Abstract
Systems for spatial and temporal control of gene expression are essential for developmental studies and are of particular importance for research in adult model organisms. We present two modified dually inducible TetON systems for tissue-specific conditional control of gene expression in zebrafish based on (i) a tetracycline inducible transcriptional activator (TetActivator) fused to the ligand binding domain of a mutated glucocorticoid receptor (TetA-GBD) and (ii) a TetActivator fused with a domain of the Ecdysone receptor (TetA-EcR). Both systems showed strong induction of tetracycline-responsive promoters upon administration of the appropriate ligands (doxycycline and dexamethasone for TetA-GBD, and doxycycline and tebufenozide for TetA-EcR), and undetectable leakiness when compared with classical TetActivators. Combinations of transgenic lines expressing TetA-GBD specifically in the heart or the CNS with different Tet-responsive transgenic lines allows conditional and tissue-specific control of gene expression in embryos and adults. Importantly, induction is fully reversible and tunable by the doses of drugs used. The TetA-EcR system avoids the possible side effects of dexamethasone and displays improved sensitivity both in zebrafish and in mammalian cells. These results show that dually inducible TetON systems are convenient tools for reversible and very tightly controlled conditional gene expression in zebrafish.
Although the zebrafish has achieved a solid status as an important vertebrate model system for embryonic development, it has only recently begun to also gain popularity as a model for human disease and other biomedical areas of research, such as cancer, physiology, or regeneration (1–3). To make full use of its potential in these areas and to facilitate more sophisticated studies of gene function during development, reliable tools for conditional, tissue-specific manipulation of gene function are required. Ideally, a system for gene overexpression would be inducible, nonleaky, allow for spatial control (tissue specificity), be reversible, reinducible, and tunable, and would work in larvae and adults. Currently, two technologies for conditional overexpression are being regularly used in zebrafish. Heat-shock promoter-driven overexpression works well in embryos and has been successfully used to study gene function during adult regeneration and homeostasis (4, 5). Although this is a robust method that appears to work in almost all cell types, its usefulness is severely limited by lack of spatial control and the pulsed nature of expression. The Cre-lox system has recently been adapted for conditional activation of gene expression in zebrafish embryos and adults, using spatially restricted, tamoxifen-inducible CreERT2 for removal of a STOP cassette from a ubiquitously expressed transgene, resulting in expression of the gene of interest after recombination (6–8). Although this technology allows for tissue-specific, conditional overexpression, it is not reversible and can be difficult to use due to leakiness of the CreERT2 and the absence of promoters that reliably drive ubiquitous expression of floxed responder transgenes. In the mouse, conditional and reversible tissue-specific gene expression can be achieved using the TetON system, which combines tissue-specific transgenic expression of a tetracycline (Tet)- or doxycycline (Dox)-inducible transcriptional activator (TetActivator) with a Tet-responsive transgene (9). The applicability of this system to zebrafish has been reported, but its usefulness was confounded by leakiness and lack of reversibility (10). Here, we present two modified, dually inducible TetON systems, which significantly reduce leakiness and display improved activity, and show that such systems can be used to achieve conditional, tissue-specific, and reversible gene expression in embryonic and adult zebrafish.
Results and Discussion
TetA-GBD Confers Very Tight and Reversible Control of Expression.
