Abstract
Human beings are remarkably skilled at recognizing faces, with the marked exception of other-race faces: the so-called “other-race effect.” As reported nearly a century ago [Feingold CA (1914) Journal of Criminal Law and Police Science 5:39–51], this face-recognition impairment is accompanied by the popular belief that other-race faces all look alike. However, the neural mechanisms underlying this high-level “perceptual illusion” are still unknown. To address this question, we recorded high-resolution electrophysiological scalp signals from East Asian (EA) and Western Caucasian (WC) observers as they viewed two EA or WC faces. The first adaptor face was followed by a target face of either the same or different identity. We quantified repetition suppression (RS), a reduction in neural activity in stimulus-sensitive regions following stimulus repetition. Conventional electrophysiological analyses on target faces failed to reveal any RS effect. However, to fully account for the paired nature of RS events, we subtracted the signal elicited by target to adaptor faces for each single trial and performed unbiased spatiotemporal data-driven analyses. This unique approach revealed stronger RS to same-race faces of same identity in both groups of observers on the face-sensitive N170 component. Such neurophysiological modulation in RS suggests efficient identity coding for same-race faces. Strikingly, OR faces elicited identical RS regardless of identity, all looking alike to the neural population underlying the N170. Our data show that sensitivity to race begins early at the perceptual level, providing, after nearly 100 y of investigations, a neurophysiological correlate of the “all look alike” perceptual experience.
Keywords: adaptation, face processing, EEG, visual cognition
Almost 100 y ago, Feingold (1) reported that human beings living in different geographical locations perceive individuals belonging to “other-races” (OR) as all looking alike: “Other things being equal, individuals of a given race are distinguishable from each other in proportion to our familiarity, to our contact with the race as whole. Thus, to the uninitiated American all Asiatics look alike, while to the Asiatics, all White men look alike.” This commonly experienced all look alike “perceptual illusion” for OR faces is at the root of one of the most robust empirical findings in face recognition: the other-race effect (ORE). The ORE refers to the marked behavioral impairment displayed by humans in recognizing OR compared to same-race (SR) unfamiliar faces (i.e., lower accuracy coupled with higher false identifications for OR faces). The scientific literature has provided clear evidence that the ORE and the popular belief that OR faces all look alike are not accounted for by the paucity of anthropometric variations in OR faces, but by a genuine lack of expertise. Although this theoretical explanation has been supported by numerous behavioral (for a review, see ref. 2), computational (e.g., refs. 3–5) and neuroimaging (6–15) studies on the ORE, the neurophysiological correlates of the all look alike perceptual experience, have never been directly investigated.
This observation is even more surprising considering that the rapid development of neuroimaging techniques has dramatically increased our knowledge of how the brain achieves visual categorization. Studies using single-cell recordings in primates and functional MRI (fMRI) in humans have shown the existence of neural populations responding preferentially to faces (e.g., 16–19) and face identity (20–23). Importantly, genuine race effects require an interaction between the race of the observer and the race of the faces. Only one fMRI study has used two groups of observers, revealing greater responses for SR compared with OR faces in the face-sensitive cortex during a face memorization task (9). Crucially, however, the all look alike effect takes place in the subsequent face recognition stage during which mechanisms related to individual face identification are engaged. For example, it is common experience to misidentify personally familiar individuals belonging to an OR group (i.e., misidentify your Chinese friend on the street, if you are Westerner), whereas this perceptual misidentification related to the all look alike effect does not occur during the memorization of familiar faces. In addition, fMRI prevents from drawing any conclusion on the time-course of neural sensitivity to race.
Human faces elicit also a particular electrophysiological signature: the N170 event-related potential (ERP) (24, 25; for a review, see ref. 26). The N170 is a bilateral occipito–temporal negative deflection peaking roughly 170 ms after stimulus onset, larger for faces compared with other visual categories. Activity in this time window is associated with the early accumulation of perceptual information leading to visual categorization, which is necessary for postsensory, decision, and motor stages (27, 28).
Numerous electrophysiological studies have investigated the early electrophysiological dynamics of the ORE (6, 7, 10–15). Several studies have failed to show any sensitivity to race on the N170 (6, 7, 14), or on its frontal counterpart, the vertex positive potential (11). These results suggest that brain activity in the N170 time window codes only for the detection of a face shape, whereas race information from faces is extracted in postperceptual stages occurring roughly between 250 and 300 ms after stimulus onset (6, 7, 14). On the other hand, several studies have reported larger N170 amplitudes for OR faces compared with SR faces (10, 13, 15). These findings, which might be partially due to uncontrolled physical differences across the stimulus set (29), are also at odds with many studies that have shown larger N170 to object categories of expertise (30), a result that would lead to the prediction of a larger N170 to SR faces. The heterogeneity of these results and the lack of sensitivity of the N170 may also be explained by the use of diverse task constraints: the detection of catch trials (7, 15), detection of colored faces (29), explicit race categorization (6), and an old–new face recognition design (14). Furthermore, previous studies used different types of OR stimuli, including Western Caucasian (WC) (6, 7, 10–13, 15, 29), East Asian (EA) (6, 7, 10, 13, 29), African American (11, 12, 14, 15, 29), and Hispanic faces (14). More importantly, all these ERP studies relied exclusively on data from the WC population, which is a methodological problem, because any effect could be confounded by physical differences in the face stimuli, preventing any firm conclusion on the ORE. A full crossover interaction between races of observers and the race of faces is necessary to assess genuine behavioral and neurophysiological ORE. To the best of our knowledge, only one electrophysiological study used two groups of observers and reported sensitivity to race for inverted faces in two groups of observers (29). Critically, none of these neuroimaging studies has yet used a paradigm optimally tapping into mechanisms devoted to face recognition, which leaves the neurophysiological bases of the all look alike effect unexplored.
