Abstract
Restricting the cytotoxicity of anticancer agents by targeting receptors exclusively expressed on tumor cells is critical when treating infiltrative brain tumors such as glioblastoma multiforme (GBM). GBMs express an IL-13 receptor (IL13Rα2) that differs from the physiological IL4R/IL13R receptor. We developed a regulatable adenoviral vector (Ad.mhIL-4.TRE.mhIL-13-PE) encoding a mutated human IL-13 fused to Pseudomonas exotoxin (mhIL-13-PE) that specifically binds to IL13Rα2 to provide sustained expression, effective anti-GBM cytotoxicity, and minimal neurotoxicity. The therapeutic Ad also encodes mutated human IL-4 that binds to the physiological IL4R/IL13R without interacting with IL13Rα2, thus inhibiting potential binding of mhIL-13-PE to normal brain cells. Using intracranial GBM xenografts and syngeneic mouse models, we tested the Ad.mhIL-4.TRE.mhIL-13-PE and two protein formulations, hIL-13-PE used in clinical trials (Cintredekin Besudotox) and a second-generation mhIL-13-PE. Cintredekin Besudotox doubled median survival without eliciting long-term survival and caused severe neurotoxicity; mhIL-13-PE led to ∼40% long-term survival, eliciting severe neurological toxicity at the high dose tested. In contrast, Ad-mediated delivery of mhIL-13-PE led to tumor regression and long-term survival in over 70% of the animals, without causing apparent neurotoxicity. Although Cintredekin Besudotox was originally developed to target GBM, when tested in a phase III trial it failed to achieve clinical endpoints and revealed neurotoxicity. Limitations of Cintredekin Besudotox include its short half-life, which demanded frequent or continued administration, and binding to IL4R/IL13R, present in normal brain cells. These shortcomings were overcome by our therapeutic Ad, thus representing a significant advance in the development of targeted therapeutics for GBM.
Keywords: targeted glioma therapeutics, immunotoxins, adenoviral vectors, GBM12 tumors, TetON system
Intense research efforts have aimed to restrict the cytotoxic effect of anticancer agents by targeting cellular receptors exclusively overexpressed on tumor cells (1–5). This specificity is particularly required when designing therapies for brain tumors, such as glioblastoma multiforme (GBM), in which tumor cells infiltrate the normal brain. GBM is the most common primary brain cancer in adults, and carries a dismal prognosis (14- to 21-mo median survival), in spite of an advanced standard of care, namely surgery, radiotherapy, and chemotherapy with temozolomide (6–8).
Targeting of cytotoxic proteins to receptors exclusively expressed on GBMs would allow selective killing of glioma cells diffusely infiltrating throughout the normal brain. Fifty to 80% of human GBMs express a variant of the IL-13 receptor, IL13Rα2 (9–12), that differs from its physiological counterpart IL4R/IL13R, expressed in normal tissues (13). Because IL13Rα2 is virtually absent from normal brain cells (14), human IL-13 was fused to a truncated Pseudomonas exotoxin (hIL-13-PE; Cintredekin Besudotox) to target IL13Rα2-expressing GBM cells (15). Due to the short half-life of the hIL-13-PE protein formulation (16, 17), multiple injections or continued delivery was necessary to achieve therapeutic levels (1, 16, 18). This approach was tested preclinically and in phase I–III clinical trials (16–18). However, in spite of high expectations, the phase III trial failed to achieve clinical endpoints (http://www.fiercebiotech.com/node/4883). Clinical trials of GBM with hIL-13-PE reported adverse events in all patients, most of which (60%) were neurological (18). Grade III or IV adverse events were observed at the dose-limiting toxicity of 1 μg/mL (18). Neurotoxicity could be related to hIL-13-PE binding to the physiological IL4R/IL13R expressed in the normal brain (19, 20). To overcome the above limitations, we developed a regulatable gene therapy strategy to express highly cytotoxic proteins in the brain tumor microenvironment, aiming to elicit tumor regression and long-term survival.
To restrict the targeted toxin exclusively to IL13Rα2+-glioma cells, a mutated form of IL-13 was engineered [IL-13.E13K, or mhIL-13 (21)], which was previously shown to bind to the GBM-associated IL13Rα2 with higher affinity than the native hIL-13, exhibiting negligible binding to the physiological receptor IL4R/IL13R (21, 22). We hypothesized that a regulatable adenoviral vector (Ad) encoding mhIL-13 fused to Pseudomonas exotoxin would provide long-term local expression leading to an effective cytotoxic response in IL13Rα2-expressing GBM cells, with minimal neurotoxicity toward the normal surrounding brain parenchyma. To further increase safety, the vector encodes mutated human IL-4 [mhIL-4, or IL-4.Y124D (21, 22)] that binds and blocks the physiological receptor IL4R/IL13R without interacting with IL13Rα2, thereby inhibiting any potential binding of mhIL-13-PE to normal brain cells and subsequent neurotoxicity.
