Abstract
Sexually dimorphic nociception and opioid antinociception is very pervasive but poorly understood. We had demonstrated that spinal morphine antinociception in females, but not males, requires the concomitant activation of spinal μ- and κ-opioid receptors (MOR and KOR, respectively). This finding suggests an interrelationship between MOR and KOR in females that is not manifest in males. Here, we show that expression of a MOR/KOR heterodimer is vastly more prevalent in the spinal cord of proestrous vs. diestrous females and vs. males. Cross-linking experiments in combination with in vivo pharmacological analyses indicate that heterodimeric MOR/KOR utilizes spinal dynorphin 1–17 as a substrate and is likely to be the molecular transducer for the female-specific KOR component of spinal morphine antinociception. The activation of KOR within the heterodimeric MOR/KOR provides a mechanism for recruiting spinal KOR-mediated antinociception without activating the concomitant pronociceptive functions that monomeric KOR also subserves. Spinal cord MOR/KOR heterodimers represent a unique pharmacological target for female-specific pain control.
Keywords: estrous cycle, sexual dimorphism, sex steroids, signaling complexes, estrogen and progesterone
Sexual dimorphism in nociception and opioid antinociception has been extensively documented in humans (1–4) and laboratory animals (5–9). Nevertheless, underlying molecular mechanisms causally associated with sex-dependent nociception and opioid antinociception remain enigmatic. For example, there is little mechanistic understanding of why women are more likely than men to experience myriad chronic pain syndromes (1–3) as well as recurrent pain, more severe levels of pain, and pain of longer duration (10). Similarly, reports of more robust κ-opioid receptor (KOR) antinociception in females vs. males (11–14) are not accompanied by compelling mechanistic rationales.
In addition to proposed genetic contributions (15), the milieu of ovarian sex steroids is thought to contribute to sex-dependent nociception (5, 6) and opioid antinociception (5, 16). However, sex steroid molecular targets and their altered functionality that are relevant to sex-dependent nociception and opioid antinociception are not defined. This laboratory reported (17) that the antinociception produced by intrathecal (i.t.) morphine results from the sex-based differential recruitment of spinal analgesic components. In males, spinal morphine antinociception results from the exclusive activation of spinal μ-opioid receptor (MOR). In contrast, in females, spinal morphine antinociception requires the concomitant activation of spinal MOR and KOR (17). The most parsimonious explanation for this sex-dependent dichotomy would be the female-specific recruitment of spinal MOR/KOR heterodimers.
We investigated the hypothesized sexually dimorphic expression in spinal cord of MOR/KOR heterodimers by comparing their presence in the spinal cord of male, proestrous and diestrous rats as well as rats subjected to ovariectomy. Here, we report that expression of MOR/KOR heterodimers in spinal cord of females was not only dependent on the stage of the estrous cycle but was also substantially greater than in spinal cord of males. Parallel in vivo quantitative pharmacological analyses indicated that heterodimeric MOR/KOR is likely to be the molecular transducer for the female-specific dynorphin/KOR component of spinal morphine antinociception.
Results
MOR and KOR Form Heterodimers in Spinal Cord.
