanced transforming potential of p110β is the result of its decreased inhibition by p85, due to the disruption of an inhibitory C2–iSH2 domain interface.
Keywords: oncogenic mutation, oncogenic transformation, lipid kinase
PI3-kinases are important regulators of cell growth and survival, and they mediate responses to extracellular stimuli through receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) (1). Disruption of normal PI3-kinase signaling is observed in cancer and many other diseases (1, 2). Class IA PI3-kinases are obligate heterodimeric proteins composed of a catalytic subunit (p110α, -β, -δ) and a regulatory subunit (p85α, p85β, p55α, p50α, and p55γ) (3). It had been suggested previously that class IA PI3-kinases function primarily downstream of RTKs, with the Src homology 2 (SH2) domains of p85 targeting the p85/p110 dimers to phosphorylated tyrosine residues in activated receptors or their adaptor molecules (1). However, recent data suggest that p110β is activated downstream of GPCRs, although it still is unclear whether p110β is activated only by GPCRs or by RTKs as well (4, 5).
p85 and p110 are multidomain proteins that interact with each other and with multiple upstream regulators and downstream effectors (6). p85 consists of an N-terminal Src homology 3 (SH3) domain, two proline-rich regions surrounding a breakpoint cluster region (BCR) homology region that can bind to small GTPases, and two SH2 domains flanking the inter-SH2 (iSH2) antiparallel coiled coil. Class IA p110 subunits are composed of an adaptor-binding domain (ABD), a Ras-binding domain (RBD), a C2 domain, and helical and kinase domains. The ABD and C2 domains of p110α bind to the p85 iSH2 domain (7, 8), and the p110α helical, C2, and kinase domains contact the p85 N-terminal SH2 (nSH2) domain (8, 9). These contacts are essential for the modulation of p110 activity and stability (7–11).
Mutations in PI3-kinase catalytic alpha polypeptide (PIK3CA; p110α) and phosphatase and tensin homolog (PTEN) are among the most common oncogenic mutations in a wide variety of cancers (12). In contrast to p110α, mutations in other class IA catalytic subunits have not been observed, although higher expression levels of p110β and p110δ have been detected in some cancers (13, 14). Kang et al. (15) showed that, unlike p110α, which is nontransforming in its wild-type state and requires mutational activation to transform cells, p110β, p110γ, and p110δ are transforming in their wild-type states when overexpressed. In this study, we compare the regulation and signaling of p110β with that of p110α and p110δ. We show that, compared with p110α, p110β is poorly inhibited by p85. Furthermore, p110β is inhibited to the same extent by wild-type p85 and by an oncogenic p85 truncation that disrupts an inhibitory contact involving the iSH2 domain of p85 and the C2 domain of p110 (8, 11, 16). This behavior is reminiscent of an oncogenic p110α-N345K mutant that also disrupts this contact, and a sequence alignment suggests that N345 in p110α is aligned with K342 in p110β. Introduction of a K342N mutation into p110β enables the differential regulation of p110β activity by wild-type and oncogenic p85 and reduces PI3K signaling and cellular transformation to levels similar to those of p110α. In contrast to p110β, p110δ seems to display an intact C2–iSH2 interface, because its regulation by p85 is similar to that observed for p110α. Our data suggest that the disruption of the C2–iSH2 interface plays an important role in the oncogenic potential of p110β but not of p110δ.
Results
p110β Regulation by p85 Suggests a Disrupted C2–iSH2 Interface.
Sequence analysis of human p110α and p110β (http://www.tcoffee.org) shows that K342 in p110β is aligned with N345 in p110α (Fig. 1A). This alignment suggests that wild-type p110β might be similar to the oncogenic mutant p110α-N345K, in which the inhibitory hydrogen bonding between N345 of the p110α C2 domain and D560 and N564 of the p85 iSH2 domain is disrupted (8, 11). p110α-N345K shows reduced inhibition by p85, and its kinase activity and transformation potential are not differentially regulated by wild-type p85 versus a truncation mutant in which C2–iSH2 contacts are disrupted (p85-572STOP) (11). In contrast, wild-type p110α is inhibited to a much greater extent by wild-type p85 than by p85-572STOP (17). The loss of differential regulation by wild-type p85 versus p85-572STOP in p110α-N345K thus defines an assay for the disruption of the C2–iSH2 interface.