Results
Construction, Rescue, and Purification of Adenoviral Vectors Encoding Mutated Human IL-13 Fused to Pseudomonas Exotoxin.
To achieve sustained expression of GBM-specific mhIL-13-PE within the tumor microenvironment, we constructed a regulatable, bidirectional Ad expressing the mhIL-13-PE cytotoxin under the control of the tetracycline-inducible promoter system. This vector also expresses a mutated form of human IL-4, IL4.Y124D (mhIL-4), which blocks the putative binding of mhIL-13-PE to the physiological IL4R/IL13R without affecting its binding to the glioma-associated IL13Rα2 (21, 22). We also constructed a control vector expressing mhIL-4 and mhIL-13 (without the PE) under the control of the tetracycline-responsive (TRE) promoter. The TetON system that regulates the activity of the bidirectional TRE promoter consists of the tetracycline-controlled transactivator rtTA2S-M2, which activates the TRE promoter in the presence of the inducer doxycycline (Dox), and the Tet repressor tTSkid, which inhibits expression in the absence of Dox, inhibiting the leakiness of the system (23, 24). The mCMV promoter drives constitutive expression of TetON. The diagram in Fig. 1A depicts the regulation of transgene expression from the therapeutic Ad. SI Appendix, Table 1 summarizes the description and denomination of all Ads and protein formulations used.
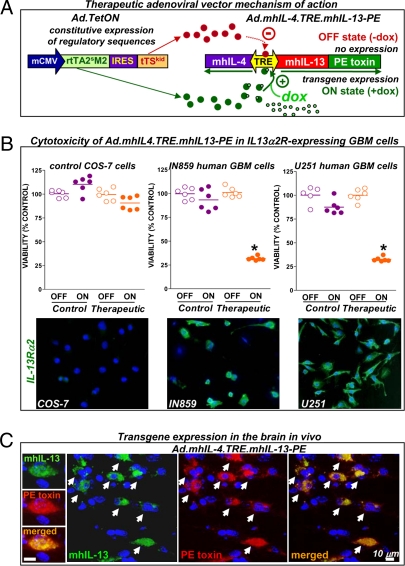
Fig. 1.
Adenovirus-mediated expression of the therapeutic cytotoxin mhIL-13-PE in vitro and in vivo. (A) Diagram showing the mechanism of Ad-mediated regulated therapeutic cytotoxin expression. Ad.mhIL-4.TRE.mhIL-13-PE expresses mhIL-4 and mhIL-13-PE under the control of the bidirectional TRE promoter, which is activated by rtTA2sM2 in the presence of Dox (ON state). In the OFF state, the transactivator is unable to induce transgene expression, which is further repressed by tTSkid. (B) Cell viability in control COS-7 cells and human GBM cells (IN859 and U251) infected with control Ad.mhIL-4.TRE.mhIL-13 or therapeutic Ad.mhIL-4.TRE.mhIL-13-PE. *P < 0.05 versus OFF transgene expression state; Student's t test. Images show expression of IL13Rα2. (C) Colocalization of therapeutic transgenes (arrows) in the mouse brain 7 d after injection of Ad.mhIL-4.TRE.mhIL-13-PE.
Bioactivity and Specificity of Therapeutic Transgenes in Vitro and in Vivo.
Adenoviral-mediated therapeutic transgene expression and GBM-specific cytotoxicity were successfully regulated in vitro by the Tet-dependent system. In the presence of Dox (ON state), Ad.mhIL-4.TRE.mhIL-13-PE led to a reduction in cell viability of >70% in IL13Rα2+ human GBM cells (IN859 and U251), whereas it did not affect the viability of control COS-7 cells, which do not express IL13Rα2 (Fig. 1B), highlighting the specificity of the cytotoxic effect. Expression of mhIL-13 and PE was detected in U251 GBM cells only in the “ON” state (SI Appendix, Fig. 1A). Release of mhIL-4 from control COS-7 cells infected with control or therapeutic Ads was also observed only in the ON state and was completely turned off in the absence of the inducer (SI Appendix, Fig. 1B), indicating that transgene expression mediated by the TRE promoter is tightly regulated.
