Abstract
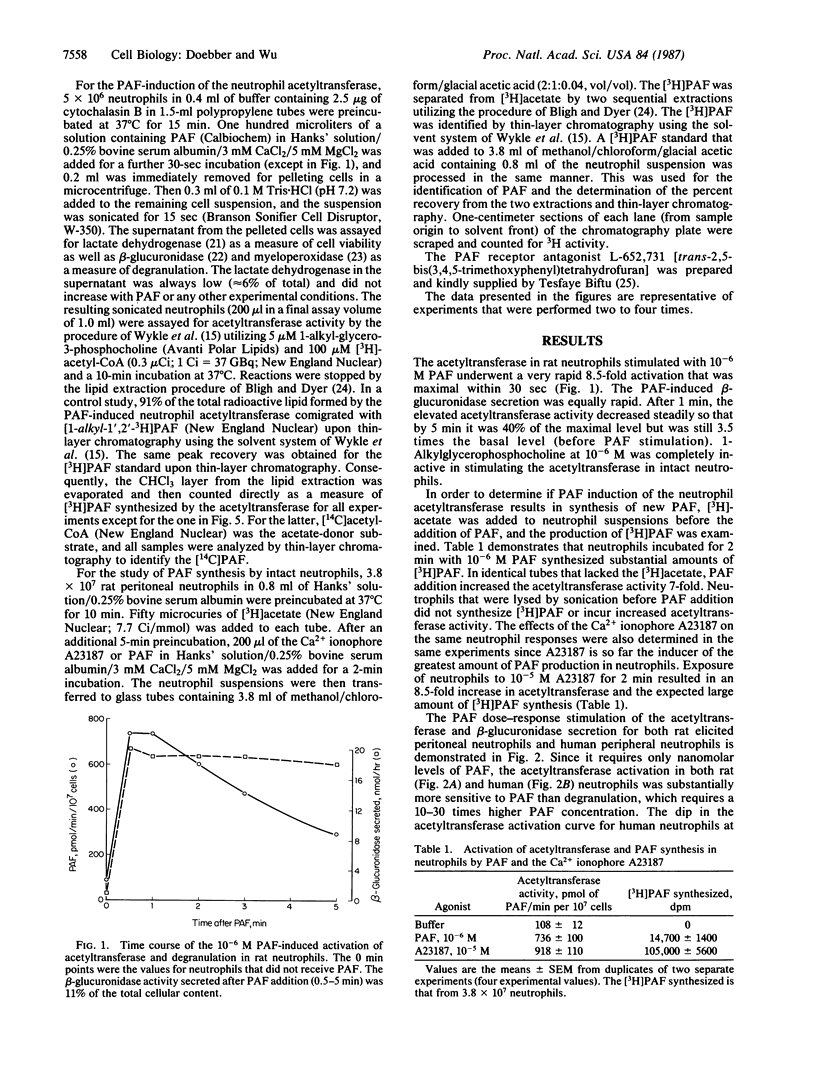
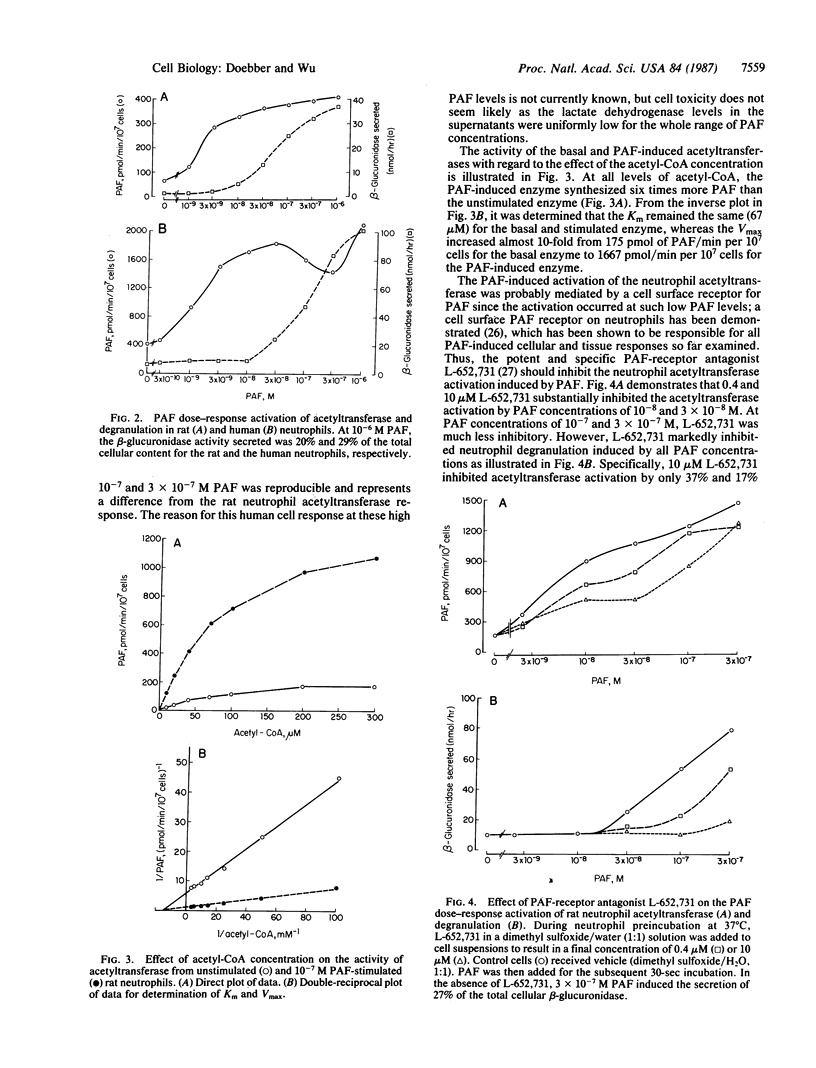
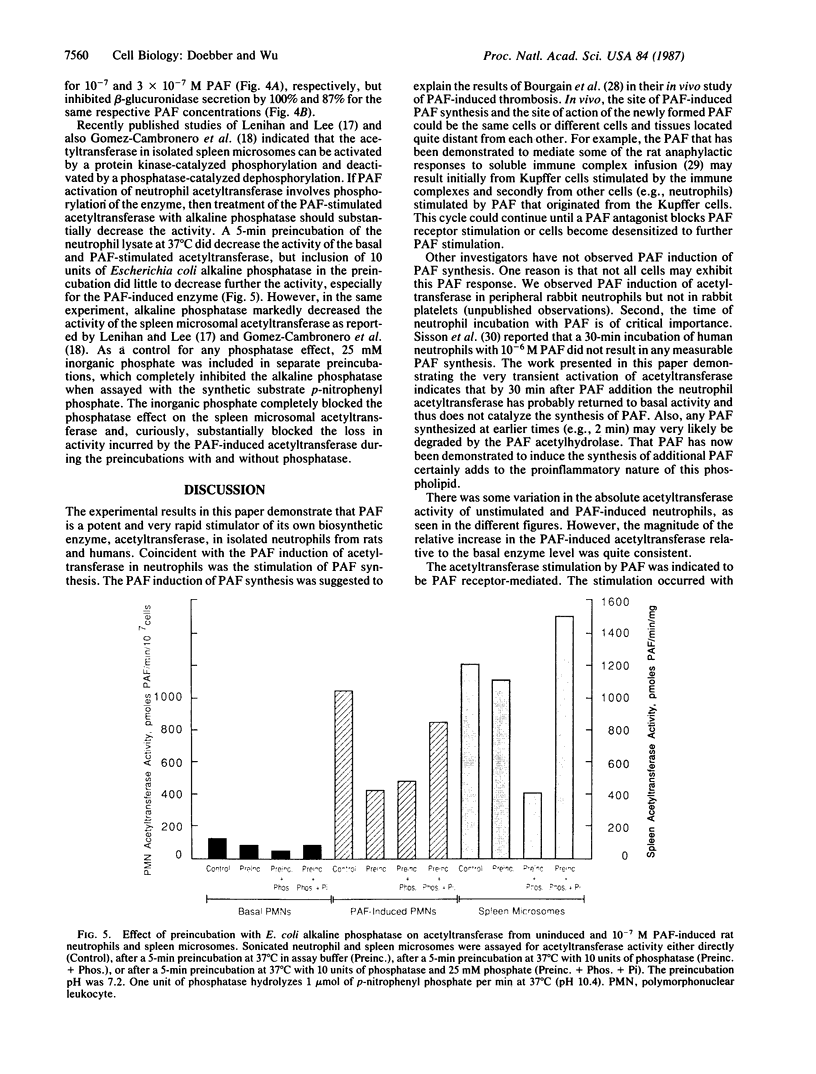
Platelet activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine; PAF) induced in isolated rat peritoneal and human peripheral neutrophils a rapid and potent activation of the PAF biosynthetic enzyme acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine O2-acetyltransferase (EC 2.3.1.67). The PAF-induced activation of the neutrophil acetyltransferase (8-10 times basal neutrophil activity) was maximal within 30 sec after PAF addition, as was the PAF-stimulated degranulation. After 1 min of PAF stimulation, the elevated acetyltransferase activity steadily decreased. Within 2 min of stimulation of neutrophils with 10(-6) M PAF, the 7-fold increase in acetyltransferase activity was coincident with substantial PAF synthesis (as measured by [3H]acetate incorporation into PAF), which was 14% of the PAF synthesis induced by the Ca2+ ionophore A23187 at 10(-5) M. PAF activation of the acetyltransferase and PAF synthesis required intact neutrophils as they did not occur in cells broken by sonication. The neutrophil acetyltransferase was 10-30 times more sensitive to activation by PAF than was degranulation as the acetyltransferase activation was evident with 10(-9) M PAF and was about maximal with 3 x 10(-8) M PAF. The unstimulated and PAF-induced acetyltransferase exhibited the same Km for acetyl-CoA (67 microM), but the Vmax for the PAF-induced enzyme (1667 pmol/min per 10(7) cells) was 10 times that of the unstimulated enzyme (175 pmol/min per 10(7) cells). The PAF induction of the acetyltransferase was less sensitive to inhibition by the specific PAF receptor antagonist L-652,731 than was PAF-induced degranulation. This, along with the differing sensitivities to PAF, suggests that acetyltransferase activation and degranulation induced by PAF either involve two different PAF receptors or involve one