Abstract
The upcoming global mechanism for reducing emissions from deforestation and forest degradation in developing countries should include and prioritize tropical peatlands. Forested tropical peatlands in Southeast Asia are rapidly being converted into production systems by introducing perennial crops for lucrative agribusiness, such as oil-palm and pulpwood plantations, causing large greenhouse gas (GHG) emissions. The Intergovernmental Panel on Climate Change Guidelines for GHG Inventory on Agriculture, Forestry, and Other Land Uses provide an adequate framework for emissions inventories in these ecosystems; however, specific emission factors are needed for more accurate and cost-effective monitoring. The emissions are governed by complex biophysical processes, such as peat decomposition and compaction, nutrient availability, soil water content, and water table level, all of which are affected by management practices. We estimate that total carbon loss from converting peat swamp forests into oil palm is 59.4 ± 10.2 Mg of CO2 per hectare per year during the first 25 y after land-use cover change, of which 61.6% arise from the peat. Of the total amount (1,486 ± 183 Mg of CO2 per hectare over 25 y), 25% are released immediately from land-clearing fire. In order to maintain high palm-oil production, nitrogen inputs through fertilizer are needed and the magnitude of the resulting increased N2O emissions compared to CO2 losses remains unclear.
Keywords: drainage, respiration, gain-loss approach, stock-difference approach
Globally, peatlands cover an area of 400 million hectare, which is equivalent to 3% of the Earth’s land area. These ecosystems store a large fraction of terrestrial carbon, as much as 528 Pg (Pg = 1 × 1015 g), or one-third of global soil carbon (1, 2). This quantity is equivalent to the amount of carbon that would be emitted to the atmosphere from burning fossil fuels at the current annual global rate (approximately 7 Pg in 2007) for the next 75 y.
One-third of the carbon stored in peatlands (191 Pg) is located in the tropics (3, 4), of which 60% is in Southeast Asia with an estimated area of 25 million hectare (Mha). The majority (84%) of Southeast Asian peatlands are found in Indonesia (around 21 Mha), whereas Malaysia harbors 2–2.5 Mha. Thailand has around 45,000 ha, and relatively small areas are found in Vietnam, Brunei, and the Philippines (5).
Tropical peatlands are an important terrestrial carbon pool, but they are highly vulnerable and have become a major source of carbon emissions that requires policy changes to allow mitigation measures to take place. During the period of 2000–2005, the deforestation rate in Indonesian peatlands was estimated around 0.1 Mha per annum (6). Adding to this, the area of peatlands burnt during the big fire in 1997 was 2.12 Mha (7).
The main driver of tropical peatlands deforestation is the development of oil-palm and pulpwood plantations (8). Indonesia and Malaysia, which currently account for 85% of the world’s supply of crude palm oil, aim at supplying Chinese, Indian, and European markets. If crude palm oil demand increases, there could be much more pressure on the forested land in the region. For example, in order to substitute 1% of fossil fuel use with biofuels for electricity production, Europe would consume the oil production of at least 2 Mha of oil-palm plantations (9).
It was estimated that converting a hectare of forest to palm-oil production yields net present values (NPV) of $3,835–$9,630 to land owners (10, 11). The conversion is more profitable than leaving the forests standing for carbon credits from voluntary markets of $614–$994 per hectare although belowground carbon in peatland is also considered. Unless post-2012 global climate policies create significant financial incentives to overcome the economic drivers of deforestation, reducing emissions from deforestation and forest degradation (REDD) will not be able to compete financially with factors that expand oil-palm agriculture (12).
The Bali Action Plan paves the way for REDD implementation as a climate change mitigation measure (13). The Action Plan invites countries to consider policy approaches and creates the opportunity for positive incentives on issues relating to improved forest management, including conservation, sustainable management of forests, and enhancement of forest carbon stocks, known as “REDD+.” Because the rules and modalities of REDD+ are to be decided, it is now the right time to promote the peatlands sector to be included in the new climate regime under the REDD+ schemes.
In this review we explore existing data for these ecosystems and suggest areas for scientific support in meeting the methodological challenges to assess greenhouse gas (GHG) emissions from tropical peatlands. Identifying research gaps with respect to improved management in these carbon-rich ecosystems merits significant elaboration to support effective REDD+ implementation.
Current Status and Trends of Tropical Peatlands
Peatland Development.
In 1981, “planned deforestation” in Indonesia was legislated, involving 30 Mha of conversion forests (14). In addition to plantation forests, most of the conversions were allocated for agricultural land development, such as oil palm. Furthermore, in early 2009, the government of Indonesia issued a regulation that allows the development of oil-palm plantations in peatlands with peat depth less than 3 m, which could potentially trigger further deforestation and peatlands degradation.
In late May 2010, however, a letter of intent (LoI) between the government of Indonesia and the government of Norway on cooperation on REDD+ was signed. The Norwegian government pledge of $1 billion will trigger (i) the development of REDD+ strategies to address key drivers forest and peatland-related emissions, (ii) the establishment of an independent REDD+ agency and an independent monitoring, reporting, and verification institution, and (iii) the financial mechanisms. Thus far, the LoI has generated extensive debates across government agencies, private sectors, and civil society regarding opportunity costs, institution settings, and new regulatory framework.