Adaptation is a well-established paradigm to reveal the nature of information coding at the perceptual and neurophysiological levels (for a review, see ref. 31). Neural activity in stimulus-sensitive regions is typically reduced when a stimulus is repeated, a phenomenon known as “repetition suppression” (RS) (31–34). RS was initially observed in monkey single-cell recordings (e.g., refs. 33–36). More recently it has been reported in human electrophysiological (e.g., refs. 37–41) and fMRI blood-oxygen level-dependent studies (e.g., refs. 23, 34, 42) using a variety of cognitive tasks. Although the precise neural computations of RS remain unclear, RS elicited by two stimuli presented in rapid succession indicates the engagement of the same (or at least a largely overlapping) neural population in the processing of both stimuli (31). Therefore, the amount of RS is related to the capacity of a neural population to discriminate stimuli and could be compared with a novelty detection mechanism (31), decreasing neural responses’ redundancy and increasing coding efficiency (31, 36, 43–45). Interestingly, the face-sensitive N170 component shows preferential RS to faces but not to other visual categories (46), as well as to face identity (47–49). Thus, RS represents a powerful tool to elucidate the time-course and the nature of the neural representations leading to the all look alike effect. In this context, it seems logical to predict stronger RS for SR compared with OR faces in the N170 time window.
To address this question, we recorded high-temporal resolution scalp ERP signals in EA and WC observers as they viewed sequences of two faces: an “adaptor” and a “target” face (Fig. S1). In each sequence, the two faces were either EA or WC, and of the same or different identities. To minimize the use of trivial image matching strategies, we changed the facial expression displayed by the adaptor and the target faces. As expected, Western and Eastern observers showed an ORE, as assessed in a separate face recognition task. Previous electrophysiological adaptation studies compared the ERPs to target faces, ignoring the response to adaptor faces (46–49). In our experiment, this conventional approach failed to reveal any significant difference across conditions. However, because RS is a signal reduction to the second stimulus of a pair, we developed a unique single-trial analysis method. We subtracted the signal elicited by the presentation of the target face to that elicited by the adaptor face independently for each pair, resulting in a single-trial RS (stRS) electrophysiological response (Fig. S2). We also used unbiased spatiotemporal data-driven analyses at all electrodes and time-points. In line with previous findings (47–49), stRS responses in the N170 time window showed larger RS for SR faces of the same identity compared with any of the other conditions in both groups of observers. This result suggests a more effective coding of identity for SR faces than OR faces. Strikingly, however, in both groups of observers OR faces elicited similar RS responses regardless of a change in facial identity, suggesting that the neural populations underlying the early face-sensitive N170 responses cannot discriminate OR face exemplars.
Results
We carried out ANOVAs independently at all electrodes and all time-points (see Methods for details). This approach led to a large number of F, t, and P values. For clarity, we report here only the minimum and maximum F or t values and their associated P values. Exact P values are reported for significant effects, unless they were smaller than 0.001.
Behavioral Results.
WC observers were better at recognizing SR (d' = 2.2; SD = 0.44) than OR faces (d' = 1.7; SD = 0.4). Likewise, EA observers were better at recognizing SR (d' = 2.1; SD = 0.41) than OR faces (d' = 1.6; SD = 0.49). A repeated-measures ANOVA performed on d' scores, with race of the observers (WC and EA) as a between-subject factor, and race of the face (WC or EA) as within-subject factor, confirmed the significance of this observation [F(2,32) = 7.19; P < 0.001].
ERP Results.
Descriptive statistics.
Consistent with previous research (26), for both groups of observers the N170 peaked bilaterally over parietal–occipital electrodes, being largest at the right hemisphere electrode PO8h (Table S1). After artifact rejection, the mean number of trials per condition was 72 (SD = 9.2).
RS results.