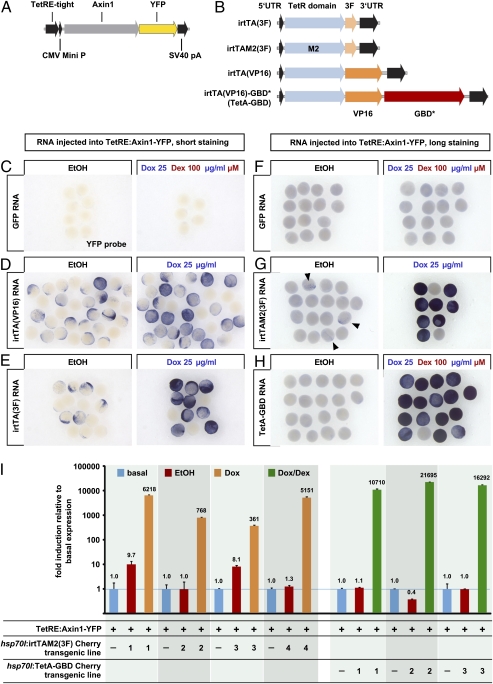
To test the activity and leakiness of different TetActivator variants in zebrafish embryos, we first established a transgenic fish line (TetRE:Axin1-YFP tud1) placing the Wnt/β−catenin inhibitor Axin1 fused to YFP under control of optimized Tet response elements (TetRE-tight, Clontech) (Fig. 1A). We then injected RNAs coding for different TetActivator variants (Fig. 1B) into transgenic embryos and assayed Axin1-YFP mRNA induction after drug or vehicle treatment by whole-mount in situ hybridization. Codon-optimized (“improved”) fusions of the reverse Tet repressor domain with either the Herpes simplex virus VP16 transactivation domain [irtTA(VP16)] or the VP16 derivative 3F [irtTA(3F)] (11), induced well but displayed severe leakiness (Fig. 1 D and E, quantification in Fig. S1A). The M2 mutant variant of the reverse Tet repressor fused with the 3F domain [irtTAM2(3F)] (12) induced Axin1-YFP RNA with much lower background, but still caused some leaky induction in solvent-treated embryos (Fig. 1G, quantification in Fig. S1B). To further reduce leakiness, we created a dually inducible activator, the function of which depends on drug-induced nuclear import in addition to activation by Dox, by fusing the ligand binding domain of a mutated glucocorticoid receptor (GBD*) to irtTA(VP16). This construct, which we termed irtTA(VP16)-GBD*, in short TetA-GBD, was able to strongly induce Axin1-YFP expression after activation with Dox and dexamethasone, (Dex) without producing detectable background in solvent-treated controls (Fig. 1H, quantification in Fig. S1B). Likewise, in mammalian HEK293 cells transiently transfected with a Tet-responsive luciferase reporter and TetActivator variants, irtTAM2(3F) displayed some leakiness, whereas TetA-GBD did not (Fig. S1C).
Fig. 1.
Dually inducible TetA-GBD activates transgene transcription in a nonleaky fashion in zebrafish embryos. (A) Transgenic Tet responder construct. (B) Human codon optimized (“improved”) variants of the reverse tetracycline-responsive transactivator (irtTA) used in this study. (C–H) YFP RNA expression detected by whole-mount in situ hybridization in TetRE:Axin1-YFP transgenic embryos injected with equimolar amounts of GFP (25 pg), irtTA(VP16) (30 pg), irtTA(3F) (25 pg), irtTAM2(3F) (30 pg), or irtTA(VP16)-GBD* (TetA-GBD, 50 pg) and treated with EtOH vehicle or 25 μg/mL Dox or 25 μg/mL Dox plus 100 μM Dex from 5 hpf for 4.5h (C–E) or 3.5h (F–H). Samples in F–H were stained significantly longer than those in C–E to reveal even low levels of leakiness. Note severe leaky induction in irtTA(VP16) and irtTA(3F) injected embryos and weak leakiness in irtM2(3F) injected embryos (arrowheads). (I) Axin1-YFP RNA expression detected by QPCR in progeny of TetRE:Axin1-YFP fish crossed with individual sublines of hsp70l:irtTAM2(3F)-p2a-mCherry or hsp70l:TetA-GBD-p2a-mCherry transgenic fish, heatshocked at 24 hpf, and treated with EtOH or 25 μg/mL Dox or Dox plus 100 μM Dex for 4 h. Levels are normalized to expression in TetRE:Axin1-YFP embryos containing no TetActivator transgene (“basal”).