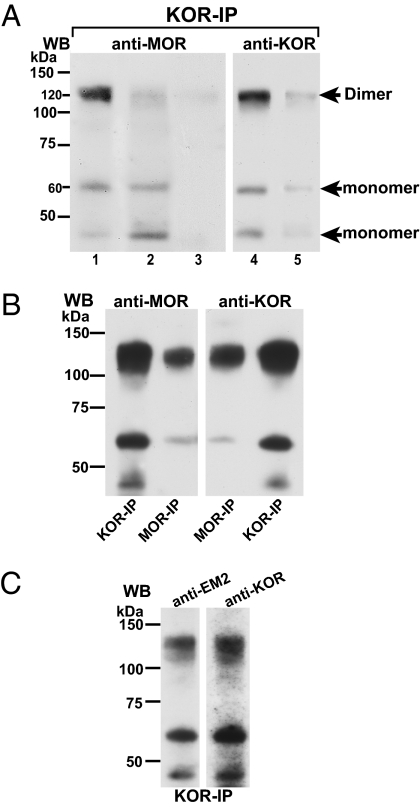
Immunoprecipitate obtained from spinal cord membranes using anti-KOR antibodies contained a ≈120-kDa molecular mass specie that was revealed in Western blot analyses using either anti-MOR or anti-KOR antibodies (Fig. 1A, lanes 1 and 4, respectively). This molecular mass corresponds to that predicted for heterodimeric MOR/KOR. MOR or KOR Western blots also revealed two smaller molecular mass species of ≈60 and ≈45 kDa, which correspond to the molecular mass of glycosylated and nonglycosylated monomeric MOR or KOR, respectively. It is likely that the presence of monomeric MOR in KOR immunoprecipitate and KOR in MOR immunoprecipitate (see below) resulted from the partial dissociation of heterodimeric MOR/KOR during processing subsequent to immunoprecipitation (IP) because the monomeric species were not detected when cross-linking preceded IP. The intensity of the ≈120-kDa MOR/KOR Western blot signal was reduced by >70% when gel electrophoresis was performed under reducing conditions (5 mM DTT and 5 mM iodoacetamide; Fig. 1A, lane 2). This reduction was accompanied by an approximately onefold increase in the intensity of the ≈45-kDa Western blot signal (Fig. 1A, lane 2 vs. lane 1), indicating that the nonglycosylated monomeric MOR specie participated in heterodimer formation. Specificity of Western blot signals was indicated by their substantial reduction in intensity when preabsorbed anti-MOR or anti-KOR antibodies were used for Western blot analysis (Fig. 1A, lane 3 vs. 1 and lane 5 vs. 4, respectively). Importantly, the presence of heterodimeric MOR/KOR could also be demonstrated by using anti-MOR antibodies (instead of anti-KOR antibodies) for IP, further substantiating the MOR/KOR identity of the ≈120-kDa molecular mass (Fig. 1B).
Fig. 1.
Spinal MOR and KOR form heterodimers. (A) Immunoprecipitate obtained from spinal cord membranes of a proestrous rat using anti-KOR antibodies was Western blotted with anti-MOR or anti-KOR antibodies (lanes 1 and 4, n = 5). In addition to ≈45- and ≈60-kDa signals, a ≈120-kDa band was visualized with either antibody. The intensity of the ≈120-kDa MOR signal was reduced >70% (lane 2) when electrophoresis was performed under reducing conditions (n = 2). Lanes 3 and 5 demonstrate loss of MOR and KOR Western blot signals, respectively, when using preabsorbed anti-sera. (B) The ≈120-kDa Western blot signal is observed in both MOR and KOR Western blots of immunoprecipitates obtained by using either anti-MOR or anti-KOR antibodies. (C) EM2 binds to MOR/KOR heterodimer. Spinal cord membranes from proestrous female rats were incubated with EM2 (10 nM, 15 min) followed by cross-linking. Identical aliquots of KOR immunoprecipitate were subjected to parallel Western blot analyses by using either anti-EM2 or anti-KOR antibodies. Notably, 10 nM EM2 is 500-fold lower than its Ki for KOR. Thus, the presence of an identical ≈120-kDa band in each Western blot that corresponded to the MOR/KOR heterodimer suggests that EM2 can bind to the MOR protomer in heterodimeric MOR/KOR (n = 3). WB, Western blot.
Endomorphin 2 (EM2) Binds to MOR/KOR Heterodimer.
To validate the presence of a MOR protomer in the ≈120-kDa specie immunoprecipitated with anti-KOR antibodies, we cross-linked the highly MOR-selective agonist EM2 (10 nM) (18) with spinal membranes before KOR IP. Immunoprecipitate so obtained was subjected to parallel Western blot analyses by using either anti-EM2 or anti-KOR antibodies. Western blots using either antibody revealed the ≈120-kDa signal, validating the presence of a MOR protomer in the ≈120-kDa signal visualized with anti-KOR antibodies (Fig. 1C), underscoring its MOR/KOR identity.
Expression of Heterodimeric MOR/KOR in Spinal Cord Is Sexually Dimorphic and Dependent on Stage of Estrous Cycle.