One of the main drawbacks of the cytotoxin protein formulation is its short half-life, and thus we assessed whether Ad.mhIL-4.TRE.mhIL-13-PE would lead to sustained therapeutic transgene expression in vivo. We tested the expression of Ad.mhIL-4.TRE.mhIL-13-PE 7 d after injection into the naïve mouse brain. Expression of mhIL-13, assessed by immunocytochemistry, was readily detected in the brain injected with either control or therapeutic Ads (SI Appendix, Fig. 2). Fig. 1C shows colocalization of mhIL-13 and PE 7 d after injection with Ad.mhIL-4.TRE.mhIL-13-PE, indicating the robustness of this system to achieve sustained expression of the cytotoxin in the ON state.
Efficacy and Neurotoxicity of Therapeutic Ad Versus Protein Formulations in Intracranial Human GBM Xenografts in Nude Mice.
Targeted toxins are delivered into the brain parenchyma surrounding the tumor after surgical resection of the main tumor mass, and hence it is crucial that these agents induce specific killing of GBM cells infiltrating the nonneoplastic brain without eliciting toxicity to normal brain cells. Thus, the cytotoxicity of Ad.mhIL-4.TRE.mhIL-13-PE and the protein formulation hIL-13-PE (Cintredekin Besudotox) that consists of the native human IL-13 fused to PE or the second-generation mhIL-13-PE that consists of the mhIL-13, that is, IL-13.E13K fused to PE, was assessed in human GBM cells and in primary cultures of human neural progenitor cell (NPC)-derived astrocytes and neurons. Whereas in human GBM cells (Fig. 2A) Ad.mhIL-4.TRE.mhIL-13-PE and both protein formulations exhibited a powerful cytotoxic effect, none of the three treatments induced substantial cytotoxicity in cultures of human NPC-derived astrocytes and neurons (Fig. 2B), indicating that, in vitro, the cytotoxic effect of all these targeted cytotoxins is specific for GBM cells.
Fig. 2.
Cytotoxicity of Ad.mhIL-4.TRE.mhIL-13-PE and the protein formulations hIL-13-PE and mhIL-13-PE in human glioma cells, astrocytes, and neurons. Human GBM U251 cells (A) and human NPC-derived astrocytes and neurons (B) were infected with control Ad.mhIL-4.TRE.mhIL-13, therapeutic Ad.mhIL-4.TRE.mhIL-13-PE, or as a control an Ad expressing the reporter gene LacZ (Ad.TRE.LacZ). Transgene expression was activated using 1 μg/mL Dox. Cells were also incubated in the presence of 1 μg/mL hIL-13-PE or mhIL-13-PE. Graphs show cell viability as determined by flow cytometric analysis of Annexin V and propidium iodide-stained cells. Insets show representative dot plots. *P < 0.05 versus Ad.TRE.LacZ or mock; one-way ANOVA followed by Tukey's test.
We then compared the in vivo therapeutic efficacy of the protein formulation of the cytotoxins and the Ad-mediated gene delivery approach in an intracranial human GBM xenograft model. To this end, we implanted U251 human GBM cells in the striatum of nude mice (SI Appendix, Fig. 3) and treated them 5 d later with a single intratumoral injection of Ad.mhIL-4.TRE.mhIL-13-PE or, as control, Ad.mhIL-4.TRE.mhIL-13, saline, or an empty Ad (Ad.0) (Fig. 3A). Whereas all mice treated with saline, Ad.0, or control Ad.mhIL-4.TRE.mhIL-13 succumbed due to tumor burden (median survival: 28, 43, and 47 d, respectively), ∼70% of the mice treated with the therapeutic Ad.mhIL-4.TRE.mhIL-13-PE survived long-term, over 100 d (Fig. 3B). To test the neurotoxicity of this gene therapeutic approach in the naïve mouse brain of immune-competent animals, we injected Ad.mhIL-4.TRE.mhIL-13-PE or, as controls, Ad.mhIL-4.TRE.mhIL-13, saline, or Ad.0 in the striatum of wild-type BALB/c mice. Seven days later, brain architecture was evaluated by Nissl staining and immunocytochemistry for tyrosine hydroxylase (TH), as an index of striatal tissue integrity, and myelin basic protein (MBP), as an index of oligodendrocyte integrity. We found that Ad.mhIL-4.TRE.mhIL-13-PE did not induce overt toxicity or local or systemic adverse side effects. The structure of the brain was preserved and there was no apparent reduction in TH expression, demyelinization (Fig. 3C), or overt inflammation (SI Appendix, Fig. 4).
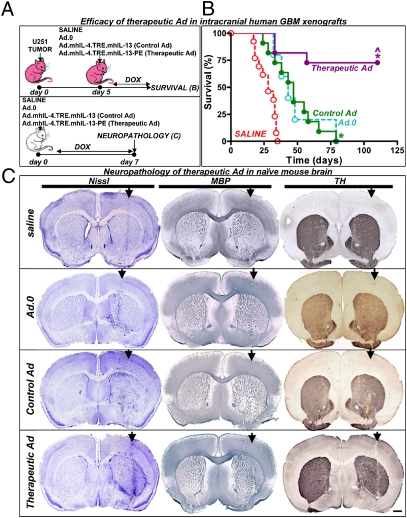
Fig. 3.