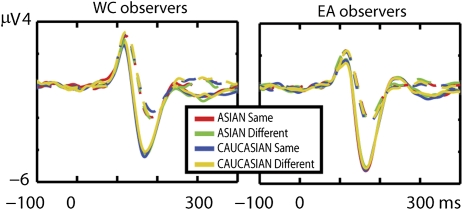
Following the conventional RS analysis, we performed a two-way ANOVA of the amplitude of the ERPs to target faces, independently at all electrodes and time-points [race of the observer (two: WC, EA) × condition (four: WC same identity, WC different identity, EA same identity, EA different identity)]. This ANOVA revealed no significant effect across the whole epoch [P > 0.05, corrected for multiple comparisons (MC) by a multivariate cluster analysis, as described in Methods] (Fig. 1). A two-way ANOVA of the amplitude of the ERPs to adaptor faces also led to null results [race of the observer (two: WC, EA) × race of the face (two: WC, EA; P > 0.05, MC corrected)] (Fig. 1).
Fig. 1.
Mean ERPs elicited by the adaptor (continuous line) and the target faces (dotted line) measured at PO8h for the four conditions (Asian faces of same identities, red line; Asian faces of different identities, green line; Caucasian faces of same identities, blue line; Caucasian faces of different identities, yellow line) for WC and EA observers. No significant differences were observed across all conditions for the ERPs elicited by both adaptor and target faces.
We carried out a two-way ANOVA of the peak latency of the P1, N170, and P2 components elicited by the target face. The race of the observer was the between-subject factor and the four conditions (as described above) were the within-subject factor. We performed the ANOVA at the electrodes where the components were the largest: PO8h and PO7h for the N170, and O2 and O1 for P1 and P2. No significant latency differences were observed for P1 [F(2,11) = 1.12; P > 0.05], N170 [F(2,11) = 2.11; P > 0.05], or P2 [F(2,11) = 1.06; P > 0.05].
We also carried out a two-way ANOVA on the peak latency of P1, N170, and P2 components elicited by the adaptor face at the electrodes described above. The factors of the ANOVA were race of the observers and conditions. Once again, no significant latency differences were observed for P1 [F(2,11) = 2.31; P > 0.05], N170 [F(2,11) = 0.51; P > 0.05], or P2 [F(2,11) = 1.2; P > 0.05].
Single-trial RS results.
Only trials accepted for both adaptor and target faces were included in the computation of the stRS ERP (see results at electrode PO8h in Fig. 2A). The mean number of trials accepted per condition thus dropped from 72 to 54 (minimum = 46; maximum = 68; standard = 6.4).
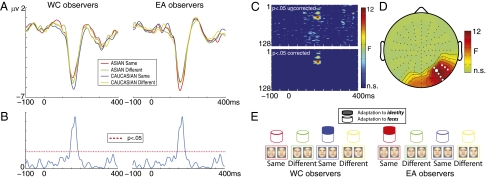
Fig. 2.
(A) Single-trial RS responses measured at PO8h, the electrode with the largest N170 for WC and EA observers. The stRS responses are obtained by averaging the single-trial differences between adaptor and target faces. Note that the more negative is the amplitude, the stronger the adaptation. (B) Time-course of the F values for the group × condition interaction from the ANOVA on the stRS amplitude at PO8h, the electrode with the largest effect size. Note the burst of significant F values coincided with the stRS peak latency. (C) Significant F values of the group × condition interaction on the stRS amplitude obtained from the ANOVA carried out independently at all electrodes and time-points, uncorrected (Left) and corrected (Right) for multiple comparisons. Electrodes are stacked up along the y axis and time is shown along the x axis. The electrode order is not based on their spatial topographical positions (which instead is correctly shown in D). (D) Topography of the F values at the latency of the maximum F, showing the electrodes displaying a significant group × condition interaction on the stRS amplitude. The white dots are the right occipito–temporal electrodes that displayed significantly larger RS responses for SR same identity faces compared with any other condition. The largest white dot indicates the electrode showing the largest effect. (E) Model. A general level of adaptation to faces is observed for all conditions (empty bars). The stRS specific to identity is only observed for same-race faces (filled bars), which is related to greater coding efficiency for this visual category.
The ANOVA (race of the observer × condition) carried out on the stRS amplitude independently at all electrodes and time-points revealed a significant interaction [maximum F(2,11) = 11.06; minimum F(2,11) = 2.75, P = 0.049, MC corrected], which was maximal at the latency of the stRS peak amplitude at PO8h (Fig. 2). This interaction was significant over a cluster of center and right occipito–parietal electrodes (Fig. 2D). The time-course of the F values of the race of the observer × condition interaction revealed a burst of significant F values in the N170 time window, synchronous with the largest RS responses (Fig. 2B). Post hoc paired t tests revealed that regardless of the race of the observers, SR same-identity trials elicited significantly larger stRS responses compared with the other conditions [at PO8h − minimum: WC: t(11) = −2.73; P = 0.02; EA: t(11) = −2.29; P = 0.04; maximum: WC: t(11) = −3.92; P = 0.002; EA: t(11) = −4.22; P = 0.001] over a cluster of right occipito–parietal electrodes only (i.e., P8, P8h, P10h, PO6, PO8, PO8h, PO10h, PO10) (Fig. 2D). RS was equivalent across the remaining conditions (Fig. 2A). Moreover, stRS responses were time-locked to stimulus onset and reliably present throughout the duration of the experiment (Fig. S3). No other significant amplitude differences were observed.