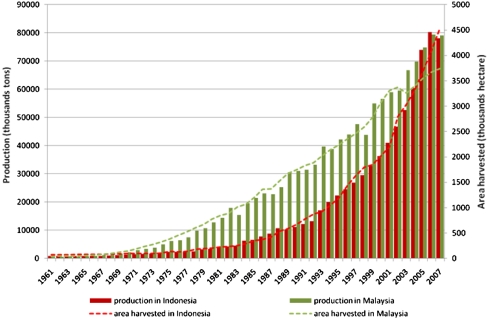
The tremendous expansion of oil-palm plantations in Indonesia and Malaysia shown in Fig. 1 (15) is partly driven by biofuels markets. Palm oil is a versatile tropical agricultural product because its production cost is the lowest and productivity is the highest among major oil crops (16). The emergence of the renewable energy market to meet climate change challenges has been considered an economic opportunity for developing countries. The expansion of oil palm increases demand for land, which causes substantial social and environmental concerns with regards to community rights, biodiversity in high-value conservation forests, and GHG emissions from removal of forest biomass and peat decomposition (17, 18).
Fig. 1.
Trends of oil-palm plantation expansions and fruit production in Indonesia and Malaysia. The different pattern in both expansion of the area and production may indicate different types of management and site quality or soil productivity.
Since the early 1990s, the government of Indonesia has subsidized the expansion of pulpwood plantations and the paper industry. As a result, the pulp and paper sector has been growing rapidly with a number of social and environmental consequences (19). Unlike the oil-palm sector, which has been driven by combination of land-use policy and rising commodity prices, the ambitious pulpwood expansion was solely policy-driven. The capacity of Indonesian mills to produce pulp was on the order of 6 million tons per year in 2004. To meet this capacity, companies relied heavily on mixed tropical hardwood harvested from natural forests. In the same period Indonesia was the second largest exporter of bleached pulp to China after Russia, amounting to 1.2 million tons (8). Assuming the domestic consumption of pulp is to be the same order of magnitude, there is still a huge shortfall in terms of supplying raw materials. If REDD measures are not driven by strong policies and adequate incentives, it will be difficult to compete with the increasing demand for pulp and paper and the overcapacity of pulp mills.
REDD Opportunities and Risks.
The latest development in international negotiations is that the REDD mechanism will go beyond just emissions from deforestation and forest degradation and will also support other activities including forest conservation, sustainable management of forests, and enhancement of forest carbon stocks. Thus, the mechanism in now referred to as REDD+. Despite the uncertainties regarding the rules and modalities for REDD+, there are emerging opportunities and challenges for countries harboring forests. In particular there are opportunities for countries with carbon-rich peat swamp forests.
Indonesia, for example, has 40 Mha of forestland classified as nonforested or degraded by the Ministry of Forestry (20). A large portion of degraded lands which are characterized by mineral soils may be allocated for sustainable pulpwood and oil-palm development. Therefore, carbon-rich peatlands can be preserved and targeted for rehabilitation as part of enhancement of sinks activities under REDD+ mechanisms.
Only planting on degraded secondary forests and degraded grasslands could be compatible with environmentally responsible expansion of palm-oil production. There is, however, a financial trade-off here because land owners often generate the capital required for plantation establishment from the proceeds of liquidating the native forests. REDD finance could be used to overcome this barrier. Moreover, fire risks should be adequately addressed in REDD+ activities. Fire protection, which usually consumes public funds, could be internalized in the REDD mechanism.
In most cases, vegetation and land fires are lit intentionally to remove vegetation residues or debris before introducing new crops or plantations. Although extensive drought because of large-scale climatic phenomena, like El Niño, is associated with widespread fires, it has never been the cause of fires (21). Nevertheless, dry climate has amplified the effects of land-use cover change (LUCC) and land management, causing carbon emission during the 2006 El Niño that were estimated to be more than 30 times greater than emissions during the 2000 La Niña (22). Although peat fires are episodic, the CO2 flux from smoldering peat fires could be at least as large as the decomposition flux from peatlands (23). Estimates for Southeast Asian emissions from fires for the period 1997–2006 were between 1.4 and 4.3 Pg per year (24). These scenario-based estimates were well within the range of estimates using intensive measurements in plots (25) that ranged between 3 and 9 Pg of CO2. A more recent study for equatorial Asia (Malaysia, Indonesia, and Papua New Guinea) using CO∶C data obtained from Moderate Resolution Imaging Spectroradiometer and Measurements of Pollution in the Troposphere sensors, respectively (22), resulted in average CO2 emissions from fires during 2000–2006 of 0.5 Pg yr-1.
The public funds made available by multilateral agencies should be able to support countries to equip themselves with a certain level of capacity to engage in REDD+ projects. These opportunities are not necessarily unique to the forest sector but can address concerns of the wider national development agenda. However, through REDD+ activities, countries would have the opportunity to improve forest governance, management capabilities, biodiversity conservation, poverty alleviation, and issues related to property rights.
There are also risks to the climate system if the expansion of the REDD+ mechanism allows for expansion of plantation operations on peatlands in the name of ecosystem rehabilitation. For example, Indonesia’s strategy for emissions reductions places emphasis on enhancing sinks through tree planting on degraded forestland over reducing deforestation (26). Plantation forestry on degraded peatlands is likely to sustain high emissions from peat soils because of the drainage while providing only modest offsets from biomass accumulations. The net effect of such a scheme is likely to be negative on the atmosphere.