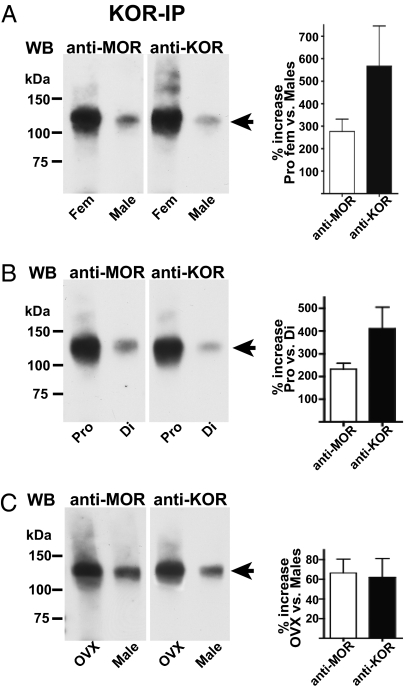
In the spinal cord of proestrous females, the ≈120-kDa signal visualized with either anti-MOR or anti-KOR antibodies was very robust (Fig. 2A). In contrast, in the spinal cord of males, the intensity of the ≈120-kDa band, visualized with either anti-MOR or anti-KOR antibody, was dramatically less. On average, the density of the ≈120-kDa signal was approximately fourfold greater in spinal cord of proestrous females vs. males (n = 4).
Fig. 2.
Spinal cord expression of MOR/KOR heterodimer is sexually dimorphic and dependent on stage of the estrous cycle. Immunoprecipitates obtained by using anti-KOR antibodies from spinal cord of proestrous females vs. males (A), proestrous vs. diestrous females (B), and ovariectomized females vs. males (C) were Western blotted using anti-MOR or anti-KOR antibodies. (A) Levels of MOR/KOR heterodimers (≈120 kDa) were strikingly higher in the spinal cord of proestrous females vs. males (n = 5). (B) MOR/KOR heterodimer expression in spinal cord of females depends on the stage of estrous cycle being approximately two- to threefold greater in the spinal cord of proestrous vs. diestrous rats (n = 3). (C) The content of heterodimeric MOR/KOR in the spinal cord of ovariectomized females remains greater (≈60%) than the content observed in the spinal cord of males (n = 3). Because the spinal cord of males have greatly reduced levels of heterodimeric MOR/KOR, we used larger amounts of spinal cord membrane protein from male rats [and therefore ovariectomized (OVX) animals] for IP as well as a longer detection time for its Western blot analysis than was used for spinal cord of diestrous rats. Consequently, the Western blots depicted in B and C should not be used to compare diestrous vs. OVX conditions. Bar graphs to the right of Western blot images illustrate corresponding quantification of densitometric comparisons of Western blot signals. Data are presented as mean ± SEM. WB, Western blot; Pro, proestrous; Di, diestrous. Arrowheads denote MOR/KOR heterodimer.
The relevance of circulating ovarian sex steroids to sex-dependent expression of spinal MOR/KOR heterodimers was investigated by comparing their expression in the spinal cord of proestrous vs. diestrous animals (Fig. 2B). Stage of cycle was found to be a critical determinant of MOR/KOR dimeric expression, being approximately threefold greater in spinal cord of proestrous vs. diestrous animals (n = 3). Notably, the content of heterodimeric MOR/KOR remained significantly greater (≈60%; P < 0.05) in the spinal cord of ovariectomized female rats vs. males (Fig. 2C; n = 3). This finding indicated that at least a portion of the sexually dimorphic expression of spinal MOR/KOR heterodimers was developmental in nature and parallels our earlier finding that ovariectomized adult females retain the female phenotypic KOR component of spinal morphine antinociception (17).
Stage of Estrous Cycle Determines KOR Participation in Spinal Morphine Antinociception.