We also carried out a two-way ANOVA (race of the observer × condition) on the peak latency of the stRS response at the electrodes showing significant race of the observer × condition interaction on the stRS amplitude (as described above). No significant latency differences were observed.
Finally, to rule out any potential significant contribution of the different facial expressions portrayed in the adaptor faces in modulating the stRS responses, we carried out two-way ANOVAs (two groups of observers, WC and EA, × five facial expressions of emotion: angry, sad, happy, surprised, and disgust) at all electrodes and time-points on stRS signals. Our data revealed that facial expressions did not modulate stRS signals (P > 0.05, MC corrected) (Fig. S4).
Discussion
This cross-cultural study investigated the early neural dynamics of the all look alike perceptual phenomenon, the root of the marked recognition impairment observed in humans for OR faces. We used an adaptation paradigm and recorded electrophysiological signals. In line with previous behavioral studies on the ORE (e.g., refs. 15, 30, 50–54), our WC and EA observers were more accurate at recognizing SR than OR faces. Importantly, in both groups of observers, our component-free spatiotemporal EEG analyses revealed larger repetition suppression (stRS) following adaptation to SR faces of the same facial identity compared with SR faces of a different identity. Consistently with previous electrophysiological studies, adaptation to face identity occurred in the N170 time window (47, 49).
In addition, we found a unique result: RS responses did not discriminate between “same” and “different” identities for OR faces in both groups of observers. This crossover interaction can be considered as an early neurophysiological signature of the perceptual all look alike effect, occurring in a time window typically associated with early categorical face processing (e.g., refs. 27, 47–49, 55). Importantly, the full crossover interaction between the race of the observers and the race of the faces demonstrates that the differences in stRS are genuinely related to race and culture of the observers, and not a consequence of differences in the visual properties of faces from different races (see also ref. 29). It is also worth noting that RS and the all look alike perceptual phenomenon were not modulated by the facial expression of the adaptor faces.
Identity-dependent RS occurred at right occipito–temporal electrodes only, in line with lesion studies suggesting that the right anterior temporal cortex is critical for face identification (e.g., 56–58). These early scalp electrophysiological effects were similar in observers from two cultures. This finding suggests that the consecutive presentation of any pair of faces elicits a comparable stRS (represented in Fig. 2E by the nonfilled bars). Neural populations coding for face shapes might be involved in these early RS responses. Whereas the presentation of any face caused some level of adaptation, the presentation of faces with “same identity” was responsible for an additional amount of adaptation only if faces were from the same race as the observer (represented in Fig. 2E by the plain bars). This race-preferential adaptation might be a result of the recruitment of a supplementary neural population coding selectively for SR facial identity. Importantly, our results converge with evidence showing the existence of face-preferential cortical areas (e.g., refs. 18, 59, 60), as well as distinct populations specifically coding for face identity (e.g., refs. 20–23). It then becomes natural to ask why identity-dependent RS responses are abolished by OR faces.
According to an influential theoretical model of the ORE (61, 62), individual faces are represented in an arbitrary multidimensional space shaped by visual and social experience. In this space, location codes for facial identity and distance from the mean face (i.e., a prototypical average face) codes for distinctiveness. In the model, SR faces have a wide multidimensional distribution, whereas OR faces have a denser, more clustered distribution. For example, SR faces are coded along many diagnostic dimensions, such as hair and eye color. In contrast, OR faces tend to lack diagnostic information (i.e., all EA faces have black hair and dark eyes), which leads to more prototypical coding, hence harder-to-discriminate faces (3). This encoding strategy emerges through experience and increases coding efficiency for SR identity to the detriment of OR faces. Evidence for such norm-based face encoding has been found in single-cell recordings in monkeys (59) and fMRI in WC observers confronted with SR faces (42). In particular, both studies show that neural responses to face identity increase with distinctiveness (i.e., the distance from the face prototype). Such evidence is in line with our observations, because we found larger stRS responses to SR faces, but not to OR faces.
At the neurophysiological level, numerous adaptation studies have interpreted RS as reflecting decreased neural redundancy and sharpening of sparser stimulus’ representation (for a review, see ref. 31), which would result in more efficient neural information coding (36, 43–45). Within this framework, RS has been considered as a neural mechanism devoted to novelty detection (31). The lack of stRS to OR faces suggests that they trigger prototypal responses, preventing novelty detection in face exemplars. In contrast, stRS to SR faces suggests a more efficient coding of this visual category.
