Abstract
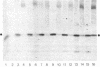
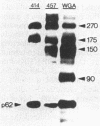
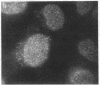
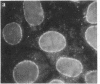
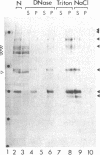
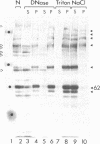
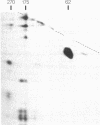
Using a monoclonal antibody (mAb 414), we previously identified a protein of 62 kDa (p62) that was localized to the nuclear pore complex by immunoelectron microscopy. We also showed that p62 binds specifically to wheat germ agglutinin. Therefore, we proposed that this nuclear pore complex protein might be a member of a recently characterized family of glycoproteins that are labeled by in vitro galactosylation of rat liver nuclei and contain O-linked monosaccharidic GlcNAc residues. In support of this, we now show that incubation with N-acetylglucosaminidase reduces the molecular mass of p62 by approximately 3 kDa because of the removal of terminal GlcNAc residues. Moreover, p62 can be galactosylated in vitro by using UDP-[3H]galactose and galactosyltransferase. We also show that most of the GlcNAc residues are added within 5 min of synthesis, when p62 is soluble and cytosolic. Thus, the addition of GlcNAc is carried out in the cytoplasm and is clearly distinct from the N- and O-linked glycosylation pathways of the endoplasmic reticulum and Golgi complex. Using another mAb with a broad specificity for nuclear GlcNAc-containing proteins, we show by immunofluorescence and protein blotting of subnuclear fractions that some of these proteins are in the interior of the nucleus, and others are most likely located in the pore complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglia F. A., Maul G. G. Nuclear ribonucleoprotein release and nucleoside triphosphatase activity are inhibited by antibodies directed against one nuclear matrix glycoprotein. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2285–2289. doi: 10.1073/pnas.80.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976 Sep;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Hogan M., Miller R., DeGaetano D. A nuclear specific glycoprotein representative of a unique pattern of glycosylation. J Biol Chem. 1987 Jan 25;262(3):1254–1260. [PubMed] [Google Scholar]
- Schwyzer M., Hill R. L. Porcine A blood group-specific N-acetylgalactosaminyltransferase. J Biol Chem. 1977 Apr 10;252(7):2346–2355. [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984 Mar 10;259(5):3308–3317. [PubMed] [Google Scholar]