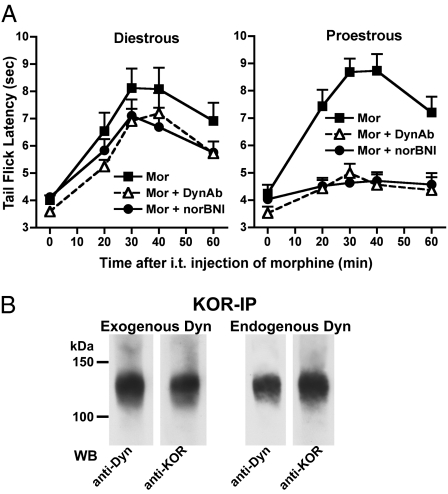
To assign functional relevance to spinal cord heterodimeric MOR/KOR, we determined whether the size of the KOR-mediated component of spinal morphine antinociception differed during proestrous vs. diestrous, i.e., whether it varied in parallel with spinal MOR/KOR expression levels. Dependence on KOR was defined as the component of spinal morphine antinociception that was eliminated by the i.t. application of the KOR-selective antagonist nor-binaltorphimine (nor-BNI). During diestrous, blockade of spinal KOR with i.t. nor-BNI produced a numeric reduction in the magnitude of spinal morphine antinociception (≈30%) that did not reach statistical significance (Fig. 3A; P > 0.05; n = 7). During proestrous, however, blockade of spinal KOR virtually abolished spinal morphine antinociception (Fig. 3A; P < 0.001; n = 7). The parallel increase in nor-BNI sensitivity and spinal expression levels of heterodimeric MOR/KOR suggested that it could be the molecular transducer for the female-specific KOR component of spinal morphine antinociception.
Fig. 3.
Contribution of dynorphin/KOR (via MOR/KOR heterodimer) to spinal morphine antinociception is stage of cycle dependent. (A) Spinal morphine (5 μg i.t.) antinociception was quantified by using the tail flick test during diestrous and proestrus, 18 h or 30 min after the i.t. application of either the KOR blocker nor-BNI (26 nmol) or affinity purified anti-dynorphin 1–17 antibodies (200 ng), respectively. Data are expressed as mean ± SEM tail flick latency (n = 7). Attenuation of spinal morphine antinociception following either blockade of spinal KOR or the i.t. application of anti-dynorphin 1–17 antibodies is stage of cycle dependent. (B) Dynorphin 1–17 is a substrate for MOR/KOR heterodimer. (Left) Spinal cord membranes of a proestrous rat were incubated with 1 μM dynorphin 1–17 and subsequently cross-linked. KOR immunoprecipitate was subjected to parallel Western blot analyses by using either anti-dynorphin 1–17 or anti-KOR antibodies. The ≈120-kDa heterodimeric MOR/KOR was visualized by using either antibody suggesting that dynorphin binds to the MOR/KOR heterodimer. (Right) Spinal dynorphin 1–17 released by the i.t. application of morphine was cross-linked in vitro. Visualization of the ≈120-kDa heterodimeric MOR/KOR by using anti-dynorphin 1–17 antibodies as well as anti-KOR antibodies indicates that dynorphin 1–17 is an in vivo substrate for the heterodimeric MOR/KOR. Mor, morphine; WB, Western blot; anti-Dyn (DynAb), anti-dynorphin antibodies.
The Intrathecal Application of KOR-Selective Agonists Do Not Produce Antinociception Using the Tail Flick Assay.
We investigated, in parallel with biochemical studies described above, antinociceptive responsiveness to the i.t. application of two KOR-selective agonists during proestrous to eliminate the possibility that spinal monomeric KOR was responsible for the increased KOR component of spinal morphine antinociception. U50,488 and U69,593 were selected because both have minimal affinity for MOR and, thus, would activate only monomeric KOR or possibly the KOR protomer of the MOR/KOR heterodimer. In these experiments, only a single concentration of U50,488H was tested because this KOR-selective agonist was used to validate the negative results of others. Consistent with earlier reports (19–22), neither U50,488H (300 nmol; n = 7) nor U69,593 (3, 10, 30, and 60 nmol; n = 4 for each dose) produced detectable antinociception by using the tail flick test, the method used to assess spinal morphine antinociception and its dependence on spinal KOR. Because the tail flick test does not detect putative antinociceptive contributions of monomeric KOR, it is highly unlikely that monomeric KOR mediates the increased nor-BNI–sensitive component of i.t. morphine antinociception, which was detected by using that test.
Stage of Estrous Cycle Determines Dynorphin Contribution to Spinal Morphine Antinociception.