Land-Use Dynamics and GHG Emissions in Tropical Peatlands
In this section, we will first review the existing methods proposed by the Intergovernmental Panel on Climate Change (IPCC) for estimating GHG flux changes associated with LUCC in tropical peatlands. Then we will examine which are the biotic and abiotic factors regulating soil GHG fluxes and how changes of these regulators following LUCC may affect the fluxes. Finally, we will calculate C losses from peat swamp forest conversion into oil-palm plantation over a period of 25 y and look at how the changes in N2O fluxes compare to C losses.
Methods for Assessing GHG Emissions from Peatlands.
The IPCC guidelines (27) propose two approaches to estimating carbon stock changes in any pool. The “gain–loss” approach includes all processes that bring about changes in a pool. Gains can be attributed to growth (i.e., biomass increases) and to transfers of carbon from another pool. Losses can be attributed to transfers of carbon from one pool to another or transfers out of the system. The “stock-difference” method is an alternative approach, which can be used where C stocks in relevant pools are measured at two points in time to assess C stock changes and an average gain or loss rate is determined over the time interval between measurements.
In the case of conversion of peatlands forest to oil-palm or Acacia sp. plantations, the stock-difference approach is likely to be the most applicable approach to biomass carbon accounting. Published values for aboveground biomass in indigenous peat forests vary widely (Table S1). Belowground biomass accounting is generally based on the ratio of belowground biomass to aboveground biomass. The few data that have been collected suggest that root-to-shoot ratios in mature dense peat forests in the tropics are very low—on the order of 0.01–0.06 (28). There are some values for dead wood and litter in the scientific literature, but no systematic survey has been done. These pools are small portions of the carbon stocks in forests on peat soils but may make up a greater portion of carbon stocks in tree cropping systems.
The main concern related to land-use change in these ecosystems is associated with the soil organic matter. The 2006 IPCC guidelines follow a stock-difference approach for both mineral and organic soils, which is not appropriate for tropical peatlands because it is impossible to estimate carbon stocks in these systems with any reasonable level of certainty. The emissions factors for drained managed forests on organic soils in the guidelines are significantly lower than those reported for forests converted to plantations or agriculture (29–31). Tropical peats differ from temperate or boreal peats because they are derived from forest vegetation and are very heterogeneous. The soil surface is often very irregular with large hummocks and hollows (29). Tropical peat soils also have logs and undecomposed branches within them. The zones between the logs and branches are of very low bulk density, often between 0.03 and 0.20 g cm-3, and there is no predictable pattern to variation in bulk density; for example, there is no discernible trend with depth (30). Additionally, the mineral contact beneath the peat is not always regular. Thus, calculating both the volume and the carbon density of tropical peat is often not possible without very intensive measurements at each site. Such intensive measurements are impractical for national inventories and for inventories that any REDD project may establish.
An accounting approach on the basis of the gain–loss method that accounts for changes in inputs and outputs from peat soils following conversion and associated with management is likely to give a more precise estimate of the impact on the atmosphere and is likely to be more practical to implement. The scientific literature has many serious gaps at the moment, and it is therefore impossible to suggest default factors for the gain–loss method. Nevertheless, generating reasonable default factors for peatlands is not technically difficult. A concerted effort to generate good numbers over a period of, say, 10 y should be sufficient to significantly enhance our ability to do proper accounting in these ecosystems and facilitate their incorporation into a REDD mechanism.
Finally, the wetlands chapter of the guidelines provides for estimation of non-CO2 GHGs. Because drainage of peatlands generally lowers CH4 emissions (32, 33), additional anthropogenic emissions are assumed to be insignificant in drained peatlands and can be excluded from inventories. The default factor for N2O emissions associated with drainage is double the factor for temperate and boreal because mineralization rates are assumed to be about 2 times greater in tropical climates than in temperate climates. Because default values are not based on empirical studies, these should be carried out to improve accounting methods. Conversion of peat forests to intensive cropping or oil palm involves significant inputs of N fertilizers. Likewise, Acacia sp. adds significant amounts of N to these soils through N fixation, which is likely to increase N2O emissions. The revised IPCC guidelines (27) provide equations for estimating N2O emissions from managed soils in a separate chapter dedicated to non-CO2 GHGs. These guidelines cover methods for estimating direct N2O emissions from application of organic and synthetic N fertilizers or crop residues, including from N-fixing crops, and drainage and management of organic soils.
Our recommendation for assessing C loss from LUCC in tropical peatlands is to combine the gain–loss and the stock-difference approaches. Loss from the peat can be estimated by using the gain–loss approach, whereas the loss from the biomass can be assessed by using the stock-difference approach. The first approach requires information on C inputs from litterfall, root mortality, and exudation and C outputs from peat decomposition (also called soil heterotrophic respiration), CH4 fluxes, and soluble and physical removal. The second approach requires estimates of aboveground C stocks prior to and following conversion. Changes in N2O fluxes should also be accounted for, especially for those LUCC where N inputs to the soil are expected to increase.
Within the REDD mechanism, factors for both methods will be required to operationalize C accounting at project and national levels. In the following sections, we will review the available literature and assess the adequacy of the existing data to provide inputs to the IPCC equations.
Regulating Factors of Soil GHG Flux Changes from Peatlands.
Agricultural development on tropical peatlands requires drainage, which initiates a process of subsidence. Subsidence may be divided into two phases, with an initial rapid consolidation followed by slow oxidation and shrinkage (31). Subsidence of peat soils is related to the depth of the water table. Subsidence increases with depth up to 50 cm below the surface, after which it remains constant (34). In addition to the CO2 emissions, these changes affect CH4 and N2O fluxes (35). Because most of these peat systems are ombrotrophic, and thus tend to be nutrient-limited, nutrient additions are likely to significantly increase both oxidation of soil organic matter, leading to increased CO2 emissions, and N2O emissions in the case of N fertilizer. These effects could be persistent and affect rehabilitation efforts.