receptor type with different receptor occupancy requirements. Escherichia coli alkaline phosphatase, which greatly decreased the activity of the acetyltransferase in spleen microsomes, had little or no effect on the basal or PAF-induced neutrophil acetyltransferase. Thus, by stimulating the activity of acetyltransferase, PAF induces in neutrophils the synthesis of more PAF, thereby probably augmenting the neutrophil response to the initial PAF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso F., Gil M. G., Sánchez-Crespo M., Mato J. M. Activation of 1-alkyl-2-lysoglycero-3-phosphocholine. Acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem. 1982 Apr 10;257(7):3376–3378. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Biftu T., Gamble N. F., Doebber T., Hwang S. B., Shen T. Y., Snyder J., Springer J. P., Stevenson R. Conformation and activity of tetrahydrofuran lignans and analogues as specific platelet activating factor antagonists. J Med Chem. 1986 Oct;29(10):1917–1921. doi: 10.1021/jm00160a020. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Bormann B. J., Huang C. K., Mackin W. M., Becker E. L. Receptor-mediated activation of a phospholipase A2 in rabbit neutrophil plasma membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):767–770. doi: 10.1073/pnas.81.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgain R. H., Maes L., Braquet P., Andries R., Touqui L., Braquet M. The effect of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (paf-acether) on the arterial wall. Prostaglandins. 1985 Aug;30(2):185–197. doi: 10.1016/0090-6980(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Cunningham F. M., Smith M. J., Ford-Hutchinson A. W., Walker J. R. Migration of peritoneal polymorphonuclear leucocytes in the rat. J Pathol. 1979 May;128(1):15–20. doi: 10.1002/path.1711280104. [DOI] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Biftu T. Platelet-activating factor (PAF) mediation of rat anaphylactic responses to soluble immune complexes. Studies with PAF receptor antagonist L-652,731. J Immunol. 