Intracranial administration of Ad.mhIL-4.TRE.mhIL-13 leads to tumor regression and long-term survival in the absence of neurotoxicity. (A) Nude mice were implanted with human U251 cells in the striatum and 5 d later they were treated with either a single intratumoral injection of control vector Ad.mhIL-4.TRE.mhIL-13 or therapeutic vector Ad.mhIL-4.TRE.mhIL-13-PE, in combination with Ad.TetON, or as controls they received saline or Ad.0. Animals were fed Dox chow. (B) Kaplan–Meier survival curves of nude mice bearing intracranial U251. *P < 0.05 versus saline, ^P < 0.05 versus control Ad.mhIL-4.TRE.mhIL-13; Mantel log-rank test. (C) Naïve wild-type BALB/c mice were intracranially injected with saline, control vector Ad.mhIL-4.TRE.mhIL-13, or therapeutic vector Ad.mhIL-4.TRE.mhIL-13-PE and Ad.TetON. Animals were fed Dox chow. Seven days postvector delivery, neuropathological analysis was assessed by Nissl staining and immunocytochemistry using antibodies against tyrosine hydroxylase (TH) and myelin basic protein (MBP). (Scale bar, 200 μm.) The arrows indicate the injection site.
When U251 GBM-bearing mice were treated with either 0.2 or 1 μg of native hIL-13-PE protein formulation, their median survival doubled (saline: 22 d; 0.2 μg: 53 d; 1 μg: 55 d; P < 0.05), but all of the animals succumbed to tumor burden by day 56 (Fig. 4A). Mice treated with the high dose of the second-generation mhIL-13-PE performed better, exhibiting a long-term survival rate of ∼40% (Fig. 4C). However, some of the mice treated with the high dose of hIL-13-PE or mhIL-13-PE had to be euthanized shortly after the treatment due to neurological deficits (Fig. 4 A and C). We then evaluated the neuropathology of these proteins in naïve immune-competent BALB/c mice injected in the brain with 0.2 or 1 μg of Cintredekin Besudotox (hIL-13-PE) (Fig. 4B) or mhIL-13-PE (Fig. 4D). Administration of Cintredekin Besudotox at either 0.2 or 1 μg was very toxic to the normal brain, leading to severe neurological side effects, loss of brain tissue (Fig. 4B), and severe neurological deficits that required euthanasia of all of the mice 3 d after injection. Although the same outcome was found when injecting 1 μg of mhIL-13-PE in the naïve brain, mice that received the lower dose of mhIL-13-PE (0.2 μg) did not show signs of overt toxicity in the brain nor displayed neurological signs (Fig. 4D).
Fig. 4.
Efficacy and neurotoxicity of hIL-13-PE and mhIL-13-PE protein formulations. (A and B) Intracranial administration of hIL-13-PE protein formulation does not lead to long-term survival and induces severe neurological toxicity. (A) Nude BALB/c mice were implanted with human U251 cells in the striatum and 5 d later they were treated with a single intratumoral injection of saline or 0.2 or 1 μg of hIL-13-PE protein. *P < 0.05 versus saline; Mantel log-rank test. (B) Naïve wild-type BALB/c mice were intracranially injected with saline or 0.2 or 1 μg of hIL-13-PE protein. Three days postdelivery, mice were euthanized due to severe neurological deficits, and neuropathological analysis was assessed by Nissl staining and immunostaining of TH and MBP. Note the severe local neurotoxicity of both doses of hIL-13-PE when injected into the normal brain parenchyma (arrows). (C and D) Intracranial administration of mhIL-13-PE protein formulation induces antitumor efficacy and leads to dose-dependent neurological toxicity. (C) Nude BALB/c mice bearing intracranial human U251 glioma were treated with a single intratumoral injection of saline or mhIL-13-PE protein. *P < 0.05 vs. saline; Mantel log-rank test. (D) Naïve wild-type BALB/c mice were intracranially injected with saline or mhIL-13-PE protein. Neuropathological analysis was performed 3 or 7 d postdelivery, depending on the toxicity. Note the severe local neurotoxicity of the high dose of mhIL-13-PE protein in the normal brain parenchyma (arrows). (Scale bars, 200 μm.)
Efficacy and Neurotoxicity of Therapeutic Ads in Intracranial Human Primary GBM Xenografts and Syngeneic Murine GBM in Immune-Competent Mice.