Carbon dioxide (CO2).
The estimated contribution of peat oxidation to the subsidence rate varies greatly, from 40% to 73% (34). Several authors have attempted to relate peat decomposition or peat subsidence rates to the depth of drainage (34, 36, 37). The approach in these studies has been problematic because most compile data from several studies and attempt to infer emissions due to drainage as if that were the only mechanism operating in the comparisons. Most of the studies used for these analyses were conceived with objectives other than quantifying this relationship. Regressions are produced without taking into account other confounding factors (differences in nutrient availability, fertilizer application, nature of the peat, land use, ecosystem productivity, etc.). Additionally, these studies mistakenly present relationships between peat drainage depth and soil respiration across all land uses as validation of the regression models and use this to infer CO2 emissions from peat decomposition.
There are two components in soil respiration, viz. heterotrophic respiration of soil microorganisms and autotrophic respiration of roots and mycorrhizae (38). Only the former contributes to peat C loss, and the autotrophic component should never be considered to be negligible, especially in tree plantations. Heterotrophic and autotrophic activity belowground may not respond equally to changes in soil water content and may respond in context-dependent ways. In a peat swamp forest in central Kalimantan, CO2 emissions were relatively steady regardless of the water table position in hummocks, formed mainly from living and dead roots and decaying debris (39). In nearby vegetation-free hollows, CO2 emission rates were progressively lower as the water table rose towards the peat surface. Thus, there was likely a relationship between heterotrophic respiration and water table depth in the hollows, but the effect may have been overcome in the hummocks because the presence of roots oxygenated the soil and allowed heterotrophic decomposition to continue apace.
Models on total soil respiration will overestimate CO2 emissions from peat decomposition. Indeed, the use of one of these proposed relationships for an oil-palm plantation with mean water table drainage levels of 50 cm (34) returns a soil heterotrophic respiration rate of 12.3 Mg of C per hectare per year, equal to the average total soil respiration rate in oil-palm plantations on peatlands (Table 2).
Table 2.
Average C fluxes in peat swamp forest and oil-palm plantations on peat
| Source | C flux, Mg of C per hectare per year |
| Peat accumulation in the forest | 0.75 ± 0.25 (1.5 ± 0.5 mm y-1) |
| Litterfall in oil palm | 1.5 ± 0.1 |
| Root mortality in oil palm | 3.6 ± 1.1 |
| Soil respiration in oil palm | 12.7 ± 2.7 |
| Root respiration in oil palm | 3.4 ± 0.4 |
| Heterotrophic respiration in oil palm | 9.3 ± 2.7 |
| Dissolved organic carbon + particulate organic carbon | 1.0 ± 0.5 |
Values are mean ± standard error. Data sources and calculation methods are presented in SI Text.
Soil fluxes of CO2 in tropical peatlands are often interactively influenced by multiple factors. Given the growing acceptance of this relationship in the climate change community and nascent efforts to reduce emissions by reflooding drained peatlands, it is imperative to develop empirical evidence for the relationship between water table depth and CO2 emissions from peat decomposition or to devise rehabilitation strategies that tackle the true underlying drivers of peatland emissions.
Assessment of peat heterotrophic respiration rates from peat subsidence is another interesting approach. However, there are still very high uncertainties associated with the parameters involved (contribution of decomposition to subsidence, organic matter decomposition rate, relationship between water level and subsidence rate, C content of peat layers and spatial variation in peat bulk density, etc.), which makes these estimates unreliable.
Methane (CH4).
Primary factors that may impact the CH4 flux following LUCC in peatlands are soil temperature, moisture, compaction and 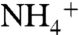 or
or 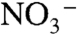 contents in the case of fertilized systems. Raised soil temperatures following LUCC may stimulate both processes of methanogenesis and methanotrophy, although methanotrophy seems to be less sensitive to temperature than methanogenesis and is more dependent on CH4 availability (40, 41). Decreased water table levels after drainage may decrease methanogenesis, increase methanotrophy, and consequently decrease the net CH4 emission to the atmosphere. Consequences of peat compaction on CH4 fluxes would be opposite to those from decreased water table levels. Finally, inhibitory effects of N fertilizers on CH4 oxidation have been reported in mineral soils. This inhibitory effect seems to be dependent on the nature of the N fertilizer and was reported to be short-term (40).
contents in the case of fertilized systems. Raised soil temperatures following LUCC may stimulate both processes of methanogenesis and methanotrophy, although methanotrophy seems to be less sensitive to temperature than methanogenesis and is more dependent on CH4 availability (40, 41). Decreased water table levels after drainage may decrease methanogenesis, increase methanotrophy, and consequently decrease the net CH4 emission to the atmosphere. Consequences of peat compaction on CH4 fluxes would be opposite to those from decreased water table levels. Finally, inhibitory effects of N fertilizers on CH4 oxidation have been reported in mineral soils. This inhibitory effect seems to be dependent on the nature of the N fertilizer and was reported to be short-term (40).
Nitrous oxide (N2O).