In an earlier report (17), this laboratory demonstrated that spinal dynorphin is a prerequisite for spinal morphine antinociception in females. If the dynorphin/KOR component of spinal morphine antinociception is mediated by heterodimeric MOR/KOR, changes in expression levels of MOR/KOR and effects of i.t. anti-dynorphin antibodies on spinal morphine antinociception should vary in parallel. Accordingly, effects of i.t. anti-dynorphin antibodies on spinal morphine antinociception was determined during proestrous vs. diestrous. The dose of anti-dynorphin antibody used was based on an earlier report from this laboratory (17). As was observed for i.t. nor-BNI, the i.t. application of anti-dynorphin antibodies during diestrous produced a numeric decrease in spinal morphine antinociception (≈11%) that did not reach statistical significance (P > 0.05; Fig. 3A). In contrast, during proestrous, i.t. anti-dynorphin antibodies essentially abolished morphine antinociception (Fig. 3A; P < 0.001). Parallel changes in the magnitude of effects of i.t. anti-dynorphin antibodies, i.t. nor-BNI, and the spinal content of dimeric MOR/KOR suggest that spinal dynorphin acts on heterodimeric MOR/KOR to provide the required KOR component of spinal morphine antinociception. Because we had demonstrated that the i.t. application of preabsorbed anti-dynorphin antibody did not alter spinal morphine antinociception in females (17), this control was not repeated to validate conclusions.
Dynorphin 1–17 Is a Substrate for Spinal MOR/KOR Heterodimers.
To validate the presumptive binding of dynorphin 1–17 to MOR/KOR heterodimers, we incubated dynorphin 1–17 with spinal cord membranes obtained from proestrous females, after which they were exposed to the cross-linking reagent disuccinimidyl suberate (DSS; see Materials and Methods and SI Materials and Methods for details), immunoprecipitated using anti-KOR antibodies, and subjected to Western blot analysis using anti-dynorphin antibodies. In addition to the expected ≈60-kDa Western blot signal (monomeric KOR), a signal of ≈120 kDa was also observed (Fig. 3B). Importantly, identical signals were observed when an adjacent lane that contained an identical aliquot of immunoprecipitate was subjected to parallel Western blot analysis using anti-KOR antibodies (Fig. 3B). These results indicate that dynorphin is a putative substrate for spinal heterodimeric MOR/KOR.
Of critical importance, endogenous spinal dynorphin 1–17 is also a substrate for the spinal MOR/KOR heterodimer. To demonstrate this attribute of dynorphin 1–17, we used i.t. morphine, shown previously by this laboratory to release endogenous dynorphin (17), which was then cross-linked in vitro and processed for KOR IP (Materials and Methods and SI Materials and Methods). Dynorphin or KOR Western blot analyses of the above immunoprecipitate also revealed a signal of ≈120 kDa (Fig. 3B), identical to that observed in spinal cord incubated in vitro with exogenous dynorphin 1–17. This finding validates that endogenous spinal dynorphin 1–17 is a substrate for heterodimeric MOR/KOR because exogenous dynorphin was not administered before cross-linking and the antibody used for Western blotting is highly selective for dynorphin 1–17 vs. its truncated forms.
Discussion
Although it is now widely accepted that G protein-coupled receptors (GPCRs) can exist as heterodimers (or homodimers), as well as monomers (23, 24), the functional significance of GPCR dimers remains unsettled. There are multiple putative consequences of heterodimeric formation: (i) amplification of the potency of agonists selective for each protomer of a dimeric GPCR (25); (ii) integration of the activity of their monomeric counterparts, i.e., occupation by a single drug of one protomer can exert a positive or negative influence on binding to the other protomer of the homo (hetero) dimer (26–28); (iii) altered β-arrestin binding and, consequently, intracellular receptor trafficking (29); and (iv) acquisition of new G protein selectivity (28, 30, 31).
These data notwithstanding, the physiological relevance of dimeric GPCRs remains enigmatic because the vast preponderance of experiments from which functional inferences have been drawn use overexpressing cells maintained in culture. Such experiments cannot shed any light on either the presence or physiological regulation of dimeric GPCRs in the central nervous system or their physiological/pathophysiological significance.