Considering the limitations of human GBM xenografts derived from cell lines, namely the loss of certain features present in human GBMs due to prolonged in vitro passage, we tested the efficacy of the therapeutic Ad in an intracranial human primary GBM12 tumor, which is a transplantable human tumor that retains the biological and histological properties of the original human GBM specimen (25, 26), including epidermal growth factor receptor amplification (26), TP53 mutation (26), and expression of IL13Rα2 (25). We implanted GBM12 cells from short-term cultures in the brain of Rag1−/− mice and treated them 5 d later with the therapeutic and control vectors or, as controls, saline or an empty Ad (Ad.0). The therapeutic vector, Ad.mhIL-4.TRE.mhIL-13-PE, led to tumor regression and long-term survival in ∼30% of the mice and increased the median survival from 20 d in control mice to 63 d (Fig. 5A). The neuropathological analysis of the brains from long-term survivors (120 d) showed complete tumor regression and did not reveal signs of neurotoxicity (Fig. 5C).
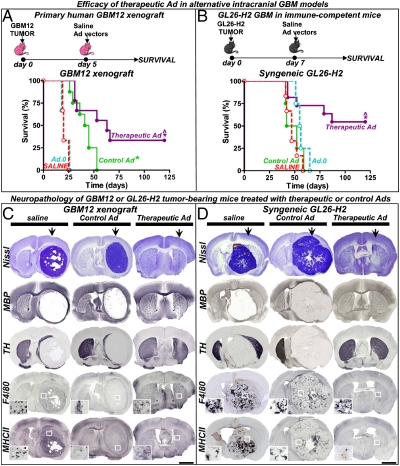
Fig. 5.
Efficacy and neurotoxicity of therapeutic Ads in alternative intracranial GBM models. (A) Rag1−/− mice were implanted with human primary GBM12 cells in the striatum and 5 d later they were treated with a single intratumoral injection of control (Ad.mhIL-4.TRE.mhIL-13) or therapeutic vector (Ad.mhIL-4.TRE.mhIL-13-PE), in combination with Ad.TetON. As controls they received saline or an empty Ad (Ad.0). (B) Immune-competent C57/B6 mice were intracranially implanted with GL26 cells expressing IL13Rα2 (GL26-H2) and treated 7 d later with the Ads. Animals were fed Dox chow. *P < 0.05 versus saline, ^P < 0.05 versus control Ad.mhIL-4.TRE.mhIL-13; Mantel log-rank test. (C and D) Neuropathology was assessed in moribund mice and long-term survivors (120 d) by Nissl staining and immunostaining of TH, MBP, F4/80 (macrophages/activated microglia), and major histocompatibility complex II (MHCII). The arrows indicate the injection site. Insets show higher-magnification microphotographs of the areas indicated with a white square. (Scale bars, 1,000 μm.) Note complete tumor regression in long-term survivors and the absence of neuropathological or inflammatory side effects.
In addition, because human GBM xenograft models do not replicate the clinical scenario in which the vectors are delivered in the setting of an immune-competent system, we tested the efficacy of our therapeutic vector in immune-competent mice bearing intracranial syngeneic gliomas that express IL13Rα2. GL26 GBM cells stably transfected to express IL13Rα2 (27) were implanted in the brain of syngeneic immune-competent C57/B6 mice. Seven days later, mice were treated by intratumoral injection of the therapeutic and control Ads or, as controls, saline or an empty Ad (Ad.0). We found that Ad.mhIL-4.TRE.mhIL-13-PE exerted a robust antitumoral effect, leading to over 50% survival for over 4 mo (Fig. 5B). The neuropathological analysis of long-term survivors did not reveal signs of neurotoxicity or overt inflammation and confirmed complete regression of the tumor (Fig. 5D; SI Appendix, Fig. 5). These results show that the efficacy of our therapeutic vector would not be curtailed in the context of a competent immune system.
Discussion
Although the development of chimeric cytotoxins targeting the IL-13 receptor in human GBM created high expectations in the past few years, their performance in clinical trials has led to modest results, with the main drawbacks being the low specificity of the toxin used, Cintredekin Besudotox, that led to dose-limiting toxicity, and the short half-life of the protein formulation, which required frequent and prolonged administrations. To overcome these limitations, we developed a therapeutic Ad that expresses mhIL-13-PE under the control of a regulatable promoter and assessed its efficacy and safety profile comparing it with Cintredekin Besudotox and with the second-generation cytotoxin protein formulation mhIL-13-PE. Our results show that a single intratumoral injection of Ad.mhIL-4.TRE.mhIL-13-PE leads to a powerful antitumor effect in vivo, yielding a survival rate of over 70%. On the other hand, a single intratumoral administration of the protein formulations led to lower antitumor efficacy; that is, Cintredekin Besudotox doubled the median survival and all of the animals succumbed to tumor burden, and the second-generation mhIL-13-PE protein formulation elicited ∼40% long-term survival. These results suggest that the short half-life of the protein formulations (16, 17) can be overcome by Ads, which allow sustained local expression.