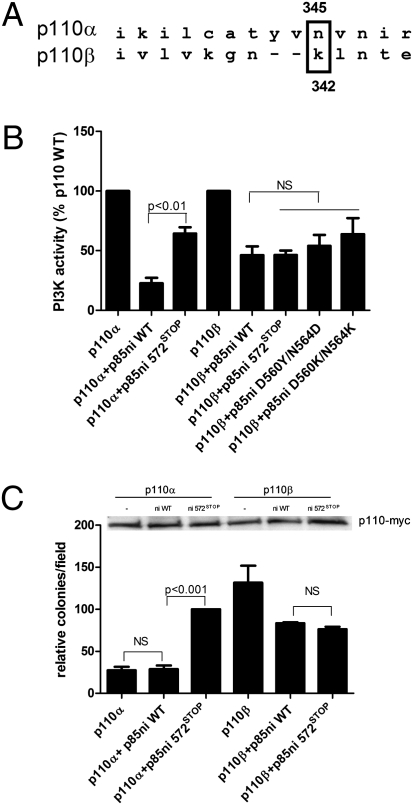
Fig. 1.
Regulation of p110β activity and transformation potential by p85. (A) Sequence alignment of p110α and p110β showing the position of the N345 position in p110α relative to K342 in p110β. (B) Recombinant p110α or p110β, produced in insect cells, was incubated for 1 h at 4 °C without or with 1 μg of wild-type or mutant p85ni and assayed for lipid kinase activity at 22 °C as described. Data are the mean ± SEM of triplicate samples from three experiments. (C) NIH 3T3 cells were transiently transfected with p110α-myc or p110β-myc alone or with wild-type or mutant p85ni. The top panel shows p110 expression levels. The cells were plated in soft agar as described, and colonies were counted after 3 wk. Colony counts are normalized to the number produced by cells expressing p110α plus p85ni572STOP. Data are the mean ± SEM of triplicate samples from three experiments. NS, not significant.
To test the hypothesis that the C2–iSH2 interface is disrupted in p110β, we used a reconstitution assay to measure inhibition of baculoviral-expressed p110 catalytic subunits by recombinant p85. p110α showed a marked inhibition (80%) after incubation with the nSH2-iSH2 fragment of p85 (p85ni), which behaves identically to full-length p85 with regard to inhibition of p110 (6). However, as previously described (11), p110α was poorly inhibited by a p85ni-572STOP mutant. In contrast, p110β showed only 40–50% inhibition by either wild-type p85ni or the p85ni-572STOP mutant (Fig. 1B). This lack of differential regulation of p110β also was observed using the p85 mutants D560K/N564K and D560Y/N564D (Fig. 1B), which also target the C2–iSH2 interface (11, 18).
To compare the effects of p85ni and p85ni-572STOP on the transformation potential of p110α and p110β in cells, we performed a soft agar colony-formation assay. In transfected NIH 3T3 cells, p110α-myc was poorly transforming when expressed alone or with wild-type p85ni. However, coexpression of p85ni 572STOP significantly enhanced the transformation potential of p110α-myc (Fig. 1C). Consistent with the kinase activity data (Fig. 1B) and previous studies (15), p110β-myc was highly transforming as compared with p110α-myc. The transformation potential of p110β-myc was the same whether coexpressed with p85ni or p85ni-572STOP, because both constructs caused a modest reduction in colony number (Fig. 1C). Similar results were obtained using full-length p85 or p85-572STOP (Fig. S1A). These data show that, unlike p110α, p110β shows no differences in regulation by wild-type versus mutant p85.
p110β-K342N Shows Differential Regulation of Lipid Kinase Activity by Wild-Type Versus Mutant p85.