To further compare the properties of these TetActivators, we created stable transgenic zebrafish lines expressing irtTAM2(3F) or TetA-GBD under control of the heat shock protein 70l promoter. We tagged both TetActivators with mCherry via the viral p2a peptide, which results in translation of separate peptides from a single ORF (13). The p2a Cherry tag did not interfere with the activating ability of TetA-GBD (Fig. S2). We crossed independent sublines of the hsp70l:irtTAM2(3F)-p2a-mCherrytud4 or the hsp70l:TetA-GBD-p2a-mCherrytud5 transgenics with the TetRE:Axin1-YFPtud1 responder fish and quantified YFP levels induced in embryos after heat shock and drug treatment using quantitative PCR. Two of four tested irtTAM2(3F) lines produced leaky Axin1-YFP induction of tenfold and eightfold in EtOH-treated embryos (lines 1 and 3 in Fig. 1I), whereas none of three tested TetA-GBD lines showed any leakiness (Fig. 1I). In addition, the TetA-GBD lines induced on average five times better after drug treatment than the irtTAM2(3F) lines, with 16,200- and 3,100-fold induction over basal expression, respectively. Thus, for the irtTAM2(3F) system, only one of four sublines combined very tight control of expression with high inducibility (line 4; 5,150-fold), whereas all TetA-GBD lines achieved greater than 10,000-fold induction without any leakiness. We conclude that the dually inducible TetA-GBD activator is superior for reliable production of a tightly controlled, strongly inducing transgenic expression system.
Tissue-Specific Inducible Expression Using TetA-GBD.
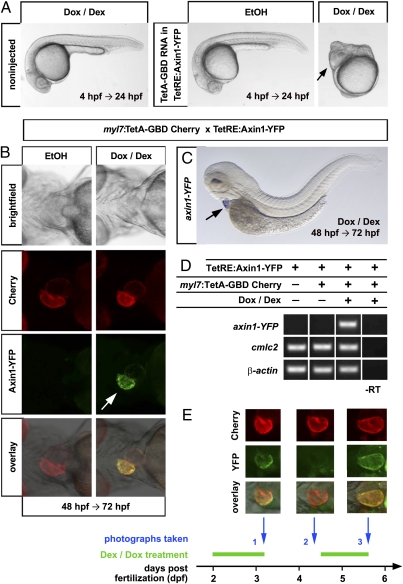
When activated during gastrulation, TetA-GBD RNA induced Axin1-YFP to levels sufficient to produce strong Wnt/β−catenin loss-of-function phenotypes, namely expansion of head structures and loss of trunk and tail (Fig. 2A). We then asked whether we could use the TetA-GBD system to achieve conditional, tissue-specific activation of gene expression. To test this, we established a transgenic line expressing the TetA-GBD-p2a-mCherry cassette specifically in the heart under control of the myosin light chain regulatory polypeptide 7 (myl7, cmlc2) promoter (myl7:TetA-GBD-p2a-mCherrytud3, in short myl7:TetA-GBD Cherry) (Fig. S3A). Treatment of double transgenic embryos with Dox and Dex for 24 h starting at 48 h postfertilization (hpf) resulted in robust activation of YFP fluorescence in the heart, whereas vehicle-treated controls showed no detectable expression (Fig. 2B). In situ hybridization for Axin1-YFP mRNA showed that induction in myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic embryos was confined to the embryonic heart (Fig. 2C), indicating that the system provides for tight spatial control of gene expression. Using semiquantitative PCR, we could not detect basal expression of Axin1-YFP in TetRE:Axin1-YFP transgenic embryos and also did not find leaky expression in myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic embryos treated with EtOH vehicle (Fig. 2D), indicating that the system provides for very tightly controlled induction. To test whether spatially restricted activation also works in other tissues, we created a transgenic line expressing TetA-GBD in the central nervous system under control of the her4.1 promoter (14) (her4.1:TetA-GBD-p2a-mCherrytud6) (Fig. S3B). When crossed with TetRE:Axin1-YFP fish, this line drove strong induction of YFP in all of the TetA-GBD-p2a-mCherry expression domains, without leakiness in vehicle-treated controls (Fig. S3C).