The explanations offered above are also compatible with two major theoretical accounts of the ORE in psychology: the social-experience model (63, 64) and the visual-expertise framework (14, 65). The social-experience model holds that the rapid extraction of race information, at the cost of individuating information, would account for the OR recognition deficit. Following this logic, OR faces would not tap into the neural population coding for identity. This prediction is clearly supported by our electrophysiological data. Along the same lines, the visual-expertise framework (14, 65) postulates that SR faces are categorized at the subordinate level (i.e., John, Jack, etc.), whereas OR faces are categorized at the basic level (i.e., East Asian, Black American, etc.). This theoretical account is also compatible with our data, in which only SR faces tap into individual face recognition.
It is worth noting that conventional electrophysiological RS measures are based on amplitude differences for target faces only (47–49) and have so far failed to show any significant effect for both groups of observers in the N170 time window. Using this approach, we also failed to replicate previously reported RS differences for SR faces of the same identity (e.g., ref. 49) (Fig. 1). The use of different images of the same individual between the adaptor and the target face (expressive vs. neutral) might explain this observation. This control increased the validity of the face recognition task, but decreased the sensitivity of conventional electrophysiological RS measures on the target face.
To increase the sensitivity of RS analyses, we implemented a unique approach. Because RS is a reduction in neural response to a target stimulus following the presentation of an adaptor stimulus, we subtracted the signal elicited by the presentation of the target face to that elicited by the adaptor face. This analysis was only performed for trials not contaminated by artifacts for both events. The amplitude of the stRS increases the signal-to-noise ratio and reflects a genuine amount of RS per event, which is a function of both amplitude and latency differences between the signals elicited by the two successively presented faces. Changes in amplitude, synchrony, or both of local field potentials may explain the modulations across events (31). Therefore, the unique stRS approach may help to elucidate the nature of the neural information coding underlying responses in electrophysiology, magnetoencephalography, and single-cell recordings, as all these techniques can finely exploit the temporal dynamics of single events.
Conclusion
The present study investigated the neural correlates of the perceptual all look alike effect, which represents the core facet of the ORE. We used an adaptation paradigm involving Western and Eastern faces and quantified single-trial spatiotemporal electrophysiological RS responses in Western Caucasian and East Asian observers. Our data show RS in the time window of the early face-sensitive N170 component, with sensitivity to face identity only for SR faces. OR faces did not show RS modulation, indicating a lack of sensitivity to face identity for this category. These results show that the discrimination of same- and other-race faces begins early, at the perceptual level. After nearly 100 y of investigations, our results are also unique in providing a neurophysiological correlate of the all look alike perceptual experience.
Methods
Participants.
Twenty-four right handed subjects took part in the experiment: 12 East Asians (six female), with an age range of 18–33 and a mean age of 25, and 12 Western Caucasian (six female), with an age range of 19–31 and a mean age of 23. All EA participants were Chinese who had been in the country for less than 1 mo and had previously never been in contact with a Western society. All participants provided written informed consent and had normal or corrected-to-normal vision. The ethical committee of the Faculty of Information and Mathematical Sciences at the University of Glasgow approved the experiments.
Stimuli.
The stimuli consisted of 20 front-view grayscale photographs of WC and EA faces (5 identities × 2 sexes × 2 races), ≈3.75° × 4.25° of visual angle, taken from the JACFEE database (66).
To limit the possibility that ERP repetition effects were the result of pixel-based low-level adaptation, instead of high-level adaptation to face identity, each identity was equally presented as a neutral face, or a face displaying five possible emotions: happy, anger, sad, disgust, and surprise. All emotions were counterbalanced across face races and conditions. All faces were cropped to remove external features by the application of the same oval mask; none had particular distinctive features and male faces were clean-shaven. The stimuli were centered in a 5.2° × 5.2° and color normalized with Adobe Photoshop CS4, by constraining all of the images in the same average template color space.
EEG Study.
Procedure.
Participants sat in a dimly lit, sound-attenuated electrically shielded booth. Viewing distance was maintained at 80 cm by a chinrest. Each trial consisted of two faces of same or different identities (Fig. S1) presented sequentially on a Samsung SyncMaster 1100 MB monitor (resolution 2,048 × 1,536 pixels, 23.5° × 30.1°, background of average luminance 25.4 cd/m2, refresh rate 80 Hz). A trial started with a black fixation cross ≈0.3° of visual angle, presented at the center of the screen for 300 ms. The first face, the adaptor, was then presented for 350 ms, followed by an interval of random duration (100–300 ms), and then by the second face, the target, for 300 ms. The offset of the second face was followed by a randomized intertrial interval between 1,300–1,500 ms. Target and adaptor faces’ identities matched in half of the trials. To minimize low-level adaptation, the adaptor face portrayed an emotion, whereas the target face was neutral. Face race and sex were consistent within trials. There were four conditions [two races (EA and WC) × two identities (same and different): SR same, SR different, OR same, and OR different (where same and different refers to the identities of the two subsequently presented faces)]. Each identity equally appeared in the same and different conditions. There were 80 trials per condition, and the order of the conditions was randomized within each block.
