Abstract
Defining the links between cell division and DNA replication is essential for understanding normal cell cycle progression and tumorigenesis. In this report we explore the effect of phosphorylation of cell division cycle 6 (Cdc6), a DNA replication initiation factor, by polo-like kinase 1 (Plk1) on the regulation of chromosomal segregation. In mitosis, the phosphorylation of Cdc6 was highly increased, in correlation with the level of Plk1, and conversely, Cdc6 is hypophosphorylated in Plk1-depleted cells, although cyclin A- and cyclin B1-dependent kinases are active. Binding between Cdc6 and Plk1 occurs through the polo-box domain of Plk1, and Cdc6 is phosphorylated by Plk1 on T37. Immunohistochemistry studies reveal that Cdc6 and Plk1 colocalize to the central spindle in anaphase. Expression of T37V mutant of Cdc6 (Cdc6-TV) induces binucleated cells and incompletely separated nuclei. Wild-type Cdc6 but not Cdc6-TV binds cyclin-dependent kinase 1 (Cdk1). Expression of wild-type Plk1 but not kinase-defective mutant promotes the binding of Cdc6 to Cdk1. Cells expressing wild-type Cdc6 display lower Cdk1 activity and higher separase activity than cells expressing Cdc6-TV. These results suggest that Plk1-mediated phosphorylation of Cdc6 promotes the interaction of Cdc6 and Cdk1, leading to the attenuation of Cdk1 activity, release of separase, and subsequent anaphase progression.
Protein modification such as phosphorylation is an important mechanism for regulating transitions through cell cycle phases to ensure DNA duplication and segregation of chromosomes into daughter cells. Polo-like kinase 1 (Plk1) is an essential mammalian mitotic kinase (1–5). Plk1 is expressed in the S, G2, and M phases of the cell cycle, and its activity peaks in mitosis (2–5). During mitosis, Plk1 localizes to several locations and it has diverse substrates and functions (5). Recently, to gain insight into a connection between cell division and DNA replication, other cell cycle functions of Plk1 have been studied. Plk1 interacts and colocalizes with minichromosome maintenance (Mcm) subunits and the origin recognition complex (Orc) 2 in the centrosome (6, 7), suggesting that Plk1 may influence components of DNA replication. Plk1 is known to phosphorylate Hbo1, a histone acetyltransferase binding to Orc1 of prereplication complex (pre-RC) (8) and topoisomerase II α (9), suggesting that Plk1-associated phosphorylation of Hbo1 or topoisomerase II α is essential for S phase.
DNA replication is tightly coordinated with cell division in order to maintain genomic integrity. In late mitosis and early G1, replication origins are licensed for replication when Orc, cell division cycle 6 (Cdc6), chromatin licensing and DNA replication factor 1 (Cdt1), and Mcm2-7 are loaded (10–12). Origins harboring pre-RC are licensed for replication but do not initiate DNA synthesis until S phase (10–12). Cdc6 plays a key role in origin licensing (13, 14). The level of Cdc6 fluctuates during the cell cycle and is controlled by a degradation mechanism that is more active in S phase than in mitosis (15). The abundance of Cdc6 through DNA synthesis indicates that Cdc6 may be required after the initiation of DNA replication. In yeast the ectopic expression of Cdc6 interferes with progression through G2 and causes a dramatic delay in entry into mitosis (13, 14). Overexpression of a dominant-negative Cdc6 mutant induces mitotic delay correlated with inhibition of mitotic cyclin-dependent kinase (CDK) activity (15). The amino-terminal domain of Cdc6 tightly binds to mitotic CDK (16), suggesting that Cdc6 acts as an inhibitor of mitotic CDK in mitosis. Recent reports show that mitotic Cdc6 stabilizes anaphase-promoting complex/cyclosome (APC/C) substrates and affects modulation of APC/CCdc20 (17). Cdc6 is also associated with the mitotic apparatus in mice throughout M phase (18). These studies suggest that Cdc6 plays a role in mitotic progression, but the details remain unclear.
In this report, we explore the interaction between Plk1, a mitotic kinase, and Cdc6, a DNA replication initiation factor. We show that Plk1 phosphorylates Cdc6 and that phosphorylation of Cdc6 by Plk1 promotes interaction of Cdc6 and Cdk1, suggesting that phosphorylation of Cdc6 by Plk1 regulates mitotic exit through Cdk1-separase.
Results
The Increased Phosphorylation of Cdc6, a Prereplication Complex Component, Was Correlated with the Level of Plk1 in M Phase.