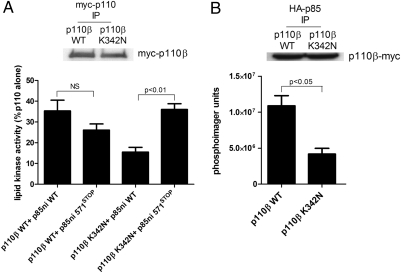
To determine whether the reduced inhibition of p110β by p85 is caused by the presence of the lysine at residue 342, we mutated the residue to aspargine (K342N) and measured the inhibition of wild-type versus K342N p110β-myc by recombinant wild-type p85ni or p85ni-572STOP. Wild-type p110β-myc showed similar levels of inhibition when incubated with either protein, but p110β-K342N-myc showed significantly greater inhibition with wild-type p85ni than with p85ni-572STOP (Fig. 2A). The activities of p85/p110β and p85-572STOP/p110β dimers were also similar, but the activity of p85-572STOP/p110β-K342N was significantly higher than that of p85/p110β-K342N (Fig. S1B). We also transfected HEK 293T cells with wild-type or K342N p110β-myc in the presence of HA-tagged wild-type p85 and measured the lipid kinase activity of p110β-myc in anti-HA-p85 immunoprecipitates (Fig. 2B). p85-bound p110β-K324N-myc had significantly lower kinase activity than p85-bound wild-type p110β-myc. These data suggest that the disruption of the C2–iSH2 interface in wild-type p110β is responsible for its high basal signaling activity.
Fig. 2.
Regulation of wild-type p110β and p110β-K342N by p85. (A) HEK 293T cells were transfected with wild-type or K342N myc-p110β. Anti-myc immunoprecipitates were incubated for 1 h at 4 °C with wild-type or mutant p85ni and were assayed for lipid kinase activity at 22 °C. Data are the mean ± SEM of triplicate samples from two experiments. The top panel shows p110 expression levels. (B) HEK 293T cells were transfected with wild-type or K342N p110β-myc and HA-tagged wild-type p85. Anti-HA immunoprecipitates were analyzed for myc-p110β levels by Western blotting (Upper) and for lipid kinase activity (Lower). Data are the mean ± SEM of triplicate samples from two experiments.
p110β-K342N Displays Lower Oncogenic Potential than Wild-Type p110β and Is Differentially Regulated by Wild-Type and Mutant p85 in Vivo.
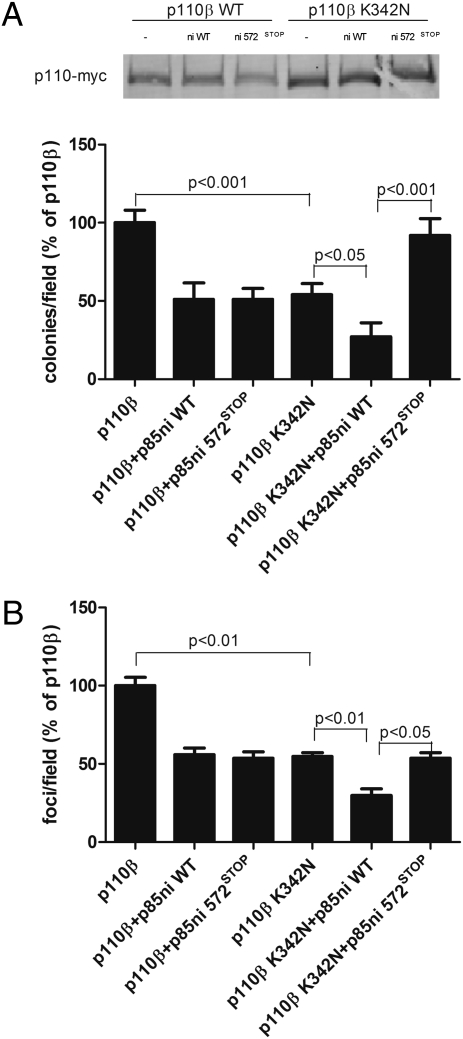
We performed soft agar colony-formation assays on wild-type and K342N p110β-myc alone or in the presence of p85ni or p85ni-572STOP. The transforming potential of p110β-K342N-myc alone was significantly less than that of wild-type p110β-myc alone (Fig. 3A). Furthermore, p110β-K342N-myc showed a differential regulation of transformation potential with p85ni and p85ni-572STOP, similar to that seen with wild-type p110α (Fig. 1B). In contrast, wild-type p110β-myc did not show differential regulation by wild-type versus mutant p85ni (Fig. 3A). The same pattern of differential regulation of K342N p110β-myc but not wild-type p110β-myc also was seen in colony-formation assays with full-length p85 or p85-572STOP (Fig. S1C). This pattern was confirmed further by a focus-formation assay, which tests the ability of cells to escape contact inhibition. As expected, p110β-K342N-myc showed a significant (∼50%) decrease in the number of foci formed compared with wild-type p110β-myc, and p85ni and p85ni-572STOP differentially regulated foci formation in cells expressing p110β-K342N-myc but not in cells expressing wild-type p110β-myc (Fig. 3B).