Clinical trials in GBM patients showed that intracranial administration of hIL-13-PE led to dose-limiting toxicity, including neurological symptoms secondary to necrotic and inflammatory processes as well as irreversible hemiparesis and the death of one patient due to neurologic decline potentially related to hIL-13-PE; these adverse side effects could be due to binding of the cytotoxin to normal brain cells which can express the physiological IL4R/IL13R (18). Further, grade III and IV imaging changes suggesting tissue damage were observed in tumor-infiltrated and normal brain parenchyma (28) and regarded as nonspecific internalization of hIL-13-PE (18). In view of these previous reports, we assessed the neurotoxicity of our therapeutic vector and performed a comparison with two cytotoxin protein formulations. Ad.mhIL-4.TRE.mhIL-13-PE did not induce overt cytotoxicity in normal human brain cells in vitro nor in the normal mouse brain in vivo, suggesting that the expression of the therapeutic transgenes encoded in this vector platform would not be toxic to normal brain tissue. On the other hand, although both protein formulations were not toxic in vitro in human astrocytes and neurons, their administration into the naïve mouse brain led to very severe neurological side effects. Although Cintredekin Besudotox exhibited neurological toxicity at low and high doses, the neurotoxicity of the second-generation mhIL-13-PE protein formulation was dose-dependent, with no neuropathological abnormalities at the low, 0.2-μg dose, supporting the notion that this toxin is more specific toward GBM and safer than Cintredekin Besudotox. These findings also highlight the crucial importance of testing neurotoxicity in vivo rather than limiting its assessment to in vitro cell-culture systems. This could be related to the fact that primary cultures of human brain cells were obtained from fetal brains, in which neurons and glial cells are still developing and may not represent closely the normal adult brain.
Our results show that a bidirectional Tet-dependent promoter can be used to successfully drive the expression of two therapeutic transgenes in a tumor model in vivo. Considering the limitations of using U251 human glioma xenografts, namely the loss of certain biological features due to continuous in vitro passage and the fact that it does not replicate the clinical scenario in which the vectors are delivered in the setting of an immune-competent system, we demonstrated the efficacy of our therapeutic vector in two additional orthotopic GBM models: human primary GBM12 xenografts and syngeneic GL26-H2 GBM in immune-competent mice. These results show that our therapeutic vector exerts a robust antitumoral effect, which would not be curtailed in the context of an immune-competent system.
In summary, the advantages of our therapy approach for GBM are as follows. First, a single intratumoral injection of this vector provides a powerful antitumor effect with negligible neurotoxicity, which constituted a serious limitation of the protein formulation hIL-13-PE, Cintredekin Besudotox, used in clinical trials. Another advantage of our vector is that the expression of the therapeutic transgenes is controlled by an inducible promoter that allows expression to be easily regulated by switching “off” transcription, through withdrawal of the inducer doxycycline. Also, expression of mhIL-4 inhibits binding of mhIL-13-PE to normal cells, which constitutes an additional safety feature of our approach. Our results highlight the suitability of Ads to deliver cytotoxins into the tumor microenvironment without the need of frequent readministration to elicit therapeutic efficacy. The major safety features of Ad.mhIL-4.TRE.mhIL-13-PE over hIL-13-PE are displayed in the diagram shown in Fig. 6. In conclusion, we demonstrate the clinically relevant in vivo and in vitro safety profile and efficacy of a regulatable, bicistronic adenoviral vector expressing mhIL-13 fused to PE (to specifically target glioma cells), together with mhIL-4, to further inhibit any potential binding of the targeted toxin to normal brain cells. These results indicate that the development of Ad.mhIL-4.TRE.mhIL-13-PE constitutes a significant advance in the implementation of targeted toxins for glioma therapeutics.
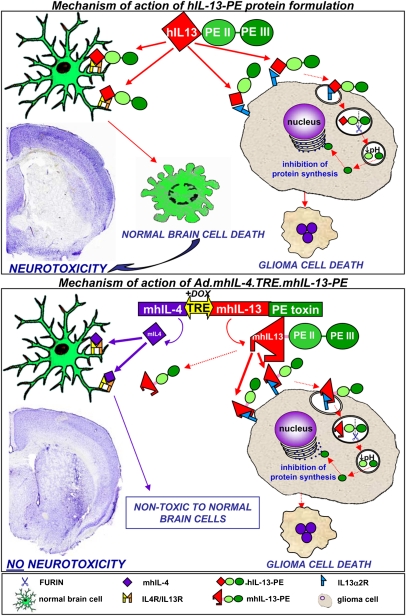
Fig. 6.