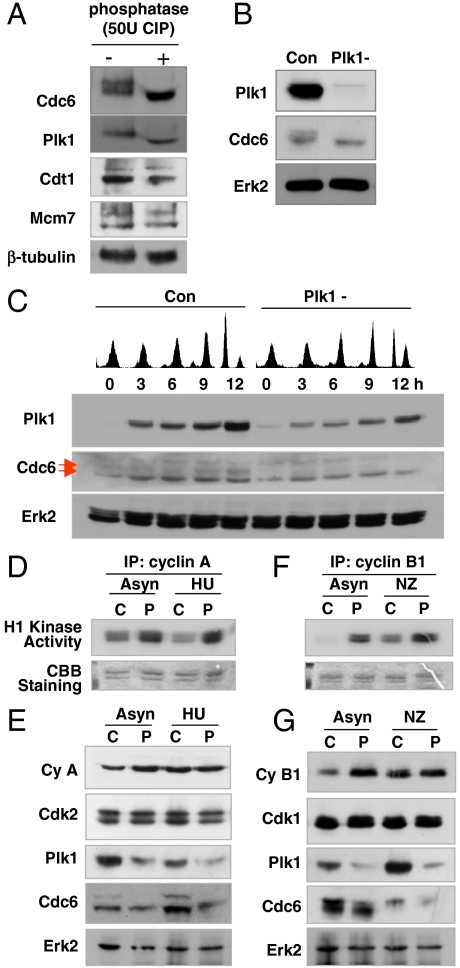
We have found that Plk1 depletion reduces the loading of Mcm proteins on chromatin and DNA synthesis, which suggests that Plk1 may affect DNA replication (19). To investigate the correlation between Plk1 and the DNA pre-RC components, the levels of pre-RC components including Orc2, Mcm7, Cdc6, and Cdt1 were observed during cell cycle progression. Cells were synchronized with a double thymidine block and released with fresh medium. Nine hours after release, most cells were in M phase and the level of Plk1 increased. The level of Cdc6 also increased, accompanied by the appearance of a more slowly migrating band. Other prereplication factors such as Orc2, Mcm7, Cdt1, and geminin were not greatly altered 9 h after release (Fig. S1A). To observe whether pre-RC components are phosphorylated in mitosis, cell extracts were treated with alkaline phosphatase, when cells were in mitosis determined by FACS analysis (Fig. 1A and Fig. S1A). Phosphatase treatment markedly altered the migration of the upper bands of Cdc6 and Plk1, indicating that the shifted bands were phosphorylated (Fig. 1A). In contrast, phosphatase had no effect on the levels of Cdt1 and Mcm7. Consistent with phosphorylation in M phase, the more slowly migrating bands of Cdc6 were detected in nocodazole-treated cells but not in hydroxyurea-treated cells (Fig. S1B).
Fig. 1.
The phosphorylation of Cdc6 does not occur in Plk1-depleted cells and is independent of the activities of cyclin-dependent kinases. (A) Cell extracts at 9 h after release from the double thymidine block were incubated with or without 50 units alkaline phosphatase (BioLabs) for 30 min at 37 °C and immunoblotted with anti-Cdc6, anti-Plk1, anti-Cdt1, anti-Mcm7, and anti-β-tubulin antibodies. (B) Cells were infected with lentivirus-expressing RNAi targeting Plk1 (Plk1-) or control virus (Con). Twenty-four hours later, cells were selected with puromycin for 2 d and then lysates were immumoblotted with anti-Plk1, anti-Cdc6, and anti-Erk2 antibodies. (C) HeLa cells were synchronized with the double thymidine block and infected with lentivirus-based RNAi targeting Plk1 (Plk1-) or with control virus (Con). Zero time is 24 h after infection. Lysates were prepared at various times and immunoblotted. FACS analysis of cell cycle progression was performed. (D–G) HeLa cells were infected with lentivirus-expressing RNAi targeting Plk1 (P) or control virus (C). Twenty-four hours later, cells were selected with puromycin for 36 h and then treated with 2 mM hydroxyurea or 100 ng/mL nocodazole for 12 h. (D and F) Cdk activity was assayed with immunoprecipitated cyclin A- or cyclin B1-dependent kinase with anticyclin A or anticyclin B1 antibody and histone H1 was used as substrate. (E and G) Extracts were immunoblotted with anticyclin A, anticyclin B1, anti-Cdk1, anti-Cdk2, anti-Plk1, anti-Cdc6, and anti-Erk2 antibodies.
Cyclin A- or Cyclin B1-Dependent Kinases Are Activated but Cdc6 is Nonphosphorylated in Plk1-Depleted Cells.
The results of phosphatase treatment of mitotic cell lysates suggest that Cdc6 may be phosphorylated by Plk1. To investigate whether the depletion of Plk1 affects the phosphorylation of Cdc6, HeLa cells were infected with lentivirus expressing human Plk1-targeting shRNA and selected with puromycin for 2 d. As shown in Fig. 1B, phosphorylation of Cdc6 was significantly reduced in Plk1-depleted cells. Plk1 depletion over 3 d induces mitotic arrest and apoptosis (20). To avoid these effects, puromycin selection was eliminated and cells were harvested 1 d after infection. Under these conditions, Plk1-depleted cells cycled slowly and apoptotic cells did not appear during the period examined. In control cells, the phosphorylation of Cdc6 was observed from 6 to 12 h after release when cells passed through G2 and mitosis. However the phosphorylation of Cdc6 was not detectable in Plk1-depleted cells (Fig. 1C), indicating that the presence of Plk1 affects the phosphorylation of Cdc6.
Cdc6 is a substrate of Cdk2 (21–23) raising the possibility that a reduction of Cdc6 phosphorylation in Plk1-depleted cells results from the low activities of CDKs. Thus, the activities of cyclin A/Cdk2 and cyclin B1/Cdk1 in Plk1-depleted cells were examined. Cyclin A- or cyclin B1-dependent kinase assays were performed using immunoprecipitation of cyclin A- or cyclin B1-dependent kinase from hydroxyurea or nocodazole-treated cells (Fig. 1 D–G). The activities of cyclin A-associated kinase (Fig. 1D) and cyclin B1-associated kinase (Fig. 1F) in Plk1-depleted cells were greater than in control cells. However, Cdc6 was hypophosphorylated in Plk1-depleted cells (Fig. 1 E and G), indicating that the phosphorylation level of Cdc6 was not related to the activities of CDKs in Plk1-depleted cells.