Denitrification and nitrification are the main processes that produce N2O. Similarly to soil respiration, variations in soil water content strongly affect soil emissions of N2O. The effect of soil water content on soil N2O effluxes has been described as a monotonic curve with maximum emissions around field capacity, which is often around 60% of water-filled pore space (WFPS). At higher water contents, N2O is primarily reduced into N2 (42). So far, no relationship has been demonstrated between the water table level and N2O emissions in tropical peatlands. It is likely that flooded conditions would enable denitrification to reduce N2O into N2 (43), resulting in lower N2O emissions. Drainage may therefore increase emissions, particularly in fertilized systems or systems with N-fixing trees. In peat soils with low pH values, denitrification of N2O into N2 at high soil water content may be partially inhibited (44). Hence, the response in N2O emissions to reduced WFPS values or decreased water table levels may vary according to intrinsic properties of the peat.
Next steps.
When the concern is the impact of LUCC on the climate, the big story in peatlands is likely to be the soil C. Research needs to focus on soil heterotrophic respiration and not total soil respiration. It is reasonable to expect that the response of the two processes will vary with respect to LUCC and management. Measurement of changes in soil heterotrophic respiration after LUCC and in response to fertilizer, in particular, is necessary and urgent. Drainage of peatlands generally lowers CH4 emissions (32, 33), and in the next section we will analyze how the decrease in soil CH4 compares to the other C flux changes in the peat C budget. We will also review the literature on soil N2O flux changes following LUCC in tropical peatlands and see how these changes compare with C flux changes.
Carbon and N2O Dynamics in Changing Land Use of Peatlands.
C losses from LUCC.
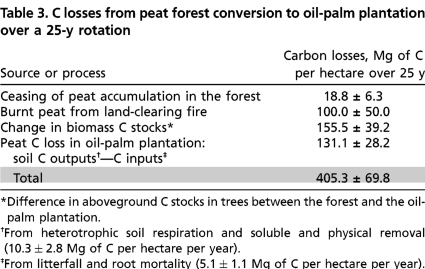
Here we present our assessment on C loss from peat swamp forest conversion into oil-palm plantation over a period of 25 y (Table 3), calculated by combining the gain–loss (for assessing C losses from the peat) and the stock-difference (for assessing C loss from the vegetation) approaches, as explained in Methods for Assessing GHG Emissions from Peatlands. Average C stocks and fluxes before (peat swamp forest) and after conversion (oil-palm plantation) are presented in Tables 1 and 2. Data sources and calculation methods are presented in the corresponding Tables S1 and S2.
Table 3.
C losses from peat forest conversion to oil-palm plantation over a 25-y rotation
*Difference in aboveground C stocks in trees between the forest and the oil-palm plantation.
†From heterotrophic soil respiration and soluble and physical removal (10.3 ± 2.8 Mg of C per hectare per year).
‡From litterfall and root mortality (5.1 ± 1.1 Mg of C per hectare per year).
Table 1.
C stocks in the aboveground biomass of the trees in peat swamp forests and oil-palm plantations on peat, and loss from the peat after land-clearing fire
| C pool | C stock, Mg of C per hectare |
| Tree aboveground biomass in peat swamp forests | 179.7 ± 38.2 |
| Tree aboveground biomass in oil palm | 24.2 ± 8.1 |
| Peat loss from land-clearing fire | 100.0 ± 50.0 |
Values are mean ± standard error. Data sources and calculation methods are presented in SI Text.
Prior to conversion, in the undisturbed peat forest, anaerobic conditions and nutrient limitations in the soil limit decomposition of the organic matter, and the balance between C inputs and outputs leads to peat accumulation (Table 2). After conversion, peat C loss in the oil-palm plantation was calculated as the balance between main soil C outputs (heterotrophic soil respiration and dissolved and particulate organic C) and inputs (litterfall and root mortality) (Table 2). This balance amounted to 5.2 ± 1.1 Mg of C per hectare per year. Additionally, land clearing by fire was assumed to be part of the land management system and calculated as a peat C stock loss (Table 1). Interruption of peat accumulation by removing forest vegetation should be accounted for and is nonnegligible. In the long run, peat loss will affect the sustainability of peatlands ecosystems.
After clearing the forest, the land is prepared for cultivation, and fire is often used. Peat C loss from these fires is significant and amounts to 25% of total C loss associated with LUCC. Therefore, REDD payment would promote zero-burning policies and peatland managers could deploy technology to support fire prevention.
Peat C loss associated with LUCC (249.9 Mg of C per hectare over 25 y) is greater than C loss from the change in aboveground biomass C stocks. However, peat losses will not cease after this period and will persist as long as management promotes organic matter oxidation. These figures demonstrate the potential for high levels of avoided emissions by including tropical peat swamp forests into the REDD mechanism and underlines the need for developing improved methods for estimating C loss from peat soils associated with LUCC.
The mean rate of peat C loss associated with oil-palm cultivation (5.2 Mg of C per hectare per year) is more than 7 times that of peat C accumulation rate in the forest (Table 2), which demonstrates how fast and intensively LUCC in tropical peatlands may affect the C cycle.
Our estimate of heterotrophic soil respiration rate in oil palm on peatland (9.3 Mg of C per hectare per year) is lower than that calculated from the linear relationship (34) of 12.3 Mg of C per hectare per year for mean water table levels of 50 cm. We would like to underline that soil heterotrophic respiration is not a direct measurement of peat C loss but has to be balanced with main C inputs to the soil in order to assess peat C loss. As a consequence, the relationships between heterotrophic soil respiration and water table level (34, 37) cannot compare with our assessment of peat C loss in the oil-palm plantation.