Fig. 2.
Tissue-specific, reversible gene expression using the dually inducible TetA-GBD/TetRE-tight system in embryonic and adult zebrafish. (A) Severe Wnt/β-catenin loss-of-function phenotypes as evidenced by posterior truncations and expanded eyes (arrow) in TetRE:Axin1-YFP embryos injected with 100 ng/μl TetA-GBD RNA and treated with Dox/Dex from 4 h postfertilization (hpf) until 24 hpf. Note that embryos treated with EtOH vehicle and noninjected embryos treated with Dox/Dex develop normally. n = 30 noninj, 7 EtOH, 9 Dox/Dex. (B) Induction of Axin1-YFP expression in ventricle (arrow) in myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic embryos treated with Dox/Dex for 24 h from 48 hpf. n = 15 EtOH, 18 Dox/Dex. (C) Heart-specific induction of Axin1-YFP RNA (arrow) after Dox/Dex treatment. n = 15. (D) Semiquantitative PCR detects axin1-YFP expression only in myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic embryos treated with Dox/Dex (lane 3), but not in EtOH treated embryos (lane 2) or embryos only containing the TetRE:Axin1-YFP transgene (lane 1). myl7 and β-actin are shown as loading controls. (E) Fast reversibility and reinducibility of Axin1-YFP induction. Fluorescent images of the heart of one individual myl7:TetA-GBD; TetRE:Axin1-YFP double transgenic embryo is shown that was treated with Dox/Dex and photographed at the times indicated. Note that YFP signal is not detectable 24 h after drug withdrawal (Middle, column 2) and reexpressed after additional 24 h of drug treatment (Middle, column 3). n = 5.
We created another responder line, placing a Dickkopf1-GFP fusion protein under control of the Tet response elements (TetRE:Dkk1b-GFPtud2) (Fig S3D) and again observed conditional induction in the heart in embryos doubly transgenic with myl7:TetA-GBD Cherry (Fig. S3E). In addition, injection of TetA-GBD RNA into the Dkk1 responder line was sufficient to produce strong Dkk1 overexpression phenotypes after drug treatment during gastrulation (Fig. S3F). In total, three of eight TetRE sublines (TetRE:Axin1-YFP or TetRE:Dkk1-GFP) that were genotyped to contain transgenic insertions were found to be inducible by the myl7 driver line. Thus, the system does not seem to be significantly more sensitive to positional effects than other zebrafish transgenic systems, indicating that useful lines can easily be created using the high efficiency of transgenesis achieved with the Tol2 or the I-SceI system (15).
An important feature of the TetON system is its reversibility. When we induced Axin1-YFP in myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic embryos for 24 h, and kept embryos in drug-free medium for another 24 h, expression was lost (Fig. 2E). Furthermore, expression could be reinduced by moving embryos back into Dox/Dex containing water (Fig. 2E). Thus, our system is fully reversible and reinducible.
Induction in Adult Fish.
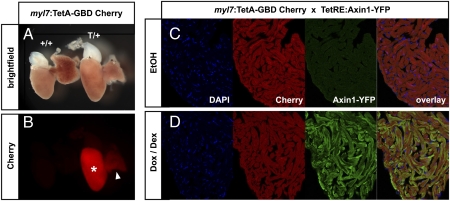
Next, we tested whether the TetA-GBD system is also suitable for conditional overexpression in adult fish. Cherry was robustly expressed in adult ventricles of myl7:TetA-GBD Cherry fish (Fig. 3 A and B). We injected adult myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic fish intraperitoneally either with Dox+Dex or EtOH once per day for 3 d, harvested and fixed hearts at day 4, and assayed for Cherry and Axin1-YFP expression on cryosections. Although both vehicle and Dox+Dex-treated hearts displayed robust Cherry fluorescence, Axin1-YFP could be detected only in Dox+Dex-treated hearts (Fig. 3 C and D). Axin1-YFP signal was found in virtually all Cherry+ cardiomyocytes at quite uniform levels. Thus, we conclude that the dually inducible TetA-GBD system can be efficiently used for tightly controlled conditional overexpression in adult zebrafish.