Cdc6 Binds to the C-Terminal Domain of Plk1 in Vitro and in Vivo.
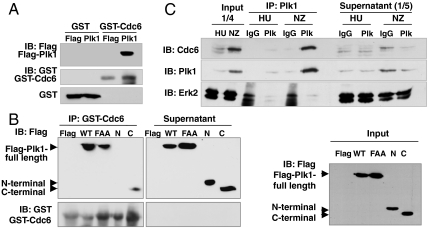
The C-terminal polo-box domain of Plk1 functions as a phosphopeptide-binding domain and may contribute to substrate specificity (24, 25). To determine whether Plk1 interacts with Cdc6 or not, in vitro GST pull-down assays were performed. Purified GST-fused Cdc6 (GST-Cdc6) was incubated with lysates of HEK 293T cells expressed with FLAG-tagged Plk1 (FLAG-Plk1). As shown in Fig. 2A, FLAG-Plk1 associated with GST-Cdc6, but not with GST. To determine whether the polo-box influences Plk1 association with Cdc6, FLAG-tagged WT, polo-box mutant which is three points mutant at W414F, V415A, and L427A (FAA), N-terminal mutant (amino acids region 1–305), and C-terminal mutant (amino acids region 306–603) of Plk1 were expressed in HEK 293T cells (Fig. 2B and Fig. S2A). Purified GST-Cdc6 fusion protein was incubated with cell lysates expressed wild type or mutant of Plk1. Shown in Fig. 2B, Left, GST-Cdc6 associated with the wild type of Plk1. However, the binding between GST-Cdc6 and Plk1-FAA mutant was markedly reduced compared to the interaction between GST-Cdc6 and Plk1-WT. GST-Cdc6 also bound the C-terminal polo-box domain, but not the N-terminal kinase domain of Plk1, indicating that the C-terminal polo-box domain of Plk1 contains the Cdc6 binding domain. Endogenous Cdc6 bound to endogenous Plk1 and ectopically expressed Plk1-WT as well (Fig. S2B). As with GST-Cdc6, endogenous Cdc6 associated with the C-terminal domain of Plk1, but not the N-terminal domain (Fig. S2B). From these results we conclude that Cdc6 associates with Plk1 through the C-terminal polo-box domain of Plk1.
Fig. 2.
Plk1 interacts with Cdc6 through its C-terminal polo-box. (A) GST-tagged Cdc6 purified from E. coli and bound Sepharase beads were incubated with lysates of HEK293T cells transfected with pCMV-FLAG or pCMV-FLAG-tagged Plk1. The FLAG-tagged Plk1 associated with GST-tagged Cdc6 was detected by immunoblot analysis with anti-FLAG antibody. GST-tagged Cdc6 was detected by anti-GST antibody. (B) FLAG, FLAG-tagged wild-type Plk1 (WT), polo-box mutant (W414F, V415A, L427A; FAA), N-terminal (N), and C-terminal (C) Plk1 were expressed in HEK293T cells for 2 d and then extracts were immunoblotted with anti-FLAG (Input). Purified GST-Cdc6 was incubated with lysates of these cells and the FLAG-Plk1 protein associated with GST-Cdc6 was detected by immunoblot with anti-FLAG antibody. GST-Cdc6 was detected with anti-GST antibody. (C) HeLa cells were treated with 2 mM hydroxyurea or 100 ng/mL nocodazole for 12 h. Extracts were immunoprecicpitated with antimonoclonal immunoglobulin G or antimonoclonal Plk1 and then immunoblotted with anti-Cdc6, anti-Plk1, or anti-Erk2 antibodies. One-fourth of total input is also shown. The supernatant is one-fifth of total proteins.
Cdc6 is a Mitotic Binding Partner and Substrate of Plk1.
Cdc6 works as a DNA replication initiator in early S phase (10–13) and Plk1 is expressed from S phase and peaks in M phase (4). To investigate when Plk1 interacts with Cdc6, coimmunoprecipitation assays were performed using lysates from cells synchronized in S phase or M phase with hydroxyurea or nocodazole, respectively. Cell lysates were immunoprecipitated with antipolyclonal Plk1 antibody and immunoblotted for Cdc6 or Plk1. As shown in Fig. 2C, endogenous Plk1 associated with endogenous Cdc6 in nocodazole-treated cells.