Soil CH4 fluxes are small compared to C release from soil heterotrophic respiration (45). For instance, net annual CH4 uptakes in an oil-palm plantation on peat of 0.0002 Mg of C per hectare per year were observed in Malaysia (46). Over 25 y, C inputs would represent 0.005 Mg of C per hectare. The same study (46) assessed a very small decrease in soil CH4 flux (0.0004 Mg of C per hectare per year) from peat swamp forest (CH4 flux of 0.0002 Mg of C per hectare per year) conversion to oil-palm plantation. Such a small C flux change (0.01 Mg of C per hectare over 25 y) is negligible compared to the overall peat C loss from LUCC (249.9 Mg of C per hectare over 25 y) and justifies not be accounted for.
In conclusion, C loss over 25 y when peat swamp forest is converted into oil palm (Table 3) is equivalent to 1,486.1 Mg of CO2 per hectare over 25 y, corresponding to a C loss rate of 16.2 ± 2.8 Mg of C per hectare per year or 59.4 ± 10.2 Mg of CO2 per hectare per year. Such an assessment for peat swamp forest conversion to Acacia sp. plantations is not feasible at the moment because of the current lack of scientific studies. Nevertheless, we could expect similar C losses as those from oil-palm plantations. Indeed, the change in biomass C stocks will be comparable. The C loss rate from Acacia sp. cultivation might also be similar. The species Acacia crassicarpa is planted on a 6–7 y rotation period, with a drainage depth of 80–100 cm, about 20 cm deeper than that required for growing oil palm (47). Larger CO2 losses from heterotrophic respiration might then be expected in Acacia sp. plantations as compared to oil-palm plantations, but the larger C inputs from both litterfall and root mortality of the Acacia sp. trees may lead to a similar peat C budget. The main difference between the two conversions would be in terms of fire risk, which is greater in Acacia sp. than in oil-palm plantations (47).
N2O emissions from fertilized crops and plantation of N2-fixing trees.
Increased N2O emissions resulting from LUCC in peatlands are likely to be a small part of the total GHG emission. Difference in soil N2O emissions between oil-palm plantations on peatlands fertilized at a rate of 100 kg of N per hectare per year and nearby natural forest was observed (48). The difference was 0.5 kg of N per hectare per year or 0.23 Mg of CO2 eq. per hectare per year [applying the N2O global warming potential of 298 over a time horizon of 100 y (49)], which is negligible compared with the total C loss associated with conversion (59.4 Mg of CO2 per hectare per year). A difference in N2O flux between intensively fertilized pineapple fields grown on peatlands and nearby peat forest was 1.4 kg of N per hectare per year—i.e., 0.7 Mg of CO2 eq. per hectare per year (50). Again, this rise in N2O emissions was negligible compared with the total C loss of 59.4 Mg of CO2 per hectare per year associated with peat swamp deforestation.
We are aware of only one study that found large differences in N2O emissions between three highly fertilized crop fields and peat forest (51). The authors found differences of 192.5, 34, and 114.5 kg of N per hectare per year, that is to say, 90.1, 15.9, and 53.6 Mg of CO2 eq. per hectare per year. In this case, the rise in N2O emissions is comparable and even larger than total C loss resulting from conversion of peat swamp forests into oil palm. Mean N2O emissions measured in the crop fields (195, 36.5, and 117 kg of N per hectare per year) are among the highest of the literature (52) probably because of the unusually high rates of fertilizer applications (832, 877, and 1,032 kg of N per hectare per year). Fertilizer application rates in oil palm are on the order of 100–300 kg of N per hectare per year (53, 54).
The increase in N2O emissions from the conversion of peat swamp forest to Acacia sp. plantations could be larger than that from the conversion to oil-palm plantation because of the deeper drainage under Acacia sp.
Concluding Remarks
In the past decade, emissions from deforestation and peat degradation due to drainage and fires in Southeast Asia, mainly Indonesia, have been the single largest source of GHG emissions from land use. Despite limited capacity to measure and monitor peatland carbon dynamics, there is an awareness of the need to include peat soils in the REDD+ mechanism. Strong political support combined with sufficient capacity and stakeholders’ participation should make REDD+ opportunities very attractive. The REDD+ mechanism offers an opportunity not only to manage peatlands more sustainably, but also to settle a number of agendas related to social and legal issues. It is timely to promote the eligibility of reducing GHGs emissions from peatlands in the new climate regime.
The framework for accounting CO2 and N2O emissions is adequate. An approach on the basis of the C gain–loss method that accounts for changes in inputs (litterfall, root mortality, and exudation) and outputs (soil heterotrophic respiration, CH4 fluxes, and soluble and physical removal) from peat soils prior to and following conversion is likely to give a more precise estimate than a stock-difference approach. Appropriate emissions factors are required to properly implement the equations, and these have only partly been developed for peat soils. Generating reasonable emissions factors for these activities is not technically difficult. A concerted effort to generate good numbers should be sufficient to significantly enhance our ability to do proper accounting in these ecosystems, which should be one of the highest priority areas for research.
Finally, research on the mechanisms of carbon cycle and how it is affected by peatland management and ecosystem rehabilitation measures is urgently needed. The current mechanistic understanding is insufficient to develop appropriate emissions mitigation recommendations. The approach of estimating changes on emissions on the basis of single factors like drainage depth should not be used for REDD+ projects or for management recommendations. Research should focus on improving both water and nutrient management to ensure that we have the best chance to advance in this area rapidly.
Supplementary Material
ACKNOWLEDGMENTS.