Fig. 3.
(A and B) The myl7:TetA-GBD Cherry transgene is expressed in the adult heart. (A) Brightfield images of whole extracted adult hearts: (Left) WT; (Right) myl7:TetA-GBD Cherry heterozygous. (B) Cherry channel. Note Cherry expression in ventricle (*) and weakly in atrium (arrowhead). (C and D) Inducibility in adult hearts. Cryosections of adult hearts of myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenic fish that had been injected intraperitoneally with EtOH (C) or 20 μg Dox plus 5 μg Dex (D). Axin1-YFP is detected by anti-GFP antibody. n = 6 EtOH and Dox/Dex.
Dose Dependency of Induction.
Next, we returned to embryos to characterize the drug dose dependency of the dually inducible TetON system. In myl7:TetA-GBD Cherry; TetRE:Axin1-YFP double transgenics Axin1-YFP RNA expression was detectable 4 h after induction, and was strongly dependent on the drug doses applied (Fig. S4 A–C). We found that Dex doses were limiting in double transgenics and when TetA-GBD RNA was injected into TetRE:Axin1-YFP embryos (Fig. S4 D–F). A 10-μg/mL quantity of Dox (19 μM) plus 100 μM Dex achieved maximal activation in double transgenics. Thus, gene activation can be tuned by adjusting drug doses. The doses of Dox+Dex needed for optimal induction did not alter early development of embryos (Fig. 2A). To assay drug toxicity in adults, we tested their effects on caudal fin regeneration, a process highly sensitive to perturbations of cellular growth and survival. We found that fish injected with the drugs or exposed to water containing the drugs showed normal speed of fin regeneration and normal morphology of regenerated fins, indicating low toxicity (Fig. S5A). We conclude that tissue-specific expression of the dually inducible TetActivator–GBD combined with responder lines containing the TetRE-tight allows for very tightly spatially and temporally controlled, reversible, and tunable gene expression in zebrafish embryos and adults.
TetA-EcR Is More Efficient than TetA-GBD.
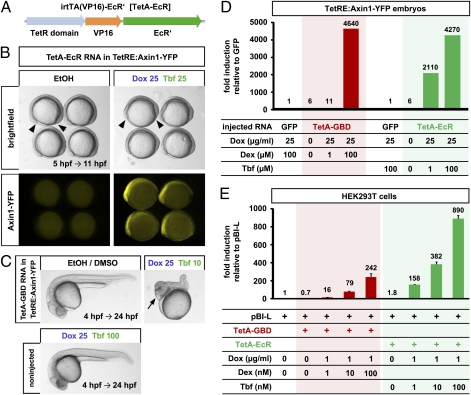
Although we found that the drug doses required for optimal induction are nontoxic to embryonic and adult fish, Dex has been shown to deplete T cells in zebrafish embryos at doses starting from 60 μM (16). Thus, caution might be warranted in use of this system for the manipulation of biological processes that also involve immune responses, including regeneration. Therefore, we set out to create a dually inducible TetActivator that is activated by small molecules having no known activity in vertebrates. We replaced the GBD* in irtTA(VP16)-GBD* with a modified ligand-binding domain (EcR’) of the Bombyx mori ecdysone receptor, which has been shown to confer inducibility to a Gal4 transactivator (17), to create TetA-EcR (Fig. 4A). In vertebrates, no EcR homologs exist and EcR agonists have no known activity; thus EcR-based systems should have few side effects (17). Injection of TetA-EcR RNA into TetRE:Axin1-YFP embryos resulted in strong activation of YFP fluorescence after induction with Dox and tebufenozide (Tbf), an ecdysone receptor agonist, without any detectable leakiness in vehicle-treated controls (Fig. 4B). Furthermore, TetA-EcR activation during gastrulation produced severe Axin1 overexpression phenotypes in TetRE:Axin-YFP embryos (Fig. 4 B and C).