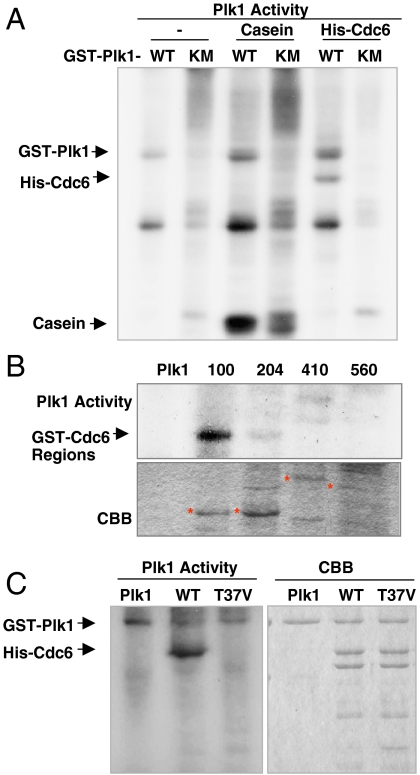
The reciprocal interaction between Plk1 and Cdc6 in vitro and in vivo suggests that Cdc6 may be a substrate of Plk1. To address this possibility, in vitro Plk1 kinase assay was carried out with purified His-tagged Cdc6 protein. GST-tagged wild-type Plk1 (WT) or kinase-defective mutant (KM) was purified from baculovirus and incubated with Casein as a positive control or purified His-Cdc6 (Fig. 3A). Plk1-WT, but not Plk1-KM, phosphorylated casein and His-Cdc6, indicating that Cdc6 is a substrate of Plk1 in vitro. To investigate the phosphorylation site of Cdc6 by Plk1, GST-tagged serial deletion mutants of Cdc6 were used for Plk1 kinase assay. Sequences at N terminus of Cdc6 (amino acids 1–100) were phosphorylated by Plk1 (Fig. 3B). Using site-directed mutants of full-length Cdc6, Plk1 assays showed that the point mutant of Cdc6 containing T37V was not detectably phosphorylated by Plk1 in vitro (Fig. 3C), indicating that Cdc6 is phosphorylated by Plk1 on threonine 37. To address whether Plk1 phosphorylates Cdc6 in cells, FLAG-tagged Plk1-WT and Plk1-KM were expressed in HEK 293T cells and then immunoblot assay was performed. Consistent with in vitro kinase assay, the Cdc6 band shift occurred in cells expressing Plk1-WT, but not in cells expressing Plk1-KM (Fig. S3). The results indicate that Cdc6 is a mitotic binding partner and substrate of Plk1.
Fig. 3.
Plk1 phosphorylates Cdc6 on residue T37. (A) Purified GST-tagged wild-type Plk1 (WT) or kinase-defective mutant (KM) was assayed with casein or His-tagged Cdc6 as a substrate and 10 μCi [γ-32P] ATP at 30 °C for 30 min. (B) GST-tagged Plk1-WT assayed with purified GST-tagged regions of Cdc6, amino acids 1–100 (100), 101–204 (204), 205–410 (410), 411–560 (560) as substrates and 10 μCi [γ-32P] ATP. (C) GST-tagged Plk1-WT was assayed with purified His-tagged full-length wild-type Cdc6 (WT) or T37V mutant Cdc6 (T37V) and 10 μCi [γ-32P] ATP. The samples were resolved with SDS-PAGE and detected by autoradiography.
The Nonphosphorylatable Mutant of Cdc6 Induces Defects in Chromosomal Segregation.
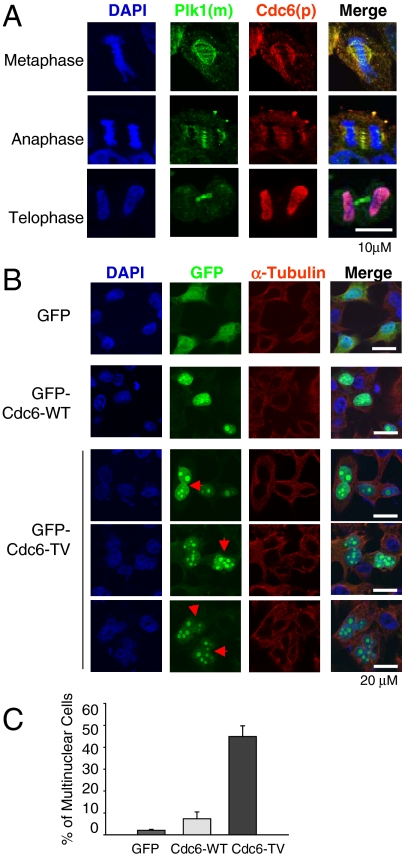
To understand the physiological role of Cdc6 phosphorylation by Plk1, HeLa cells were synchronized in mitosis and stained for Cdc6 and Plk1 (Fig. 4A). Plk1 was detected in the centrosome and kinetochore during prophase, attached to the microtubule and the spindle pole in metaphase, localized in the central spindle in anaphase, and seen in the midbody in telophase (Fig. 4A). Cdc6 colocalized with Plk1 during metaphase and anaphase at the spindle pole and the central spindle. In telophase, Cdc6 translocated to the newly formed nuclei (Fig. 4A). Colocalization of Cdc6 and Plk1 to the central spindle was consistently observed with different antibodies (Fig. S4).
Fig. 4.
The nonphosphorylatable mutant of Cdc6 induces defects in chromosomal segregation. (A) HeLa cells grown on coverslips were synchronized with the double thymidine block. Nine hours after release, the cells were fixed and stained with antimonoclonal Plk1 (Upstate; green) and antipolyclonal Cdc6 (PreteinTech; red). Nuclear DNA was stained by DAPI. (Scale bar: 10 μm.) (B and C) GFP, GFP-tagged wild-type Cdc6 (Cdc6-WT), T37V mutant Cdc6 (Cdc6-TV) were expressed in HeLa cells for 2 d. Cells were fixed and stained with anti-GFP and anti-α-tubulin. (Scale bar: 20 μm.) The populations of binucleated cells were counted and quantified. The means ± SD (error bars) from three determinations obtained in each of three experiments.