Subjects performed an orthogonal task that required pressing the “s” key on the keyboard every time one of the two faces within a trial was presented upside-down and the “k” key when both faces were inverted. This orthogonal task was designed to avoid potential signal modulations because of attentional confounds linked to the race of the stimuli (9). Inverted faces appeared in ≈12.5% of the trials: 20 with the adaptor or the target face inverted and 20 with both faces inverted. Each identity appeared twice as inverted.
The experiment consisted of eight blocks of 45 trials each (360 trials in total with 80 trials × 4 conditions and 40 trials with inverted faces) and lasted ≈20 min.
EEG recording and analysis.
We acquired EEG data with a 128-channel Biosemi Active Two EEG system (BioSemi). Four additional electrodes (UltraFlat Active electrodes; BioSemi) attached below and at the outer canthi of both eyes measured the vertical and horizontal electro-oculograms. Analog signal was digitized at 1,024 Hz and band-pass filtered online between 0.1 and 200 Hz. Electrode offsets were kept between ± 20 μV. Participants were asked to minimize blinking, head movement, and swallowing.
We used EEGLAB (68), Matlab 7.5 (2007b), and BESA 5.2 to perform EEG analyses. In BESA, EEG data were referenced to an average reference. Noisy electrodes were rejected on a subject-by-subject basis. The signal was low-pass filtered at 40 Hz with a slope of 6dB. Single trials were corrected for horizontal and vertical eye movements and blinking artifacts by principle components analysis, as implemented in BESA. First, we identified eye movements on the continuous signal using their specific topographical distribution. Then we manually selected portions of the signal showing the topographical configuration of interest. Finally, we averaged the selected epochs to create a subject-specific template for each artifact. The first principle components analysis component of each artifact was removed. This component accounted for 89–99% of the variance (mean = 91%). Artifacts were rejected based on absolute abnormal values larger than 120 μV. Trials were averaged across an epoch of −100 ms to +500 ms, and the average 100 ms of prestimulus activity was removed from every time-point, independently at each electrode. Trials including inverted faces were excluded from the analysis. Channels contaminated by artifacts were interpolated using the EEGLAB topoplot function. Consistently with previous studies, we first analyzed the ERP amplitude to the adaptor and to the target faces separately. For adaptor faces, we carried out a two-way ANOVA with two groups of observers (WC and EA) × two races of faces (WC and EA). We performed another two-way ANOVA for target faces, with two groups of observers (WC and EA) × four conditions (WC faces same identities, WC faces different identities, EA faces same identities, EA faces different identities). These ANOVAs were performed independently at all electrodes and time-points, with ERP amplitude as a dependent variable. This analysis makes no a priori assumption about where and when to look for effects in the ERP signal. Post hoc t tests were then carried out between paired conditions.
The ANOVA on both the target and adaptor faces failed to demonstrate any RS effects across the whole ERP. However, this type of analysis makes the implicit assumption that adaptor and target faces are independent and therefore fails to recognize the paired nature of the experimental design. We thus developed a stRS response. Based on the definition of RS, which refers to a stimulus specific reduction of neural activity, we computed the stRS response by subtracting the activity elicited by the target face from the activity elicited by the adaptor. We rejected from the analysis the signal elicited by both adaptor and target face if either one of the two epochs was contaminated by artifacts (for more details about the procedure see Fig. S2). We then carried out a two-way ANOVA [two races of the observers (EA and WC) × four conditions (WC faces same identities; WC faces different identities; EA faces same identities; EA faces different identities)] independently at all electrodes and time-points, with stRS as a dependent variable. To correct for the increase in type I errors because of multiple comparisons, we used bootstrap (67) and a multivariate clustering technique (68). We centered the data so that each condition had a mean of zero; then we used the bootstrap to derive an estimate of the sampling distribution of our statistic in a condition in which the null hypothesis of no difference in means is true. In each bootstrap, we sampled subjects with replacement and carried out the repeated-measures ANOVAs described above, independently at all electrodes and time-points. Then the significant F values (P < 0.05) were grouped in spatiotemporal clusters (68). For each bootstrap, we computed the sum of F values in every cluster and saved the maximum cluster sum across clusters. We repeated this procedure 599 times, leading to 600 F cluster sums for each main effect and for each interaction. After sorting the 600 cluster sums, we selected the 95th percentile as our cluster threshold to assess statistical significance. The significant F values from the original ANOVAs were clustered, and the sum of F values inside each cluster was compared with the bootstrap cluster threshold for that test. If an observed cluster sum was equal or larger than the threshold sum obtained under H0, all of the time-points and the electrodes inside that cluster were considered significant.
Behavioral Study.