Fig. 3.
Transformation potential of wild-type p110β or p110β-K342N. (A) NIH 3T3 cells were transiently transfected with wild-type or K342N p110β-myc alone or with wild-type or mutant p85ni. The top panel shows p110 expression levels. The cells were plated in soft agar, and colonies were counted after 3 wk. Colony counts were normalized to the number produced by cells expressing p110β alone. Data are the mean ± SEM of triplicate samples from three experiments. (B) 3T3 cells were transiently transfected with wild-type or K342N p110β-myc alone or with wild-type or mutant p85ni and were left to grow to confluence for 2 wk. Foci were counted and normalized to the number produced by cells expressing p110β alone. Data are the mean ± SEM of two experiments.
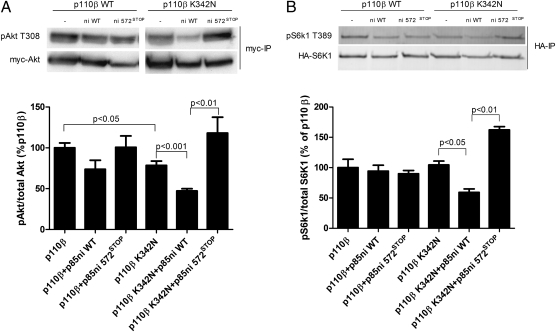
We also tested the effects of the p110β-K342N mutant on downstream signaling. We transfected HEK 293T cells with wild-type or K342N p110β-myc, alone or with p85ni or p85ni-572STOP, in the presence of myc-Akt or HA-labeled ribosomal protein S6 kinase (HA-S6K1). Wild-type p110β-myc resulted in a high basal level of pT308-Akt, but the expression of p110β-K342N-myc caused a significantly lower level of basal Akt phosphorylation (Fig. 4A). Furthermore, pT308-Akt levels were differentially regulated by p85ni and p85ni-572STOP in cells expressing p110β-K342N-myc but not in cells expressing wild-type p110β-myc (Fig. 4A). Similarly, pT389-S6K1 levels showed differential regulation by p85ni and p85ni-572STOP only in cells expressing K342N p110β-myc, not in cells expressing wild-type p110β-myc (Fig. 4B).
Fig. 4.
Signaling downstream of wild-type or K342N p110β. (A) HEK 293T cells were transiently transfected with myc-Akt plus wild-type or K342N p110β-myc alone or with wild-type or mutant p85ni. Cells then were starved overnight, and Akt immunoprecipitates were blotted with anti-pT308-Akt antibody. Data are the mean ± SEM of three experiments. (B) HEK 293T cells were transiently transfected with HA-S6K1 plus wild-type or K342N p110β-myc, alone or with wild-type or mutant p85ni. Cells then were starved overnight, and S6K1 immunoprecipitates were blotted with anti-pT389-S6K1 antibody. Data are the mean ± SEM from two or three experiments.
Differential Regulation of p110α by Wild-Type or Mutant p85 Is Caused by the C2 Domain.