Next, the effect of T37V mutant of Cdc6 (Cdc6-TV) on mitosis was studied. Cells expressing GFP-tagged Cdc6-TV were stained with anti-GFP and anti-α-tubulin. Over 40% cells expressing Cdc6-TV were binucleated or had incompletely separated nuclei compared to 7% of cells expressing Cdc6-WT (Fig. 4 B and C), indicating that the nonphosphorylatable Cdc6 mutant may affect chromosomal segregation and cytokinesis.
The Phosphorylation of Cdc6 by Plk1 Regulates the Activity of Separase Through the Association with Cdk1.
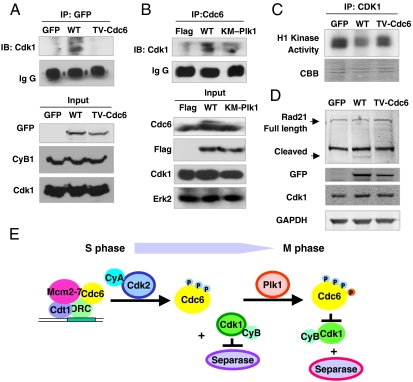
Chromosomal segregation in mitosis is triggered by activation of separase (26). In vertebrates Cdk1-dependent inhibition of separase is a securin-independent pathway for anaphase control (27, 28). The association of separase and Cdk1 leads to inactivation of protease activity (28). To investigate the effects of Cdc6 phosphorylation by Plk1 on Cdc6 interaction with cyclin B1/Cdk1, coimmunoprecipitation assays were done. GFP-tagged Cdc6 was immunoprecipitated from GFP-tagged Cdc6-WT or Cdc6-TV transfected cells, and immunoblotted for Cdk1. Cdc6-WT but not Cdc6-TV interacted with Cdk1 (Fig. 5A). We also examined the association between Cdc6 and Cdk1 in FLAG-tagged Plk1-WT or Plk1-KM transfected cells. Endogenous Cdc6 showed a different phosphorylation pattern after expression of Plk1-WT compared to Plk1-KM (Fig. 5B, Input). Immunoprecipitation assay showed that Cdc6 interacted with Cdk1 in Plk1-WT, but not in Plk1-KM expressing cells (Fig. 5B), suggesting that phosphorylation of Cdc6 by Plk1 induces the interaction of Cdc6 with Cdk1.
Fig. 5.
Phosphorylation of Cdc6 by Plk1 regulates separase activity via interaction with Cdk1. (A) GFP-tagged mock (GFP), wild-type Cdc6 (WT), and T37V mutant of Cdc6 (TV) were expressed in HeLa cells for 2 d. Extracts were immunoprecipitated with anti-GFP, and then immunoblotted with anti-Cdk1 antibody. Input was analyzed by Western blot analysis with anti-GFP, anti-Cdk1, and anticyclin B1 antibodies. (B) FLAG-tagged mock, Plk1-WT, and Plk1-KM proteins were expressed in HeLa cells for 2 d and then the cells were treated with nocodazole for 12 h. Extracts were immunoprecipitated with anti-Cdc6 and then immunoblotted with anti-Cdk1. Input was analyzed by Western blot analysis with anti-Cdc6, anti-FLAG, anti-Cdk1, and anti-Erk2 antibodies. (C and D) Cells were prepared as described in Fig. 5A. (C) Extracts were immunoprecipitated with anti-Cdk1 and Cdk1 activity was assayed with histone H1 and 10 μCi [γ-32P] ATP at 30 °C for 30 min. Activity was detected by autoradiography. (D) Extracts were immunoblotted with anti-Cdk1, anti-Rad21, anti-GFP, anti-GAPDH antibodies. Three independent experiments were done. (E) Plk1-mediated phosphorylation of Cdc6 leads the activation of separase. Cdc6 phosphorylated by Plk1 binds with Cdk1 and inhibits its activity. Thus, separase may be released from Cdk1, which promotes chromosomal segregation in mitosis.
To determine whether the association of Cdc6 with Cdk1 affects Cdk1 activity, histone H1 kinase assays were performed on Cdk1 immunoprecipitates. Expression of WT Cdc6 significantly lowered the activity of Cdk1 compared to expression of the nonphosphorylatable mutant of Cdc6 or the GFP control (Fig. 5C), indicating that the phosphorylation of Cdc6 leads to inhibition of Cdk1 activity.
To determine whether the activity of separase is affected by the association of Cdc6 and Cdk1, the cleavage of Cohesin/Rad 21 was assayed. The cleavage of Cohesin/Rad21 was much greater in Cdc6-WT expressing cells than in GFP and Cdc6-TV mutant expressing cells. Expression of WT Cdc6 but not mutant Cdc6 was correlated with activation of separase (Fig. 5D), indicating that the phosphorylation of Cdc6 by Plk1 and subsequent binding of Cdk1 leads to activation of separase and initiation of anaphase.