Subsequent to the EEG experiment, the same participants performed a behavioral task that directly assessed the ORE. In an encoding phase, participants had to memorize 20 faces of each race, presented for 3 s each, with 5-s inter-stimulus interval (ISI). Subsequently, subjects performed a forced-choice old–new recognition task with 20 old and 20 new faces.
Faces were selected from a set of stimuli different from the set used in the EEG experiment (69). Participants did two blocks of the old/new face recognition task per race. The order of the blocks was pseudorandom and counterbalanced across observers. Faces were blocked by race during both the encoding and the recognition phase. The encoding stage was followed by a 1-min pause, after which participants pressed the “s” key on the computer keyboard for old faces and the “k” key for new faces. Each face remained on the computer screen for 200 ms (3-s ISI). Participants did not know the ratio of old to new faces and did not receive feedback on their responses.
Supplementary Material
Acknowledgments
R.C. was supported by The Economic and Social Research Council and Medical Research Council (ESRC/RES-060-25-0010).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005751107/-/DCSupplemental.
References
- 1.Feingold CA. The influence of environment on identification of persons and things. Journal of Criminal Law and Police Science. 1914;5:39–51. [Google Scholar]
- 2.Meissner C, Brigham J. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychol Public Pol L. 2001;7:3–35. [Google Scholar]
- 3.Caldara R, Hervé A. Simulating the ‘other-race’ effect with autoassociative neural networks: further evidence in favor of the face-space model. Perception. 2006;35:659–670. doi: 10.1068/p5360. [DOI] [PubMed] [Google Scholar]
- 4.O'Toole AJ, Deffenbacher KA, Abdi H, Bartlett JC. Simulating the ‘other-race effect’ as a problem in perceptual learning. Connect Sci. 1991;3:163–178. [Google Scholar]
- 5.O'Toole AJ, Deffenbacher KA, Valentin D, Abdi H. Structural aspects of face recognition and the other-race effect. Mem Cognit. 1994;22:208–224. doi: 10.3758/bf03208892. [DOI] [PubMed] [Google Scholar]
- 6.Caldara R, Rossion B, Bovet P, Hauert CA. Event-related potentials and time course of the “other-race” face classification advantage. Neuroreport. 2004;15:905–910. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- 7.Caldara R, et al. Face versus non-face object perception and the ‘other-race’ effect: A spatio-temporal event-related potential study. Clin Neurophysiol. 2003;114:515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, et al. Racial distinction of the unknown facial identity recognition mechanism by event-related fMRI. Neurosci Lett. 2006;397:279–284. doi: 10.1016/j.neulet.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 9.Golby AJ, Gabrieli JD, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nat Neurosci. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann MJ, et al. The other-race effect for face perception: An event-related potential study. J Neural Transm. 2007;114:951–957. doi: 10.1007/s00702-007-0624-9. [DOI] [PubMed] [Google Scholar]
- 11.Ito TA, Thompson E, Cacioppo JT. Tracking the timecourse of social perception: The effects of racial cues on event-related brain potentials. Pers Soc Psychol Bull. 2004;30:1267–1280. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- 12.Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures of attention to the race and gender of multiply categorizable individuals. J Pers Soc Psychol. 2003;85:616–626. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- 13.Stahl J, Wiese H, Schweinberger SR. Expertise and own-race bias in face processing: An event-related potential study. Neuroreport. 2008;19:583–587. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka JW, Pierce LJ. The neural plasticity of other-race face recognition. Cogn Affect Behav Neurosci. 2009;9:122–131. doi: 10.3758/CABN.9.1.122. [DOI] [PubMed] [Google Scholar]
- 15.Walker PM, Silvert L, Hewstone M, Nobre AC. Social contact and other-race face processing in the human brain. Soc Cogn Affect Neurosci. 2008;3:16–25. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- 18.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 23.Winston JS, Henson RN, Fine-Goulden MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- 24.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bötzel K, Grüsser OJ. Electric brain potentials evoked by pictures of faces and non-faces: A search for “face-specific” EEG-potentials. Exp Brain Res. 1989;77:349–360. doi: 10.1007/BF00274992. [DOI] [PubMed] [Google Scholar]
- 26.Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage. 2008;39:1959–1979. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Schyns PG, Petro LS, Smith ML. Dynamics of visual information integration in the brain for categorizing facial expressions. Curr Biol. 2007;17:1580–1585. doi: 10.1016/j.cub.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. J Neurosci. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vizioli L, Foreman K, Rousselet GA, Caldara R. Inverting faces elicits sensitivity to race on the N170 component: A cross-cultural study. J Vis. 2010;10:1–23. doi: 10.1167/10.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Rossion B, Curran T, Gauthier I. A defense of the subordinate-level expertise account for the N170 component. Cognition. 2002;85:189–196. doi: 10.1016/s0010-0277(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 31.Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 33.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 34.Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 36.Sobotka S, Ringo JL. Stimulus specific adaptation in excited but not in inhibited cells in inferotemporal cortex of macaque. Brain Res. 1994;646:95–99. doi: 10.1016/0006-8993(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 37.Schendan HE, Kutas M. Time course of processes and representations supporting visual object identification and memory. J Cogn Neurosci. 2003;15:111–135. doi: 10.1162/089892903321107864. [DOI] [PubMed] [Google Scholar]