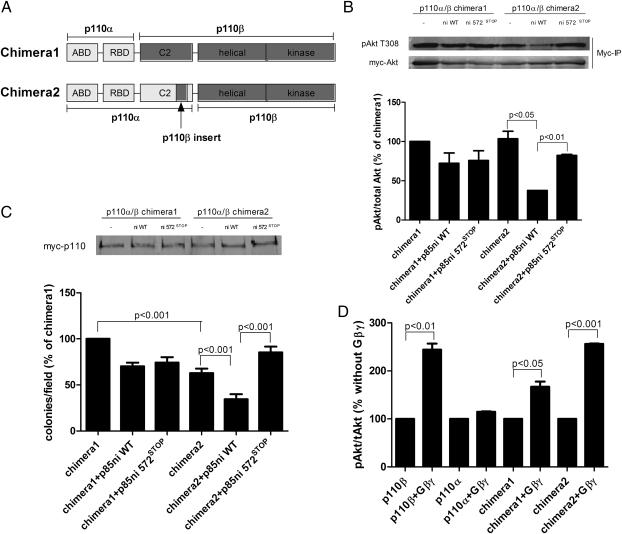
To test directly the role of the C2–iSH2 contacts in the regulation of p110α and p110β by p85, we created chimeric p110 molecules (described in detail in Materials and Methods). Chimera 1 contained the ABD and RBD domains of p110α with the C2, helical, and kinase domains of p110β (19), and chimera 2 contained the ABD, RBD, and C2 domains of p110α with the helical and kinase domains of p110β (Fig. 5A). Both chimeras have normal lipid kinase activity as compared with wild-type p110β (ref. 19 and Fig. S2). In cells expressing chimera 1 (which has the C2 domain from p110β), we observed no difference in pT308-Akt levels in the presence of p85ni or p85ni-572STOP (Fig. 5B). However, similar to p110β-K342N, cells expressing chimera 2 (which has the C2 domain of p110α) showed a much lower level of pT308-Akt in the presence of wild-type p85 as opposed to p85ni-572STOP (Fig. 5B). We then performed soft agar colony-formation assays on chimera 1 and chimera 2, alone or in the presence of p85ni or p8ni 572STOP. The transforming activity of chimera 2 was ≈60% of that of chimera 1 (Fig. 5C). Furthermore, chimera 2, but not chimera 1, showed differential suppression of its transformation potential by p85ni versus p85ni-572STOP (Fig. 5C). The loss of transformation activity in chimera 2 and its acquisition of differential regulation by wild-type versus mutant p85ni was not caused by a loss of interactions with Gβγ; although activation of chimera 1 by Gβγ was somewhat reduced for unknown reasons, activation of chimera 2 was similar to that seen with wild-type p110β (Fig. 5D). These data identify the C2 domain as a source of the distinct regulation of p110α and p110β by p85.
Fig. 5.
Transformation potential of p110α/β chimeras. (A) Schematic representation of the structures of the two chimeric p110α/β molecules. Construction of the chimeras is described in detail in Materials and Methods. (B) HEK 293T cells were transiently transfected with myc-Akt plus myc-tagged chimera 1 or chimera 2 alone or with wild-type or mutant p85ni. Cells were starved overnight, and Akt immunoprecipitates were blotted with anti-pT308-Akt antibody. Data are the mean ± SEM of two experiments. (C) NIH 3T3 cells were transiently transfected with myc-tagged chimera 1 or chimera 2 alone or with wild-type or mutant p85ni. The top panel shows p110 expression levels. The cells were plated in soft agar, and colonies were counted after 3 wk. Colony counts were normalized to the number produced by cells expressing chimera 1 alone. (D) HEK 293T cells were transfected with myc-Akt and p85 plus p110α-myc, p110β-myc, or myc-tagged chimera 1 or chimera 2, with or without Gβγ subunits. Akt immunoprecipitates were blotted with anti-Akt and anti-pT308-Akt antibodies. Data are the mean ± SEM of three experiments.
p110δ Is Regulated Differentially by Wild-Type and Mutant p85, Suggesting an Intact C2–iSH2 Interface.