This work was generously supported by the contributions of the governments of Australia (Grant Agreement 46167), Finland (Grant Agreement HELM023-29), and Norway (Grant Agreement GLO-3945 GLO-09/764) to the Center for International Forestry Research (CIFOR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911966107/-/DCSupplemental.
References
- 1.Gorham E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol Appl. 1991;1:182–195. doi: 10.2307/1941811. [DOI] [PubMed] [Google Scholar]
- 2.Immirzi CP, Maltby E. Exeter, UK: Department of Geography, University of Exeter; 1992. The global status of peatlands and their role in carbon cycling, a report for Friends of the Earth by the Wetland Ecosystems. Research Group Report 11. [Google Scholar]
- 3.Post WM, et al. Carbon pools and world life zones. Nature. 1982;298:156–159. [Google Scholar]
- 4.Page SE, Rieley JO. Tropical peatlands: A review of their natural resource functions, with particular reference to Southeast Asia. Int Peat J. 1998;8:95–106. [Google Scholar]
- 5.Association of Southeast Asian Nations (ASEAN) Sustainable Management of Peatlands: Wise Use, Prevention of Fires and Rehabilitation. Jakarta: ASEAN Peatlands Management Initiative, ASEAN Secretariat; 2005. [Google Scholar]
- 6.Ministry of Forestry. Reducing Emissions from Deforestation and Forest Degradation in Indonesia: Methodology and Strategies. Jakarta: Ministry of Forestry of the Republic of Indonesia; 2007. [Google Scholar]
- 7.Tacconi L. Bogor: Center for International Forestry Research; 2003. Fires in Indonesia: Causes, cost, and policy implication. CIFOR Occasional Paper 38. [Google Scholar]
- 8.Murdiyarso D, Kanninen M. Forests and climate change: An outlook of Asian forests in the new climate regime. In: Loh C, Stevenson A, Tay S, editors. Climate Change Negotiations: Can Asia Change the Game? Singapore: Hong Kong Civic Exchange–Singapore Institute of International Affairs; 2008. pp. 74–87. [Google Scholar]
- 9.Reinhardt G, Rettenmaier N, Gärtner S. Rain Forest for Biodiesel? Ecological Effects of Using Palm Oil as a Source of Energy. Frankfurt: World Wildlife Fund Germany; 2007. p. 52. [Google Scholar]
- 10.Butler RA, Koh LP, Ghazoul J. REDD in the red: Palm oil could undermine carbon payment schemes. Conservation Lett. 2009;2:67–73. [Google Scholar]
- 11.Ministry of Forestry. Consolidation Report on Reducing Emissions from Deforestation and Forest Degradation in Indonesia. Jakarta: Ministry of Forestry of the Republic of Indonesia; 2008. [Google Scholar]
- 12.Stern Nicholas. Stern Review: The Economics of Climate Change. Cambridge: Cambridge Univ Press; 2006. [Google Scholar]
- 13.United Nations Framework Convention on Climate Change. Report of the Conference of the Parties on its 13th Session, Part Two: Action Taken by the Conference of the Parties at its 13th Session. 2007. pp. 3–8. Document FCCC/CP/2007/6/Add.1.
- 14.Murdiyarso D, Adiningsih E. Climatic anomalies, Indonesian vegetation fires and terrestrial carbon emissions. J Mitig Adapt Strat Glob Change. 2007;12:101–112. [Google Scholar]
- 15.Food and Agriculture Organization Statistical Database. 2006. [accessed March, 2010]. http://faostatfao.org/
- 16.Yusoff S, Hansen SB. Feasibility study of performing a life cycle assessment on crude palm oil production in Malaysia. Int J Life Cycle Assess. 2007;12:50–58. [Google Scholar]
- 17.Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P. Land clearing and the biofuel carbon debt. Science. 2008;319:1235–1238. doi: 10.1126/science.1152747. [DOI] [PubMed] [Google Scholar]
- 18.Danielsen F, et al. Biofuel plantations on forested lands: Double jeopardy for biodiversity and climate. Conservation Biol. 2008;23(2):348–358. doi: 10.1111/j.1523-1739.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 19.Barr C. Pulp industry and plantation development in Indonesia: Experiences and potential lessons learned for China; Proceedings of the International Forum on Investment and Finance in China’s Forestry Sector; Beijing: Forestry Economics and Development Research Center-Forest Trend-CIFOR; 2006. [Google Scholar]
- 20.Ministry of Forestry. Forestry Statistics of Indonesia. Jakarta: Department of Forestry, Ministry of Forestry; 2009. [Google Scholar]
- 21.Murdiyarso D, et al. Policy responses to complex environmental problems: Insights from a science-policy activity on transboundary haze from vegetation fires in Southeast Asia. Agric Ecosyst Environ. 2004;104:47–56. [Google Scholar]
- 22.van der Werf GR, et al. Climate regulation of fire emissions and deforestation in equatorial Asia. Proc Natl Acad Sci USA. 2008;105:20350–20355. doi: 10.1073/pnas.0803375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rein G, Cohen S, Simeoni A. Carbon emissions from smoldering peat in shallow and strong fronts. Proc Combustion Inst. 2009;32:2489–2496. [Google Scholar]
- 24.Hooijer A, et al. PEAT-CO2, assessment of CO2 emissions from drained peatlands in SE Asia. Delft Hydraulics Report. 2006. Q3943.