This study not only demonstrates the presence of MOR/KOR heterodimers in a naturally occurring integrated tissue but also its sexually dimorphic presentation and regulation by ovarian sex steroids. Although a portion of the sexually dimorphic presentation of heterodimeric MOR/KOR is genetic/developmental (resistant to ovariectomy), the striking influence of the stage of the estrous cycle on spinal levels of heterodimeric MOR/KOR reveals that its formation is dynamic and plastic, depending on physiological status. This plasticity could suggest that the physiological significance of spinal heterodimeric MOR/KOR is contextually determined and dependent on physiological state. This perspective is supported by our finding that the MOR/KOR heterodimer is differentially recruited by i.t. morphine depending on sex and stage of estrous cycle, i.e., heterodimeric MOR/KOR mediates the much larger KOR component of spinal morphine antinociception that occurs during proestrous vs. diestrous.
Females have greater antinociception responsiveness to KOR ligands than do males (11–14, 32), but compelling mechanistic formulations have not undergirded these observations. Dimeric MOR/KOR represents a female-specific signaling molecule that could underlie reports of enhanced KOR antinociception in women vs. men. This formulation is supported by (i) the female-specific expression in spinal cord of heterodimeric MOR/KOR that parallels the manifestation of a dynorphin/KOR component of spinal morphine antinociception; (ii) the inability of either i.t. U50,488 or U69,593, both of which activate monomeric KOR, to produce antinociception by using the tail flick test during proestrous, during which time the same nociceptive test indicates a substantially augmented KOR component of spinal morphine antinociception; and (iii) the absence of a dynorphin/KOR component of spinal morphine antinociception in males (17) despite the presence in their spinal cord of monomeric KOR.
We suggest that activation of the KOR protomer within the MOR/KOR heterodimer (by dynorphin 1–17) concomitant with the activation of its MOR protomer has effects not manifested when monomeric KOR is activated alone. In this regard, it should be noted that individual blockade of either spinal MOR or KOR in females essentially abolishes i.t. morphine antinociception, indicating the requirement for their concomitant activation (17). It is possible, if not likely, that dynorphin 1–17 also binds and activates the MOR protomer of the MOR/KOR heterodimer given the high affinity of dynorphin 1–17 for MOR (KI = 2.6 ± 0.9 nM) albeit less than that for KOR (KI = 0.3 ± 0.09 nM) (33). Thus, dynorphin 1–17 signaling via spinal MOR/KOR heterodimers could enable the selective recruitment and integration of two anatomically and functionally distinct opioid pathways by using a single ligand, i.e., participating cells would be only those that express both MOR and KOR.
There is precedent that heterodimers acquire novel pharmacological characteristics and functions not manifest by their component protomers. These characteristics include new selectivity of G protein coupling (26, 30, 31, 34, 35), activation of new signaling cascades (36), altered agonist affinities (34), and altered intracellular trafficking (37). These functionalities notwithstanding, we do not yet know the particular attributes of the MOR/KOR heterodimer, not shared by its KOR protomer when activated individually, that are advantageous to the transduction of spinal KOR antinociceptive signals. With current technology, it is not possible to ascertain the fractions of the monomeric spinal MOR and KOR populations that exist as heterodimers. Nevertheless, heterodimers such as MOR/KOR may be part of a large heterooligomeric array, a small fraction of which could actually affect functioning of the entire cluster, as has been suggested (38).
The enhanced formation of dimeric MOR/KOR during proestrus vs. diestrous indicates that ovarian sex steroids regulate levels of MOR/KOR. This finding suggests that the role of MOR/KOR in spinal antinociception could be related to reproductive status. Sex-dependent differential attributes of spinal opioid antinociception may have evolved to better suit sex-dependent functions. The hunter/warrior function assumed by males predisposes them to greater severity and frequency of traumatic injury and attendant somatic pain than females. In contrast, the reproductive function of females predisposes them to experience visceral pain of greater severity and frequency than their male counterparts. We envision that this differential vulnerability to painful experience was a major driving force behind the evolution of sex-dependent divergent antinociceptive systems. A heterodimeric MOR/KOR antinociceptive pathway that is selectively recruited during recurrent physiological states could enable coping with the visceral discomfort/pain associated with those conditions without compromising the ability to harness the more robust monomeric MOR-coupled antinociceptive system, if needed.