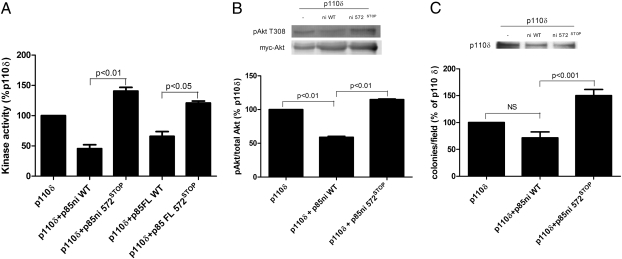
We also examined the alignment of p110α with p110δ. Although an alignment based on the two crystal structures (20, 21) suggests that p110α-N345 aligns with p110δ-N334, the loop immediately C-terminal to N334 in p110δ is not seen in the crystal structure, presumably because it is mobile. Therefore we could not predict whether the C2–iSH2 domain contact is intact in the p85/p110δ dimer, so we tested p110δ for differential regulation by wild-type or mutant p85 as an assay for the presence of the C2–iSH2 interface.
In a reconstitution assay, recombinant p110δ was inhibited by ≈40% by wild-type p85 (Fig. 6A), similar to effects seen with p110β (Fig. 1B). Unlike p110β, however, p110δ was minimally inhibited by p85-572STOP. This result is similar to the loss of p110δ inhibition by p85 mutants observed by Jaiswal et al. (18). The same pattern was seen for Akt activation in cells transfected with p110δ alone or with p85; expression of p110δ did lead to activation of Akt, but this activation was differentially regulated by wild-type p85ni and p85ni-572STOP (Fig. 6B). Finally, we performed soft agar colony-formation assays to test the transforming potential of p110δ and its regulation by p85. p110δ was transforming on its own, as had been observed previously (15). This transforming potential was slightly decreased by coexpression with p85ni and was significantly increased by coexpression with p85ni 572STOP (Fig. 6C). These data suggest that the C2–iSH2 interface is intact in p110δ and that its transforming potential is caused by other factors.
Fig. 6.
Regulation of p110δ activity, signaling, and transformation by wild-type and mutant p85. (A) HEK 293T cells were transfected with myc-p110δ as described in Materials and Methods. Anti-myc immunoprecipitates were incubated for 1 h at 4 °C with wild-type or mutant full-length p85 and were assayed for lipid kinase activity at 22 °C. Data are the mean ± SEM of triplicate samples from two experiments. (B) HEK 293T cells were transiently transfected with myc-Akt, plus untagged p110δ alone or with wild-type or mutant p85ni. Cells then were starved overnight, and Akt immunoprecipitates were blotted with anti-pT308-Akt antibody. Data are the mean ± SEM of two experiments. (C) NIH 3T3 cells were transiently transfected with untagged p110δ alone or with wild-type or mutant p85ni. The top panel shows p110 expression levels. The cells were plated in soft agar, and colonies were counted after 3 wk. Colony counts are normalized to the number produced by cells expressing p110δ alone. Data are the mean ± SEM of triplicate samples from two experiments.
Discussion
In this study, we have determined a mechanistic basis for the higher basal transforming potential of p110β (Fig. S3). The inhibitory contacts between the p110 C2 and the p85 iSH2 domains that are present in p110α are disrupted in p110β. This disruption leads to a higher basal activity of the p85/p110β dimer and subsequent constitutively activated downstream signaling. Stabilizing the C2–iSH2 contacts by mutating Lys342 to asparagine in p110β leads to decreased tumorigenesis and signaling similar to the levels in wild-type p110α. Consistent with this model, the transforming capacity of p110β is reduced in a chimeric molecule containing the C2 domain of p110α.
Previous studies have identified p110β as a key player in tumor formation, particularly in PTEN-null models and cell lines (22–25). It also has been shown that p110β, as well as p110δ and p110γ, are transforming in their wild-type state (15); the authors noted the alignment of K342 in p110β with N345 in p110α and hypothesized that p110β might be similar to the p110α-N345K mutant. This suggestion was rebutted by Amzel et al. (20), whose alignment shows N345 of p110α corresponding to N344 of p110β. The discrepancy results from small differences in the placement of gaps in the alignment, which are localized differently by different alignment algorithms.
Our data are clearly consistent with the alignment of K342 in p110β with N345 of p110α. p110β acts like the p110α-N345K mutant in that it is highly transforming, it has high basal Akt and S6K1 activation, and its lipid kinase activity is not regulated differentially by p85ni versus p85ni-572STOP. The p85-572STOP mutant acts by disrupting the C2–iSH2 interface, and it has no phenotype when paired with a p110α mutant in which this interface is already disrupted (11). In p110β-K342N, the C2–iSH2 contact is similar to that in wild-type p110α, decreasing transforming potential and restoring differential regulation by wild-type and mutant p85.