- 25.Page SE, et al. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature. 2002;420:61–65. doi: 10.1038/nature01131. [DOI] [PubMed] [Google Scholar]
- 26.Indonesia Second National Communication under the United Nations Framework Convention on Climate Change (UNFCCC): Summary for Policy Makers. Jakarta: Government of Indonesia; 2009. [Google Scholar]
- 27.IPCC. IPCC guidelines for national greenhouse gas inventories, prepared by the National Greenhouse Gas Inventories Programme. In: Eggleston HS, et al., editors. Hayama: Institute for Global Environmental Strategies; 2006. [Google Scholar]
- 28.Brady MA. Vancouver: University of British Columbia; 1997. Organic matter dynamics of coastal peat deposits in Sumatra, Indonesia. PhD thesis. [Google Scholar]
- 29.Hirano T, et al. Carbon dioxide balance of a tropical peat swamp forest in Kalimantan, Indonesia. Glob Change Biol. 2007;13:412–425. [Google Scholar]
- 30.Kool DM, Buurman P, Hoekman DH. Oxidation and compaction of a collapsed peat dome in Central Kalimantan. Geoderma. 2006;137:217–225. [Google Scholar]
- 31.Wösten JHM, Ismail AB, van Wijk ABM. Peat subsidence and its practical implications: a case study in Malaysia. Geoderma. 1997;78:25–36. [Google Scholar]
- 32.Furukawa Y, et al. Effect of changing groundwater levels caused by land-use changes on greenhouse gas fluxes from tropical peat lands. Nutr Cycl Agroecosyst. 2005;71:81–91. [Google Scholar]
- 33.Hirano T, Jauhiainen J, Inoue T, Takahashi H. Controls on the carbon balance of tropical peatlands. Ecosystems. 2009;12:873–887. [Google Scholar]
- 34.Couwenberg J, Dommain R, Joosten H. Greenhouse gas fluxes from tropical peatlands in Southeast Asia. Glob Change Biol. 2009;16:1715–1732. [Google Scholar]
- 35.Hadi A, et al. Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutr Cycl Agroecosyst. 2005;71:73–80. [Google Scholar]
- 36.Rieley JO, Page SE. Carbon budgets under different land uses on tropical peatlands. In: Rieley JO, Banks CJ, Page SE, editors. Future of Tropical Peatlands in Southeast Asia as Carbon Pools and Sinks. CARBOPEAT, International Peat Society, University of Leicester; 2008. pp. 27–32. [Google Scholar]
- 37.Hooijer A, et al. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences. 2010;7:1505–1514. [Google Scholar]
- 38.Ryan MG, Law BE. Interpreting, measuring, and modeling soil respiration. Biogeochemistry. 2005;73:3–27. [Google Scholar]
- 39.Jauhiainen J, et al. Carbon fluxes from a tropical peat swamp forest floor. Glob Change Biol. 2005;11:1788–1797. [Google Scholar]
- 40.Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: A review. Eur J Soil Biol. 2001;37:25–50. [Google Scholar]
- 41.Striegl RG. Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere. 1993;26:715–720. [Google Scholar]
- 42.Davidson EA, Vitousek PM, Matson PA. Soil emissions of nitric-oxide in a seasonally dry tropical forest of Mexico. J Geophys Res. 1991;96:15439–15445. [Google Scholar]
- 43.Inubushi K, et al. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere. 2003;52:603–608. doi: 10.1016/S0045-6535(03)00242-X. [DOI] [PubMed] [Google Scholar]
- 44.Šimek M, Cooper JE. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci. 2002;53:345–354. [Google Scholar]
- 45.Jauhiainen J, et al. Carbon flux controls and land use change in tropical peatland; Proceedings of the International Symposium and Workshop ‘Carbon-Climate-Human Interactions—Carbon Pools, Fire, Mitigation, Restoration and Wise Use’, Yogyakarta, Indonesia; 2007. pp. 27–31. http://www.geog.le.ac.uk/carbopeat/media/pdf/yogyapapers/p25.pdf. [Google Scholar]
- 46.Melling L, Hatano R, Goh KJ. Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol Biochem. 2005;37:1445–1453. [Google Scholar]
- 47.Pirard R, Cossalter C. Will They Help Eliminate Fiber Shortfalls at Sumatran Pulp Mills or Feed the China Market? Bogor: Center for International Forestry Research; 2006. The revival of industrial forest plantations in Indonesia’s Kalimantan provinces. CIFOR Working Paper 37. [Google Scholar]
- 48.Melling L, Hatano R, Goh KJ. Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Sci Plant Nutr (Tokyo) 2007;53:792–805. [Google Scholar]
- 49.Forster P, et al. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. pp. 129–234. [Google Scholar]
- 50.Furukawa Y, et al. Effect of changing groundwater levels caused by land-use changes on greenhouse gas fluxes from tropical peat lands. Nutr Cycl Agroecosyst. 2005;71:81–91. [Google Scholar]
- 51.Takakai F, et al. Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, Central Kalimantan, Indonesia. Soil Sci Plant Nutr (Tokyo) 2006;52:662–674. [Google Scholar]
- 52.Stehfest E, Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst. 2006;74:207–228. [Google Scholar]
- 53.Caliman JP, Togatorop E, Martha B, Samosir R. Aerial fertilization of oil palm. Better Crops Int. 2002;16:10–14. [Google Scholar]
- 54.Chew PS, Pushparajah E. Nitrogen management and fertilization of tropical plantations tree crops. In: Bacon PE, editor. Nitrogen Fertilization and the Environment. New York: Dekker; 1995. pp. 225–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.