The sex- and ovarian steroid-dependent recruitment of spinal cord MOR/KOR heterodimers would provide a way to influence the balance between antinociceptive vs. pronociceptive functions of the spinal dynorphin/KOR opioid system, which has long been debated. It is tempting to speculate that the chemical pairing of KOR and MOR enables the recruitment of spinal KOR-mediated antinociception without being compromised by the concomitant pronociceptive functions that spinal KOR also subserves (see ref. 39 for review). Whereas antinociception would be favored by the enhanced formation of heterodimeric MOR/KOR, its impaired generation could be pronociceptive. Impaired formation of heterodimeric MOR/KOR could be a biological determinant of a variety of chronic pain states that are vastly more pervasive in women than men.
Materials and Methods
Please refer to SI Materials and Methods for a more extensive description of the experimental procedures used.
Experimental Animals, Determination of Stage of Estrous Cycle, Adult Ovariectomy, i.t. Drug Administration, and Determination of Tail Flick Latency.
Experiments used male and female adult (250–300 g) Sprague–Dawley rats (Charles River). The Animal Care and Use Committee of State University of New York Downstate approved all experimental procedures. Histology of vaginal smears was used to evaluate stage of cycle. Established procedures were used to ovariectomize female rats, quantify tail flick latency, and administer drugs to the i.t. space (see SI Materials and Methods for details and references).
Membrane Preparation, IP, and Western Blot Analysis.
Spinal cord membranes were prepared and solubilized as described by this laboratory (see SI Materials and Methods for references and detailed description). Briefly, immunoprecipitates to be compared were always obtained and processed in parallel. Two sequential IP procedures were performed by using either an N-terminally directed anti-KOR antibody (aa 1–70; Santa Cruz Biotechnology) or an anti-MOR antibody generated against the carboxyl-terminal 50 aa of MOR (40) (generously provided by Thomas Cote, Uniformed Services, University of the Health Sciences, Bethesda, MD). This antibody was also used for MOR Western blotting. The first IP was performed in solution as described (41) The second IP used antibodies that were immobilized on columns, which were prepared and eluted according to manufacturer (Pierce) instructions. The first IP (in solution) was used to concentrate MOR/KOR from a large amount (400–600 μg) of membrane. The second IP, which used immobilized antibody, enabled elimination of IgG, which would have confounded Western blot detection of monomeric MOR and KOR species. The initial eluate contained virtually all of the MOR/KOR dimer, effectively concentrating it, whereas the monomeric species were contained in both the first and second column eluates. Because only the first eluate was used for MOR or KOR Western blot analyses, the double IP method facilitated obtaining samples for Western blot analysis enriched in MOR/KOR heterodimers. Anti-MOR and anti-KOR Western blot analysis was performed as previously reported and described in SI Materials and Methods. In contrast to the N-terminally directed anti-KOR antibody used for KOR IP, the anti-KOR antibody that was used for KOR Western blot analysis was generated against aa 262–275 of KOR (Pierce). Affinity purified anti-dynorphin 1–17 and anti-EM2 antibodies were purchased from Bachem and Neuromics, respectively.
Cross-Linking EM2 and Dynorphin 1–17 to MOR/KOR Heterodimers.
Membranes from female (proestrous) spinal cord were incubated in vitro with either EM2 or dynorphin 1–17, and cross-linked with 5 mM DSS as per manufacturer instructions (Pierce). Using DSS, we also cross-linked endogenously released dynorphin to MOR/KOR heterodimers.
Statistical Analysis.
Significance of differences in the magnitude of the Western blot signals was assessed by using the two-tailed Student's t test. A mixed linear model was used to assess effects on tail flick latency of drug treatment, stage of estrus cycle, and their interaction.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009923107/-/DCSupplemental.