Our alignment of p110α-N345 with p110β-K342 is supported by the biochemical experiments but cannot be considered definitive, given the lack of a p110β structure. We therefore calculated energy scores for the comparative protein structure models that were built for the alignment reported here, as opposed to one in which p110β K342 is shifted two positions toward the N terminus, as predicted by Amzel et al. (20). Although the differences in the potentials scores were not statistically significant, they showed a trend toward a lower energy state for the alignment of p110α-N345 with p110β-K342 (−13.38 ± 0.29, as opposed to −13.21 ± 0.33 for the alignment of Amzel et al.).
We also compared p110α with p110δ. Our biochemical data clearly show that, despite its higher intrinsic transforming activity (15), p110δ is differentially regulated by wild-type versus mutant p85. This result suggests that p110β is unique among class IA PI3-kinase subunits in its failure to form the inhibitory C2–iSH2 interface (11). The mechanisms for the enhanced transforming activity of p110δ remain to be discovered.
Although our data provide insight into the transforming potential of p110β, they do not explain a number of its other isoform-specific characteristics. It is unlikely that differences in the C2–SH2 interface affect Gβγ regulation of p110β, especially because we show that the chimera containing the ABD, RBD, and C2 domains of p110α but the helical and kinase domains of p110β is activated by Gβγ. However, the loss of p85 inhibition might be partially responsible for the minimal activation of p110β in response to receptor tyrosine kinase activation (5). Given that phosphopeptide activation of Class IA PI3-kinase involves a disinhibition of p110 (10), the partial loss of p85 inhibition in p110β would be expected to lead to a corresponding loss of activation when tyrosine phosphorylated proteins bind to the p85 nSH2 domain. This prediction also is consistent with a recent study showing that p85 mutations disrupting either the nSH2–helical domain interface or the iSH2–C2 domain interface act primarily through p110α rather than p110β (26). Although we observed ≈50% less phosphopeptide activation of p85/p110β dimers as compared with p85/p110α dimers in vitro, Nurnberg and coworkers (27) reported similar levels of phosphopeptide activation of p85/p110α and p85/p110β dimers. Further experiments will be needed to clarify the relationship between the two known inhibitory interfaces in p85/p110α, involving the p85 nSH2 domain with the p110α helical domain and the p85 iSH2 domain with the p110α C2 domain.
In summary, our data show that the lack of an inhibitory C2–iSH2 interface in p85/p110β is at least partially responsible for its high oncogenic potential as compared with p110α. These data provide a logical basis for targeting the C2–iSH2 interface to modulate the activity of p110β.
Materials and Methods
Construction of p110 Chimera.
Chimera 1 (ABD-RBDalpha/C2-helical-kinasebeta) has been described previously (18). To construct chimera 2 (ABD-RBD-C2alpha/helical-kinasebeta), we initially linked the ABD, RBD, and C2 domains of p110α to the helical and kinase domains of p110β using a number of junction sites in the C2-helical linker. None of these constructs were active. We then modeled the structure of p110β on the crystal structure of p110α using the Modeler program (28) and defined the interdomain contacts using the NCONT module in the CCP4 computational suite (29). The C2 domain of p110β has an insert (residues 408–432) not present in p110α that is predicted to be present at the intramolecular C2–helical domain interface. We hypothesized that the lack of this insert in the p110α C2 domain might disrupt its packing with the helical domain of p110β. We therefore modified the C2 domain of p110α by inserting residues 408–432 of p110β in place of residues 409–416 of p110α. Unlike the inactive ABD-RBD-C2alpha/helical-kinasebeta chimera, the ABD-RBD-C2(insert)alpha/helical-kinasebeta had normal activity as compared with p110β (Fig. S2).
Sequence Alignment.
Sequence alignment of human p110α and human p110β was done using the Tcoffee alignment software (www.tcoffee.org).
Protein Modeling.
Comparative protein structure models were built with the M4T approach (30–32) using MODELER (28). Energy scores were obtained using PROSAII statistical pair-wise potential (33).