Fig. 4.
An ecdysone receptor-based dually inducible TetActivator. (A) TetA-EcR construct. (B) Induction of Axin1-YFP in TetRE:Axin1-YFP transgenic embryos injected with 100 pg of TetA-EcR RNA, treated with 25 μg/mL Dox and 25 μM Tebufenozide (Tbf) from 5 hpf and photographed at 11 hpf. Note shortened body axes (arrowheads) indicative of Axin1 gain-of-function phenotypes in drug-treated embryos. n = 10/group. (C) Severe Axin1 overexpression phenotype (posterior truncations and expanded anterior fates; arrow) in 25 μg/mL Dox and 10 μM Tbf drug-treated embryos at 24 hpf. Note that embryos treated with EtOH/DMSO vehicle and noninjected embryos treated with even higher doses of Dox/Tbf develop normally. n = 32 noninj, 6 EtOH/DMSO, 6 Dox/Tbf. (D) TetA-EcR induces more strongly than TetA-GBD at low drug doses in zebrafish. TetRE:Axin1-YFP embryos were injected with equimolar amounts of GFP (25 pg), TetA-GBD (50 pg) or TetA-EcR (55 pg) RNA, treated with the indicated drugs at 5 hpf for 3 h, YFP expression was quantified using QPCR and is shown relative to GFP controls treated with the same drugs. Zero indicates EtOH vehicle only. (E) TetA-EcR is more efficient than TetA-GBD in mammalian cells. HEK293 cells were transiently transfected with the Tet responsive luciferase reporter pBI-L and the activators TetA-GBD or TetA-EcR, and luciferase levels were measured after treatment with indicated doses of drugs. Levels are shown relative to cells transfected with pBI-L only. Error bars represent SEM.
To compare the leakiness and efficiency of the TetA-GBD and TetA-EcR systems, we injected equimolar amounts of TetA-GBD and TetA-EcR RNA into TetRE:Axin1-YFP embryos and quantified Axin1-YFP RNA levels using quantitative PCR after induction with equivalent doses of drugs. Compared with the basal expression of Axin1-YFP in embryos injected with GFP instead of a TetActivating construct, both TetA-GBD and TetA-EcR produced virtually no leaky induction in the absence of inducing drugs, and greater than 4,000-fold induction with high doses of inducers (Fig. 4D). Interestingly however, whereas TetA-GBD failed to induce with a low dose of Dex (1 μM) plus 25 μg/mL Dox, TetA-EcR induced 2,100-fold with 1 μM Tbf plus 25 μg/mL Dox (Fig. 4D). We conclude that TetA-EcR induces more efficiently than TetA-GBD without increasing leakiness.
We also wondered how our modified, ecdysone-dependent TetON system compares with the previously described ecdysone-inducible Gal4-UAS system, which has been shown to confer tissue-specific, inducible gene expression in zebrafish (17), but the leakiness of which has not been characterized. We created Gal4-VP16-EcR′, which, like TetA-EcR, uses the VP16 transactivating domain and mutated ecdysone receptor domain, but the Gal4 DNA binding domain instead of the reverse TetRepressor. When injected into 4xUAS:GFPhzm3 transgenic embryos (18), this construct had constitutive activity (Fig. S6), which is in agreement with what Esengil et al. reported for a similar construct containing the wild-type EcR domain (17). In contrast, Gal4-VP16-F-EcR′, which contains only a subset of the VP16 domain (17), showed much reduced leakiness (sixfold), but also induced poorly (50-fold at 100 μM Tbf) (Fig. S6). Thus, in our hands, Gal4-VP16-F-EcR' is less active than reported by Esengil et al. (17), which could be due to the fact that we used a transgenic responder, whereas Esengil et al. transiently injected the responder, or due to the properties of the UAS elements used in the responder constructs. We conclude that the dually inducible ecdysone dependent TetON system provides for much stronger induction than the ecdysone inducible Gal4-UAS system (4,200-fold vs. 50-fold), at least with transgenic responder constructs.