- 38.Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cereb Cortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- 39.Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004;21:1674–1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol. 2000;111:694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 41.Doniger GM, et al. Visual perceptual learning in human object recognition areas: A repetition priming study using high-density electrical mapping. Neuroimage. 2001;13:305–313. doi: 10.1006/nimg.2000.0684. [DOI] [PubMed] [Google Scholar]
- 42.Loffler G, Yourganov G, Wilkinson F, Wilson HR. fMRI evidence for the neural representation of faces. Nat Neurosci. 2005;8:1386–1390. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- 43.Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat Neurosci. 2002;5:883–891. doi: 10.1038/nn900. [DOI] [PubMed] [Google Scholar]
- 44.Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- 45.Gutnisky DA, Dragoi V. Adaptive coding of visual information in neural populations. Nature. 2008;452:220–224. doi: 10.1038/nature06563. [DOI] [PubMed] [Google Scholar]
- 46.Kovács G, et al. Electrophysiological correlates of visual adaptation to faces and body parts in humans. Cereb Cortex. 2006;16:742–753. doi: 10.1093/cercor/bhj020. [DOI] [PubMed] [Google Scholar]
- 47.Caharel S, d'Arripe O, Ramon M, Jacques C, Rossion B. Early adaptation to repeated unfamiliar faces across viewpoint changes in the right hemisphere: Evidence from the N170 ERP component. Neuropsychologia. 2009;47:639–643. doi: 10.1016/j.neuropsychologia.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Jacques C, Rossion B. The speed of individual face categorization. Psychol Sci. 2006;17:485–492. doi: 10.1111/j.1467-9280.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 49.Jacques C, d'Arripe O, Rossion B. The time course of the inversion effect during individual face discrimination. J Vis. 2007;7:3. doi: 10.1167/7.8.3. [DOI] [PubMed] [Google Scholar]
- 50.Michel C, Caldara R, Rossion B. Same-race faces are perceived more holistically than other-race faces. Vis Cogn. 2006;14:55–73. [Google Scholar]
- 51.Michel C, Rossion B, Han J, Chung CS, Caldara R. Holistic processing is finely tuned for faces of one's own race. Psychol Sci. 2006;17:608–615. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka JW, Kiefer M, Bukach CM. A holistic account of the own-race effect in face recognition: Evidence from a cross-cultural study. Cognition. 2004;93:B1–B9. doi: 10.1016/j.cognition.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Blais C, Jack RE, Scheepers C, Fiset D, Caldara R. Culture shapes how we look at faces. PLoS ONE. 2008;3:e3022. doi: 10.1371/journal.pone.0003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodes G, Tan S, Brake S, Taylor K. Expertise and configural coding in face recognition. Br J Psychol. 1989;80:313–331. doi: 10.1111/j.2044-8295.1989.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 55.Rousselet GA, Husk JS, Bennett PJ, Sekuler AB. Time course and robustness of ERP object and face differences. J Vis. 2008;8:1–18. doi: 10.1167/8.12.3. [DOI] [PubMed] [Google Scholar]
- 56.Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain. 1995;118:1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126:792–803. doi: 10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- 58.Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 59.Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442:572–575. doi: 10.1038/nature04951. [DOI] [PubMed] [Google Scholar]
- 60.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 61.Valentine T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. Q J Exp Psychol A. 1991;43:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- 62.Valentine T, Endo M. Towards an exemplar model of face processing: The effects of race and distinctiveness. Q J Exp Psychol A. 1992;44:671–703. doi: 10.1080/14640749208401305. [DOI] [PubMed] [Google Scholar]
- 63.Levin DT. Classifying faces by race: The structure of face categories. J Exp Psychol Learn Mem Cogn. 1996;22:1364–1382. [Google Scholar]
- 64.Levin DT. Race as a visual feature: Using visual search and perceptual discrimination tasks to understand face categories and the cross-race recognition deficit. J Exp Psychol Gen. 2000;129:559–574. doi: 10.1037//0096-3445.129.4.559. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka JW, Taylor BJ. Object categories and expertise: Is the basic level in the eye of the beholder? Cognit Psychol. 1991;23:457–482. [Google Scholar]
- 66.Matsumoto D, Ekman P. Japanese and Caucasian Facial Expressions of Emotion (JACFEE) San Francisco State University, San Francisco: Intercultural and Emotion Research Laboratory, Department of Psychology; 1988. [Google Scholar]
- 67.Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. 2nd Ed. San Diego: Academic Press; 2005. [Google Scholar]
- 68.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Bang S, Kim D, Choi S. In: Asian Face Image Database. Lab IM, editor. Pohang, Korea: Postech; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.