Cdc6 Depletion Induces Mitotic Defects and Multinucleated Cells.
To test whether Cdc6 influences chromosomal segregation, Cdc6 was depleted with a Cdc6 targeting lentivirus. Twenty-four hours after infection, cells were selected with puromycin for 2 d, fixed and stained with anti-phosphohistone H3 (S10), a mitotic marker, and anti-Plk1. The level of phosphohistone H3 increased twofold in Cdc6-depleted cells compared to control cells (Fig. S5 A and B). Depletion of Cdc6 also increased the level of binucleated cells to 27% compared to 4% in control cells (Fig. S5 A and C). FACS analysis performed 4 d after infection showed that the population of polyploid cells was 11.3% in Cdc6-depleted cells compared to 5% in control cells, and the population of subgenomic DNA was 23% in Cdc6-depleted cells compared to 3.7% in control cells (Fig. S5D). Depletion of Cdc6 also led to an increase the protein levels of Cdk1 and cyclin B1 (Fig. S5E). Cells failing to complete chromosomal segregation and cytokinesis accounted for 60% of the population of Cdc6-depleted cells (Figs. S5F and S6B). This phenotype was reduced to around 35% in Cdc6-WT-rescued cells or 15% in Cdc6-TV-expressed cells compared to control cells depleting Cdc6 (Fig. S6). Taken together, the results indicate that Cdc6 may have a critical role in chromosomal segregation and cytokinesis by regulating separase activity.
Discussion
Cdc6 is an essential regulator of DNA replication and plays a key role in origin licensing (10, 11, 13). The role of Cdc6 in mitosis is less well studied. Here we show that in mitotic cells Plk1 interacts with and phosphorylates Cdc6, and Plk1 and Cdc6 colocalize to the spindle pole and the central spindle. Phosphorylation of Cdc6 by Plk1 affects binding between Cdc6 and Cdk1 as well as Cdk1 activity. The interaction of Cdc6 with Cdk1 results in inhibition of Cdk1 activity, which is required for activation of separase and exit from mitosis. Mutation of Cdc6 results in cells exhibiting a high percentage of binucleation and incompletely separated chromosomes. The depletion of Cdc6 also induces mitotic defects in chromosomal segregation and cytokinesis, and binucleated cells with high levels of cyclin B1 and Cdk1.
Cdc6 is phosphorylated after initiation of DNA replication, and most of these sites depend on cyclin-dependent kinases (15, 21, 22). The phosphorylation of Cdc6 by cyclin A-Cdk2 is thought to play a role in translocation of Cdc6 from the nucleus to the cytoplasm to prevent DNA rereplication (21–23). However, after S phase is initiated, an elevated Cdc6 is still found in the nucleus and is hyperphosphorylated on serine 54 (29) before the completion of mitosis. Results presented here showing that endogenous Cdc6 associates with Plk1 and is localized with Plk1 at the spindle pole in metaphase and at the central spindle in anaphase (see Fig. 4A) suggest a role of Cdc6 in mitosis.
The phosphorylation of Cdc6 on threonine 37 may be critical for mitotic exit by regulating the interaction of Cdc6 and cyclin B1 or Cdk1. Cdc6 interacts with and inhibits mitotic CDK in vitro and in vivo through its amino terminus (30–33). Overexpression of a dominant-negative mutant of Cdc6 refractory to Skp/Cullin/F-box (SCF)CDC4-mediated proteolysis induces a mitotic delay correlated with inhibition of mitotic CDK activity (15, 30). This mutant inhibits CDK activity more efficiently than WT Cdc6 because of its abundance (15, 32). Recently it has been reported that mitotic Cdc6 stabilizes APC/C substrates by a mechanism that is partially independent of mitotic CDK (17). Cdc6 down-regulates APC/Cdc20 before cells exit mitosis through physical interaction with Cdc55, a subunit of PP2A phosphatase (17). Although it has been shown that overexpressed Cdc6 delays mitotic entry and progression in both a CDK-dependent and -independent manner, modest levels of Cdc6 also acts to promote mitotic exit through inhibition of Cdk1 (33, 34). Removal of the Cdc6 N terminus confers cytokinetic defects in Saccharomyces cerevisiae deficient in Sic1 and Cdh1, suggesting that Cdc6, Sic1, and Cdh1 can cooperate to regulate mitotic CDK activity to facilitate mitotic exit (33, 34). Our studies also show that the nonphosphorylatable N-terminal mutant results in incompletely separated chromosomes, inducing failure of mitotic exit (Fig. 4 and Fig. S6). In addition, Cdc6 depletion experiments reveal that endogenous Cdc6 is required to complete chromosomal segregation and cytokinesis in mitosis.