1986 Jun 15;136(12):4659–4668. [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Shen T. Y. Platelet activating factor intravenous infusion in rats stimulates vascular lysosomal hydrolase secretion independent of blood neutrophils. Biochem Biophys Res Commun. 1984 Dec 28;125(3):980–987. doi: 10.1016/0006-291x(84)91380-9. [DOI] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Velasco S., Mato J. M., Sánchez-Crespo M. Modulation of lyso-platelet activating factor: acetyl-CoA acetyltransferase from rat splenic microsomes. The role of cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1985 Jun 30;845(3):516–519. doi: 10.1016/0167-4889(85)90219-8. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Lam M. H., Biftu T., Beattie T. R., Shen T. Y. trans-2,5-Bis-(3,4,5-trimethoxyphenyl)tetrahydrofuran. An orally active specific and competitive receptor antagonist of platelet activating factor. J Biol Chem. 1985 Dec 15;260(29):15639–15645. [PubMed] [Google Scholar]
- Lee T., Lenihan D. J., Malone B., Roddy L. L., Wasserman S. I. Increased biosynthesis of platelet-activating factor in activated human eosinophils. J Biol Chem. 1984 May 10;259(9):5526–5530. [PubMed] [Google Scholar]
- Lenihan D. J., Lee T. C. Regulation of platelet activating factor synthesis: modulation of 1-alkyl-2-lyso-sn-glycero-3-phosphocholine:acetyl-CoA acetyltransferase by phosphorylation and dephosphorylation in rat spleen microsomes. Biochem Biophys Res Commun. 1984 May 16;120(3):834–839. doi: 10.1016/s0006-291x(84)80182-5. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Henson P. M. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986 Oct 15;137(8):2653–2661. [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Demopoulos C. A., Pinckard R. N. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J Immunol. 