To test whether the TetA-EcR system also works in mammalian cells and to quantify its performance relative to the TetA-GBD inducer, we transiently transfected human HEK293 cells with a Tet-responsive luciferase reporter together with TetA-GBD or TetA-EcR, and measured luciferase activity after treatment with different doses of drugs. We found that both inducers displayed virtually no leaky induction in vehicle-treated cells, but strongly activated luciferase activity when treated with the appropriate drugs in a dose-dependent manner (Fig. 4E). Interestingly, TetA-EcR induced more strongly than TetA-GBD at all doses of inducers tested, in particular at very low doses, where TetA-EcR achieved 160-fold induction at 1 nM Tbf plus 1 μg/mL Dox, 10 times more than TetA-GBD at equivalent doses of Dex plus Dox.
We found that embryos grown in high doses (higher than required for optimal induction) of Dox plus Tbf developed normally (Fig. 4C) and that exposure or injection of adults with the drugs neither caused toxicity nor affected fin regeneration (Fig. S5 B–E), indicating that the TetA-EcR system will be applicable to studies in adult fish. We conclude that the TetA-EcR/Tbf system represents a very tight, yet efficient dually inducible TetActivator system for zebrafish and mammalian cells that is superior to the TetA-GBD/Dex system due to its increased sensitivity and low incidence of side effects of drugs used.
Conclusions.
In summary, the dually inducible TetON systems described here represent an important addition to the genetic toolbox available for zebrafish research. They allow for tissue-specific, inducible, reversible and tunable control of gene expression in embryos and adults. Although other systems for conditional gene expression have been adapted for use in zebrafish, such as the Cre-Lox system or previous installments of the TetON system (6–8, 10), the main advantages of the systems presented here are their reversibility and very low leakiness. Thus, they will be particularly useful for applications where reversibility is desired, for example for manipulation of pathways that have temporally distinct roles in the same tissue, or for studying the effects of transient manipulation of progenitor cell pools, for example during regeneration. In addition, the very low leakiness of our systems should facilitate applications involving overexpression of oncogenes or of very potent modifiers of signaling pathways, which, due to their toxicity or teratogenicity, are difficult to use with less tightly controlled systems. A very interesting application that benefits from very tight control of expression and reversibility of induction will be targeted ablation of cells via overexpression of toxins, which will facilitate the development of new models of tissue regeneration.
Materials and Methods
DNA constructs and additional methods are described in SI Materials and Methods.
Nomenclature.
Constructs consisting of a fusion of the reverse TetRepressor (rTetR) with a transactivating domain are called TetActivator (rtTA), the type of transactivating domain is noted in parentheses: rtTA(VP16) or rtTA(3F). Human codon optimized variants of TetActivator constructs have been used, which we term “i” for “improved,” that is, irtTA(VP16) and irtTA(3F). The variants irtTA(VP16), irtTA(3F), and irtTA(VP16)-GBD* have been described elsewhere (11), as has irtTAM2(3F) (12).
Establishment and Identification of Transgenic Fish Lines.
The transgenic lines created in this project have been registered with the Zebrafish Information Network under the designations TetRE:Axin1-YFPtud1, TetRE:Dkk1b-GFPtud2, myl7:TetA-GBD-P2A-mCherrtud3, hsp70l:irtTAM2(3F)-P2A-mCherrytud4, hsp70l:TetA-GBD-P2A-mCherrytud5 and her4.1:TetA-GBD-P2A-mCherrytud6. All lines were made by injection of circular plasmid DNA into fertilized eggs together with Tol2 transposase RNA or with the I-SceI meganuclease for the hsp70l lines.
In induction experiments, fish heterozygous for a TetActivator construct were crossed to responder fish, embryos carrying the TetActivator constructs were identified by mCherry fluorescence, and treated with either EtOH vehicle or appropriate drugs. Embryos negative for the TetActivator were used to determine basal level of responder expression. Note that the TetRE:Axin1-YFP tud1 transgenic line carries at least two independent functional integrations; thus in crosses of transgenic carriers with WT fish, more than 50% of the progeny are inducible when supplied with TetActivator.