Activation and inactivation of Cdk1 are correlated with mitotic entry and exit (35). Cdk1 is highly activated at the beginning of mitosis and during mitotic progress (35). To pass through anaphase and exit mitosis, Cdk1 must be inactivated, which occurs when cylcin B is degraded (27, 35). In budding yeast, the Cdk inhibitors Sic1 and Cdc6 may contribute to down-regulation of Cdk1 activity in mitosis (31, 32). The N terminus of Cdc6 is necessary and sufficient for interaction Cdk1 (16, 31) and phosphorylation of Cdc6 increases Cdk1 binding (31). Before anaphase, separase is inhibited by association with securin or Cdk1 (27, 28). The degradation of cyclin B1 and securin releases separase triggering chromosomal segregation (26, 28). In Cdc6-depleted cells, the levels of cyclin B or Cdk1 remain elevated, which may suppress the activation of separase. Chromosomal segregation was incomplete and cells were binucleated or displayed incompletely separated chromosome. In addition, the cells expressing the Cdc6-TV mutant showed a similar phenotype. The Cdc6-TV mutant cannot associate with Cdk1 and cannot inhibit Cdk1 activity. These data lead us to propose the following model (see Fig. 5E): In mitosis, Plk1 phosphorylates Cdc6 which binds to Cdk1 and inhibits its activity. This sequential action leads to the release and activation of separase in a securin-independent manner, to promote chromosomal segregation.
Materials and Methods
Plasmid Construction.
Full-length human Cdc6 was amplified by PCR and subcloned into pET-28a (His fusion), pGEX-4T-1 (GST fusion), and pCS2 + GFP (GFP fusion) vector (36, 37). FLAG-tagged Plk1-WT, K82M, FAA, N terminus, and C terminus and baculovirus encoding WT GST-Plk1 and GST-Plk1-K82M were described previously (24). The T37V mutant of Cdc6 was generated by replacing ACA with GTA (forward AGTGATGCCAAACTAGAACCAGTAAATGTCCAAACCGTAACCTG; reverse CAGGTTACGGTTTGGACATTTACTGGTTCTAGTTTGGCATCACT).
Lentivirus-Based RNAi Plasmid Preparation, Virus Production, and Infection.
The lentivirus-based RNAi transfer plasmids targeting human Cdc6 (GenBank release no. NM_001254) at positions 1314 to 1333 (GGACAATGCTGCAGTTCAATT) (pLKO-Puro1.-Cdc6) or control plasmid (pLKO-Puro.1) was prepared and lentivirus was generated and infected as described previously (19, 20).
Phosphatase Treatment.
HeLa cells were synchronized with the double thymidine block. Nine hours after release, cells were harvested and lysed in phosphatase buffer (150 mM NaCl, 1% CHAPS, 10mM Hepes, pH 7.5) and either left untreated or incubated for 30 min at 37 °C with alkaline phosphatase (BioLabs). At the end of the reaction, the lysates were immunoblotted with specific antibodies as described in SI Materials and Methods.
GST Pull-Down Assay.
GST fused Cdc6 expressed in Escherichia coli strain BL21 and purified using glutathione-sepharose 4B beads were incubated with the lysates of HEK 293T cells that had been expressed with FLAG-tagged Plk1 as described in SI Materials and Methods.
Kinase Assay.
For Cdk assays, cyclin A or cyclin B was immunoprecipitated from lysates from cells treated with hydroxyurea for 12 h or nocodazole for 12 h, respectively. Activity was assayed with histone H1 as detailed in SI Materials and Methods.
Plk1 kinase activity was assayed with purified His-tagged full-length Cdc6, GST-tagged regions of Cdc6, or casein as described in SI Materials and Methods.
Immunofluorescence.
For immunofluorescence, cells were fixed, permeabilized, incubated in 0.1% Triton X-100-PBS containing 3% BSA for blocking, incubated with antimonoclonal Plk1 (Upstate) and anti-Cdc6 (ProteinTech Group) antibodies, and analyzed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Eleanor Erikson, Jean Dahl, and Diana Kornet for critically reading the manuscript. We thank Dr. Thomas L. Benjamin (Harvard Medical School, Boston) for the generous support and materials. We thank Drs. Seung Ki Lee and Linhua Liu (Seoul National University, Seoul, Korea) for providing Cdc6 cDNA. This work was supported by National Institutes of Health Grant GM 59172, and R.L.E. is the John F. Drum American Cancer Society Research Professor.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [NM_001254 (*Human Cdc6) and NM_005030 (Human Plk1)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013557107/-/DCSupplemental.