References
- 1.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128–134. doi: 10.1007/s11926-001-0008-3. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA, Naliboff B, Lee O, Munakata J, Chang L. Review article: Gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther. 1999;13(Suppl 2):65–69. doi: 10.1046/j.1365-2036.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- 4.Larijani GE, Goldberg ME, Gratz I, Warshal DP. Analgesic and hemodynamic effects of a single 7.5-mg intravenous dose of morphine in patients with moderate-to-severe postoperative pain. Pharmacotherapy. 2004;24:1675–1680. doi: 10.1592/phco.24.17.1675.52335. [DOI] [PubMed] [Google Scholar]
- 5.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: Effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 6.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 7.Coyle DE, Sehlhorst CS, Mascari C. Female rats are more susceptible to the development of neuropathic pain using the partial sciatic nerve ligation (PSNL) model. Neurosci Lett. 1995;186:135–138. doi: 10.1016/0304-3940(95)11304-f. [DOI] [PubMed] [Google Scholar]
- 8.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: Relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- 9.Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol. 2002;452:163–173. doi: 10.1016/s0014-2999(02)02274-4. [DOI] [PubMed] [Google Scholar]
- 10.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 11.Gear RW, et al. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 12.Gear RW, et al. Sexual dimorphism in very low dose nalbuphine postoperative analgesia. Neurosci Lett. 2003;339:1–4. doi: 10.1016/s0304-3940(02)01438-6. [DOI] [PubMed] [Google Scholar]
- 13.Gear RW, et al. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 14.Gear RW, et al. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 15.Mogil JS, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sternberg WF, et al. Neonatal testosterone exposure influences neurochemistry of non-opioid swim stress-induced analgesia in adult mice. Pain. 1995;63:321–326. doi: 10.1016/0304-3959(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. J Pharmacol Exp Ther. 2007;322:654–660. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- 18.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 19.Stevens CW, Yaksh TL. Dynorphin A and related peptides administered intrathecally in the rat: A search for putative kappa opiate receptor activity. J Pharmacol Exp Ther. 1986;238:833–838. [PubMed] [Google Scholar]
- 20.Przewłocka B, Dziedzicka M, Lasoń W, Przewłocki R. Differential effects of opioid receptor agonists on nociception and cAMP level in the spinal cord of monoarthritic rats. Life Sci. 1992;50:45–54. doi: 10.1016/0024-3205(92)90196-v. [DOI] [PubMed] [Google Scholar]
- 21.Piercey MF, Einspahr FJ. Spinal analgesic actions of kappa receptor agonists, U-50488H and spiradoline (U-62066) J Pharmacol Exp Ther. 1989;251:267–271. [PubMed] [Google Scholar]
- 22.Schmauss C. Spinal kappa-opioid receptor-mediated antinociception is stimulus-specific. Eur J Pharmacol. 1987;137:197–205. doi: 10.1016/0014-2999(87)90223-8. [DOI] [PubMed] [Google Scholar]
- 23.Angers S, Salahpour A, Bouvier M. Dimerization: An emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–435. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 24.George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 25.Maggio R, Scarselli M, Novi F, Millan MJ, Corsini GU. Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole and ropinirole. J Neurochem. 2003;87:631–641. doi: 10.1046/j.1471-4159.2003.02038.x. [DOI] [PubMed] [Google Scholar]
- 26.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco R, et al. Evidence for adenosine/dopamine receptor interactions: Indications for heteromerization. Neuropsychopharmacology. 2000;23(4 Suppl):S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 28.Galvez T, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc Natl Acad Sci USA. 2004;101:1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SP, et al. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 31.Mellado M, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtman JR, Jr, Wala EP. Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacol Biochem Behav. 2006;83:100–108. doi: 10.1016/j.pbb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Garzón J, Sánchez-Blázquez P, Höllt V, Lee NM, Loh HH. Endogenous opioid peptides: Comparative evaluation of their receptor affinities in the mouse brain. Life Sci. 1983;33(Suppl 1):291–294. doi: 10.1016/0024-3205(83)90500-3. [DOI] [PubMed] [Google Scholar]
- 34.George SR, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 35.Rashid AJ, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 37.Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–38824. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- 38.Park PS, Sum CS, Pawagi AB, Wells JW. Cooperativity and oligomeric status of cardiac muscarinic cholinergic receptors. Biochemistry. 2002;41:5588–5604. doi: 10.1021/bi011746s. [DOI] [PubMed] [Google Scholar]
- 39.Lai J, Ossipov MH, Vanderah TW, Malan TP, Jr, Porreca F. Neuropathic pain: The paradox of dynorphin. Mol Interv. 2001;1:160–167. [PubMed] [Google Scholar]
- 40.Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Côté TE. Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized mu-opioid receptors from rat brain: Coimmunoprecipitation of the G proteins G(alpha o), G(alpha i1), and G(alpha i3) J Neurochem. 2000;74:1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarti S, Chang A, Gintzler AR. Subcellular localization of mu-opioid receptor G(s) signaling. J Pharmacol Exp Ther. 2010;333:193–200. doi: 10.1124/jpet.109.165142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.