Complete materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Steven C. Almo and Mark E. Girvin for helpful discussions, and Dr. Bart Vanhaesebroeck, Queen Mary University of London, for cDNA constructs. This work was supported by National Institutes of Health Grant GM55692 and National Cancer Institute Grant CA100324, by a gift from the Janey Fund (to J.M.B), and by Grant P30CA013330 from the Albert Einstein College of Medicine Cancer Center and Grant DK020541 from the Albert Einstein College of Medicine Diabetes Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008739107/-/DCSupplemental.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci USA. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosu H, et al. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 5.Guillermet-Guibert J, et al. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci USA. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Yan Y, Backer JM. Regulation of class IA PI3Ks. Biochem Soc Trans. 2007;35:242–244. doi: 10.1042/BST0350242. [DOI] [PubMed] [Google Scholar]
- 7.Miled N, et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007;317:239–242. doi: 10.1126/science.1135394. [DOI] [PubMed] [Google Scholar]
- 8.Huang CH, et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744–1748. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 9.Mandelker D, et al. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci USA. 2009;106:16996–17001. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, et al. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: Stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, et al. Regulation of Class IA PI 3-kinases: C2 domain-iSH2 domain contacts inhibit p85/p110alpha and are disrupted in oncogenic p85 mutants. Proc Natl Acad Sci USA. 2009;106:20258–20263. doi: 10.1073/pnas.0902369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bénistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000;19:5083–5090. doi: 10.1038/sj.onc.1203871. [DOI] [PubMed] [Google Scholar]
- 14.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez C, et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shekar SC, et al. Mechanism of constitutive phosphoinositide 3-kinase activation by oncogenic mutants of the p85 regulatory subunit. J Biol Chem. 2005;280:27850–27855. doi: 10.1074/jbc.M506005200. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal BS, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yip SC, et al. Over-expression of the p110beta but not p110alpha isoform of PI 3-kinase inhibits motility in breast cancer cells. Cell Motil Cytoskeleton. 2004;59:180–188. doi: 10.1002/cm.20032. [DOI] [PubMed] [Google Scholar]
- 20.Amzel LM, et al. Structural comparisons of class I phosphoinositide 3-kinases. Nat Rev Cancer. 2008;8:665–669. doi: 10.1038/nrc2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berndt A, et al. The p110delta structure: Mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol. 2010;6:117–124. doi: 10.1038/nchembio0310-244b. [DOI] [PubMed] [Google Scholar]
- 22.Wee S, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciraolo E, et al. Phosphoinositide 3-kinase p110beta activity: Key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008 doi: 10.1126/scisignal.1161577. 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill KM, et al. The role of PI 3-kinase p110beta in AKT signally, cell survival, and proliferation in human prostate cancer cells. Prostate. 2010;70:755–764. doi: 10.1002/pros.21108. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, Hillmann P, Hofmann BT, Hart JR, Vogt PK. Cancer-derived mutations in the regulatory subunit p85α of phosphoinositide 3-kinase function through the catalytic subunit p110α. Proc Natl Acad Sci USA. 2010;107:15547–15552. doi: 10.1073/pnas.1009652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maier U, Babich A, Nürnberg B. Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms β and gamma. J Biol Chem. 1999;274:29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- 28.Fiser A, Sali A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative Computational Project, Number 4 The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Fuentes N, Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. Comparative protein structure modeling by combining multiple templates and optimizing sequence-to-structure alignments. Bioinformatics. 2007;23:2558–2565. doi: 10.1093/bioinformatics/btm377. [DOI] [PubMed] [Google Scholar]
- 31.Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. MMM: A sequence-to-structure alignment protocol. Bioinformatics. 2006;22:2691–2692. doi: 10.1093/bioinformatics/btl449. [DOI] [PubMed] [Google Scholar]
- 32.Rai BK, Fiser A. Multiple mapping method: A novel approach to the sequence-to-structure alignment problem in comparative protein structure modeling. Proteins. 2006;63:644–661. doi: 10.1002/prot.20835. [DOI] [PubMed] [Google Scholar]
- 33.Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins. 1993;17:355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.