Gene therapy-mediated delivery of mhIL-13-PE leads to antitumor efficacy and long-term survival in the absence of neurological toxicity. The diagram depicts the mechanism of action of the therapeutic strategy. The targeting of IL-13α2 receptor overexpressed in glioma cells has been approached by constructing an Ad encoding the truncated form of Pseudomonas exotoxin fused to a mutated form of human IL-13 (mhIL-13, IL-13.E13K), which has higher affinity for the glioma-associated IL13Rα2 receptor and negligible binding to the physiological IL13/IL4R. Binding of mhIL-13-PE to IL13Rα2 promotes its internalization into glioma cells. Domain II (PE II) mediates the translocation of the toxin into the endosomes by endocytosis. Once in the endosomes, furin mediates proteolytic cleavage that activates the catalytic domain III (PE III). Due to the low pH of the endosome, the processed fragment of the toxin is translocated to the cytosol and inhibits protein synthesis, leading to glioma cell death. The absence of IL13Rα2 protects normal cells from mhIL-13-PE cell death. The safety of our approach has been further enhanced by coexpressing a mutated form of IL-4 (mhIL-4, IL-4.Y124D) encoded in the therapeutic Ad which acts as an antagonist of the physiological receptor comprising the IL-13α1R and IL-4αR chains. Secreted IL-4 would inhibit the already negligible binding of mhIL-13-PE to normal cells, without affecting the binding of the mhIL-13-PE to GBM cells.
Materials and Methods
SI Appendix, Table 1 summarizes the description and denomination of all adenoviral vectors and protein formulations used in this work.
Plasmid Construction and Engineering.
The construction of all plasmids used to develop the adenoviral vectors used in this study is described in SI Appendix, SI Materials and Methods and depicted in SI Appendix, Fig. 6.
Development of Adenoviral Vectors.
Ad.TetON, Ad.mhIL-4-biTRE-mhIL-13, Ad.mhIL-4-biTRE-mhIL-13-PE, and Ad.TRE.LacZ are first-generation, replication-defective serotype 5 adenoviral vectors with deletions in their E1 and E3 regions. Ads were generated by cotransfection of pΔE1-[mCMV-rtTA2SM2-IRES-tTSKid-pA] (SI Appendix, SI Materials and Methods), pΔE1-[mhIL-4-biTRE-mhIL-13], and pΔE1-[mhIL-4-biTRE-mhIL-13-linker-Pseudomonas exotoxin] with pJM17 (Microbix Biosystems) in 293 or DTR-293 cells (29) (SI Appendix, SI Materials and Methods).
In Vitro Expression and Cytotoxicity of Therapeutic Ads.
Cell lines.
Viability of GBM cells was evaluated by flow cytometry analysis after staining with Annexin V-propidium iodide 72 h after Ad infection or incubation with 1 μg/mL of hIL-13-PE or mhIL-13-PE. For a brief description, see SI Appendix, SI Materials and Methods.
Human neural and glial cell cultures.
Human fetal brain tissues (between 10 and 15 wk postconception) were obtained from the Birth Defects Laboratory at the University of Washington. The method of collection conformed to the guidelines recommended by the National Institutes of Health for the collection of such tissues and set out by all involved institutions. Institutional review board (IRB) approval was obtained for these studies (IRB no. 21505). Human neural progenitor cell cultures were prepared from freshly dissected fetal brain cortical tissue as previously described (30–32). For a brief description, see SI Appendix, SI Materials and Methods.
To evaluate toxicity toward normal brain cells in vitro, human NPC-derived astrocyte and neuronal cultures were infected with control and therapeutic Ads or incubated with cytotoxin protein formulations as described above.
Determination of cell viability.
Cell viability was determined by flow cytometry of Annexin V+PI-stained cells as described in SI Appendix, SI Materials and Methods (33, 34).
Transgene expression in vitro.
Transgene expression was determined in vitro by immunofluorescence and ELISA as described in SI Appendix, SI Materials and Methods.
In Vivo Efficacy and Neuropathology of Therapeutic Ads.
Animals.
Human GBM xenografts.
Intracranial U251 xenografts in athymic nude BALB/c mice and human primary GBM12 (kindly donated by Evanthia Galanis, Department of Oncology, Mayo Clinic and Foundation, Rochester, MN) xenografts in Rag1−/− mice were established as previously described (25, 26, 35) (SI Appendix, SI Materials and Methods).