1980 Jun;124(6):2919–2924. [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Benveniste J. Platelet-activating factor (PAF-acether) and macrophages. II. Phagocytosis-associated release of PAF-acether from rat peritoneal macrophages. Cell Immunol. 1981 Jan 15;57(2):281–292. doi: 10.1016/0008-8749(81)90087-3. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Lewis R. A., Razin E., Austen K. F. Antigen-initiated release of platelet-activating factor (PAF-acether) from mouse bone marrow-derived mast cells sensitized with monoclonal IgE. J Immunol. 1983 Dec;131(6):2958–2964. [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L., Miller C. H., Lewis J. C., Waite M., Bass D. A., McCall C. E., DeChatelet L. R. 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants. Am J Pathol. 1981 Apr;103(1):70–78. [PMC free article] [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Sklar L. A., Hyslop P. A., Oades Z. G., Omann G. M., Jesaitis A. J., Painter R. G., Cochrane C. G. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J Biol Chem. 1985 Sep 25;260(21):11461–11467. [PubMed] [Google Scholar]
- Stahl P., Rodman J. S., Schlesinger P. Clearance of lysosomal hydrolases following intravenous infusion. Kinetic and competition experiments with beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):594–605. doi: 10.1016/0003-9861(76)90471-9. [DOI] [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Iñarrea P., Alvarez V., Egido J. Vascular actions of synthetic PAF-acether (a synthetic platelet-activating factor) in the rat: evidence for a platelet independent mechanism. Immunopharmacology. 1982 Apr;4(2):173–185. doi: 10.1016/0162-3109(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Valone F. H., Goetzl E. J. Specific binding by human polymorphonuclear leucocytes of the immunological mediator 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycero-3-phosphorylcholine. Immunology. 1983 Jan;48(1):141–149. [PMC free article] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]