Drug Treatments of Zebrafish Embryos.
Doxycycline hyclate (Sigma) was dissolved in 50% EtOH and maintained as a stock solution of 50 mg/mL = 97 mM in the dark at −20 °C. Dexamethasone (Sigma) was dissolved in 100% EtOH and maintained as a stock of 10 mg/mL = 25 mM in the dark at −20 °C. Tebufenozide (Sigma) was dissolved in 100% DMSO and maintained as a stock solution of 17.6 mg/mL = 50 mM at −20 °C.
Dox was used at doses ranging from 10 μg/mL to 100 μg/mL when applied to E3 medium, with little difference in induction efficiency and strength detectable when used with the dually inducible TetA-GBD. Dex was used at doses ranging from 1 μM to 100 μM, with maximal induction achieved at 100 μM. Tbf was used at doses ranging from 1 μM to 100 μM, with 25 μM already achieving maximal induction. For TetA-GBD, we routinely use 25 μg/mL Dox plus 100 μM Dex for optimal induction, and for TetA-EcR 25 μg/mL Dox plus 25 μM Tbf. Treatments of embryos were performed either on dechorionated embryos or on embryos in chorions, with little difference in induction efficiency observed. Drugs were diluted in E3 medium, and a maximum of 30 embryos were treated in 4 mL E3 in 30-mm dishes kept in the dark at 28 °C.
Supplementary Material
Acknowledgments
We thank Reinhard Köster (Helmholtz Centre Munich, Neuherberg, Germany) for the 4xUAS:GFP transgenic fish line, Avinash Chekuru and Sumit Jaiswal for technical assistance, and Marika Fischer and Katrin Sippel for excellent fish care. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB655 (“Collaborative Research Center 655: Cells into tissues: Stem cell and progenitor commitment and interactions during tissue formation”) (to G.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007799107/-/DCSupplemental.
References
- 1.Ingham PW. The power of the zebrafish for disease analysis. Hum Mol Genet. 2009;18:R107–R112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- 2.Stoletov K, Klemke R. Catch of the day: Zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 3.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 4.Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- 6.Hesselson D, Anderson RM, Beinat M, Stainier DY. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc Natl Acad Sci USA. 2009;106:14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hans S, Kaslin J, Freudenreich D, Brand M. Temporally-controlled site-specific recombination in zebrafish. PLoS ONE. 2009;4:e4640. doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieger K, Belbellaa B, Le Guiner C, Moullier P, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CJ, et al. Conditional expression of a myocardium-specific transgene in zebrafish transgenic lines. Dev Dyn. 2005;233:1294–1303. doi: 10.1002/dvdy.20485. [DOI] [PubMed] [Google Scholar]
- 11.Anastassiadis K, et al. A predictable ligand regulated expression strategy for stably integrated transgenes in mammalian cells in culture. Gene. 2002;298:159–172. doi: 10.1016/s0378-1119(02)00979-4. [DOI] [PubMed] [Google Scholar]
- 12.Urlinger S, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provost E, Rhee J, Leach SD. Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis. 2007;45:625–629. doi: 10.1002/dvg.20338. [DOI] [PubMed] [Google Scholar]
- 14.Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Grabher C, Wittbrodt J. Recent advances in meganuclease-and transposon-mediated transgenesis of medaka and zebrafish. Methods Mol Biol. 2008;461:521–539. doi: 10.1007/978-1-60327-483-8_36. [DOI] [PubMed] [Google Scholar]
- 16.Langenau DM, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci USA. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esengil H, Chang V, Mich JK, Chen JK. Small-molecule regulation of zebrafish gene expression. Nat Chem Biol. 2007;3:154–155. doi: 10.1038/nchembio858. [DOI] [PubMed] [Google Scholar]
- 18.Distel M, Wullimann MF, Köster RW. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA. 2009;106:13365–13370. doi: 10.1073/pnas.0903060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.