Syngeneic GBM tumor model in immune-competent mice.
GL26 cells that were engineered to express IL13Rα2 [GL26-H2 cells (27)] were implanted in the brain of immune-competent C57/B6 mice as described in SI Appendix, SI Materials and Methods. Seven days later, mice were treated with the Ads as described above.
Neuropathology.
To evaluate the neuropathology, naïve female wild-type BALB/c mice (6–12 wk) were injected in the right striatum with the Ads and protein formulations as described in SI Appendix, SI Materials and Methods. Mice were euthanized as described in SI Appendix, SI Materials and Methods 7 d after treatment, or when overt signs of neurotoxicity developed. Transgene expression and neuropathological analysis were performed in coronal brain sections as described in SI Appendix, SI Materials and Methods (33).
Statistical Analysis.
P values of less than 0.05 were used to determine the null hypothesis to be invalid. The statistical tests used are indicated in the figure legends and in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Ira Pastan (Laboratory of Molecular Biology, National Cancer Institute, Bethesda, MD) for kindly donating the plasmid encoding PE38, Dr. Kenji Kohno (Laboratory of Molecular and Cell Genetics, Graduate School of Biological Sciences, Nara Institute of Science and Technology, Takayama, Ikoma, Nara, Japan) for the generous donation of the plasmid encoding the diphtheria toxin-resistant EF2, and Dr. Evanthia Galanis (Department of Oncology, Mayo Clinic and Foundation, Rochester, MN) for the kind donation of human primary GBM12 tumors. We thank Drs. S. Melmed, L. Fine, and Mark Greene for their support and academic leadership. We thank the expert assistance of Patricia Lin from the Flow Cytometry Core Facility and Dr. John Young and the staff from the Department of Comparative Medicine at Cedars-Sinai Medical Center. Our work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) Grants 1R21-NS054143, 1U01 NS052465, and 1R01 NS057711 (to M.G.C.) and Grants 1R01 NS 054193 and R01 NS061107 (to P.R.L.), The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics (to P.R.L. and M.G.C., respectively), The Linda Tallen and David Paul Kane Foundation Annual Fellowship, The Drown Foundation, and the Board of Governors at Cedars-Sinai Medical Center. M.C. is supported by NIH/NINDS Grant 1F32 NS058156.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008261107/-/DCSupplemental.
References
- 1.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 2.Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor α chain: A novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 4.Sampson JH, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer RW, Tyler MA, Ulasov IV, Lesniak MS. Targeted therapies for malignant glioma: Progress and potential. BioDrugs. 2009;23:25–35. doi: 10.2165/00063030-200923010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanu OO, et al. Glioblastoma multiforme: A review of therapeutic targets. Expert Opin Ther Targets. 2009;13:701–718. doi: 10.1517/14728220902942348. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Grandi P, et al. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9:505–517. doi: 10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada H, et al. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88:245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor α 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 11.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor α2 in glioblastoma multiforme: Implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 12.Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48:103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 13.Hershey GK. IL-13 receptors and signaling pathways: An evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 14.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 15.Debinski W, Pastan I. An immunotoxin with increased activity and homogeneity produced by reducing the number of lysine residues in recombinant Pseudomonas exotoxin. Bioconjug Chem. 1994;5:40–46. doi: 10.1021/bc00025a006. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 17.Vogelbaum MA, et al. Convection-enhanced delivery of Cintredekin Besudotox (interleukin-13-Pe38qqr) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: Phase 1 study of final safety results. Neurosurgery. 2007;61:1031, 1037–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion. [DOI] [PubMed] [Google Scholar]
- 18.Kunwar S, et al. Cintredekin Besudotox Intraparenchymal Study Group Direct intracerebral delivery of Cintredekin Besudotox (IL13-PE38QQR) in recurrent malignant glioma: A report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, et al. Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: Non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunol Immunother. 2000;49:319–324. doi: 10.1007/s002620000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, et al. In vivo expression of the interleukin 4 receptor α by astrocytes in epilepsy cerebral cortex. Cytokine. 2000;12:1656–1661. doi: 10.1006/cyto.2000.0773. [DOI] [PubMed] [Google Scholar]
- 21.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 22.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Rα2. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong W, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong W, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen C, et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannini C, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor α2-expressing glioma. J Neurooncol. 2003;64:117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 28.Parney IF, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, et al. Prostate-specific expression of the diphtheria toxin A chain (DT-A): Studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer. Cancer Res. 2002;62:2576–2582. [PubMed] [Google Scholar]
- 30.Svendsen CN, et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 31.Svendsen CN, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 32.Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Candolfi M, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: Efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtin JF, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candolfi M, et al. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.