References
- 1.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 3.Takaki T, Trenz K, Costanzon V, Petronczki M. Polo-like kinase a reaches beyond mitosis-cytokinesis, DNA damage response, and development. Curr Opin Cell Biol. 2008;20:650–660. doi: 10.1016/j.ceb.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petronczki M, Lenart P, Peters JM. Polo on the rise—from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Tsvetkov L, Stern DF. Interaction of chromatin-associated Plk1 and Mcm7. J Biol Chem. 2005;280:11943–11947. doi: 10.1074/jbc.M413514200. [DOI] [PubMed] [Google Scholar]
- 7.Stuermer A, et al. Mouse pre-replicative complex proteins colocalise and interact with the centrosome. Eur J Cell Biol. 2007;86(1):37–50. doi: 10.1016/j.ejcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Wu ZQ, Liu X. Role for Plk1 phosphorylation of Hbo1 in regulation of replication licensing. Proc Natl Acad Sci USA. 2008;105:1919–1924. doi: 10.1073/pnas.0712063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Wang Y, Liu X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase II alpha in cell cycle progression. J Biol Chem. 2008;283:6209–6221. doi: 10.1074/jbc.M709007200. [DOI] [PubMed] [Google Scholar]
- 10.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 11.Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14:R778–786. doi: 10.1016/j.cub.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene. 2005;24:2827–2843. doi: 10.1038/sj.onc.1208616. [DOI] [PubMed] [Google Scholar]
- 13.Nishitani H, Nurse P. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell. 1995;83:397–405. doi: 10.1016/0092-8674(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 14.Bueno A, Russell P. Dual functions of Cdc6: A yeast protein required for D4NA replication also inhibits nuclear division. EMBO J. 1992;11:2167–2176. doi: 10.1002/j.1460-2075.1992.tb05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins G, Drury LS, Diffley JF. Separate SCFCDC4 recognition elements target Cdc6 for proteolysis in S phase and mitosis. EMBO J. 2001;20:4836–4845. doi: 10.1093/emboj/20.17.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura S, Seki T, Tanaka S, Diffley JF. Phosphorylation-dependent binding of mitotic cyclins to Cdc6 contributes to DNA replication control. Nature. 2004;431:1118–1123. doi: 10.1038/nature03024. [DOI] [PubMed] [Google Scholar]
- 17.Boronat S, Campbell JL. Mitotic Cdc6 stabilizes APC substrates by a partially Cdc28-independent mechanism and this stabilization is suppressed by deletion of Cdc55. Mol Cell Biol. 2007;27:1158–1171. doi: 10.1128/MCB.01745-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illenye S, Heintz NH. Functional analysis of bacterial artifical; chromosomes in mammalian cells: Mouse Cdc6 is associated with the mitotic spindle apparatus. Genomics. 2004;83:66–75. doi: 10.1016/s0888-7543(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 19.Yim H, Erikson RL. Polo-like kinase 1 depletion induces DNA damage in early S prior to caspase activation. Mol Cell Biol. 2009;29:2609–2621. doi: 10.1128/MCB.01277-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26:2093–2108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Wells NJ, Hunter T. Multistep regulation of DNA replication by cdk phosphorylation of Hscdc6. Proc Natl Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen BO, Lukas J, Sorensen CS, Bartek J, Helin K. Phosphorylation of mammalian Cdc6 by cyclin A/Cdk2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delmolino LM, Saha P, Dutta A. Multiple mechanisms regulate subcellular localization of human CDC6. J Biol Chem. 2001;276:26947–26954. doi: 10.1074/jbc.M101870200. [DOI] [PubMed] [Google Scholar]
- 24.Jang YJ, Ma S, Terada Y, Erikson RL. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 25.Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- 26.Waizengger I, Gimenez-Abian JF, Wernic D, Peters JM. Regulation of human separase by securin binding and autocleavage. Curr Biol. 2002;12:1368–1378. doi: 10.1016/s0960-9822(02)01073-4. [DOI] [PubMed] [Google Scholar]
- 27.Stemmann O, Gorr IH, Boos D. Anaphase topsy-turvy. Cell Cycle. 2006;5(1):11–13. doi: 10.4161/cc.5.1.2296. [DOI] [PubMed] [Google Scholar]
- 28.Gorr IH, Boss D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol Cell. 2005;19:135–141. doi: 10.1016/j.molcel.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Alexandrow MG, Hamlin JL. Cdc6 chromatin affinity is unaffected by serine-54 phosphorylation, S-phase progression, and overexpression of cyclin A. Mol Cell Biol. 2004;24:1614–1627. doi: 10.1128/MCB.24.4.1614-1627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenwood E, Nishitani H, Nurse P. Cdc18p can block mitosis by two independent mechanisms. J Cell Sci. 1998;111:3101–3108. doi: 10.1242/jcs.111.20.3101. [DOI] [PubMed] [Google Scholar]
- 31.Elsasser S, Lou F, Wang B, Campbell JL, Jong A. Interaction between yeast Cdc6 protein and B-type cyclin/Cdc28 kinases. Mol Biol Cell. 1996;7:1723–1735. doi: 10.1091/mbc.7.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calzada A, Sanchez M, Sanchez E, Bueno A. The stability of the Cdc6 protein is regulated by cyclin-dependent kinase/cyclin B complexes in Saccharomyes cerevisiae. J Biol Chem. 2000;275:9734–9741. doi: 10.1074/jbc.275.13.9734. [DOI] [PubMed] [Google Scholar]
- 33.Calzada A, Sacristan M, Sanchez E, Bueno A. Cdc6 cooperates with Sic1 and Hct1 to inactivate mitotic cyclin-dependent kinases. Nature. 2001;412:355–358. doi: 10.1038/35085610. [DOI] [PubMed] [Google Scholar]
- 34.Archambault V, et al. Genetic and biochemical evaluation of the importance of Cdc6 in regulating mitotic exit. Mol Biol Cell. 2003;14:4592–4604. doi: 10.1091/mbc.E03-06-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavet O, Pines J. Progressive activation of Cyclin B1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yim H, Jin YH, Park BD, Choi HJ, Lee SK. Caspase-3-mediated cleavage of Cdc6 induces nuclear localization of p49-truncated Cdc6 and apoptosis. Mol Biol Cell. 2003;14:4250–4259. doi: 10.1091/mbc.E03-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, et al. ATR activity stabilizes Cdc6 and delays G2/M phase entry during hydroxyurea-induced S-phase arrest of HeLa cells. Int J Biochem Cell Biol. 2009;41:1410–1420. doi: 10.1016/j.biocel.2008.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.