Abstract
We examined whether reduced levels of Apolipoprotein A-I (apoA-I) in ovarian cancer patients are causal in ovarian cancer in a mouse model. Mice expressing a human apoA-I transgene had (i) increased survival (P < 0.0001) and (ii) decreased tumor development (P < 0.01), when compared with littermates, following injection of mouse ovarian epithelial papillary serous adenocarcinoma cells (ID-8 cells). ApoA-I mimetic peptides reduced viability and proliferation of ID8 cells and cis-platinum–resistant human ovarian cancer cells, and decreased ID-8 cell-mediated tumor burden in C57BL/6J mice when administered subcutaneously or orally. Serum levels of lysophosphatidic acid, a well-characterized modulator of tumor cell proliferation, were significantly reduced (>50% compared with control mice, P < 0.05) in mice that received apoA-I mimetic peptides (administered either subcutaneously or orally), suggesting that binding and removal of lysophosphatidic acid is a potential mechanism for the inhibition of tumor development by apoA-I mimetic peptides, which may serve as a previously unexplored class of anticancer agents.
Ovarian cancer has the highest mortality rate among all gynecologic malignancies (1). At the time of diagnosis, over 85% of patients with ovarian cancer present with advanced stage III or IV disease characterized by intraperitoneal, lymphatic, or distant spread of disease; the poor prognosis associated with ovarian cancer is attributed to a lack of symptoms at early stages of the disease, as well as a lack of biomarkers for the detection of early-stage disease. Moreover, despite appropriate surgery and receiving highly effective first-line chemotherapy, ≈20 to 30% of patients with advanced-stage disease continue to have evidence of residual disease during treatment and never have a complete clinical response. Thus, there is an immediate need for both biomarkers and therapeutic targets for treating ovarian cancer (2).
Kozak et al. used surface-enhanced laser desorption and ionization TOF-MS and identified 14 protein biomarkers, which comprised three separate protein panels that reliably identified early-stage malignant ovarian neoplasia with high sensitivity and specificity (3). Kozak et al. demonstrated that 3 of the 14 differentially expressed proteins are lower in the serum of patients with early-stage ovarian neoplasia compared with normal individuals (4). The three ovarian cancer biomarkers, apolipoprotein A-I (apoA-I), transthyretin, and transferin (4, 5), when used as a panel, were better predictors of early-stage ovarian cancer when compared with serum CA125 levels (2, 4–6). More recently (09/12/2009), the U.S. Food and Drug Administration cleared the first laboratory test that can indicate the likelihood of ovarian cancer, OVA1 Test (www.medicalnewstoday.com/articles/163761.php), which utilizes apoA-I, transthyretin, transferin, CA 125, and β2-microglobulin.
ApoA-I is the major protein in HDL and plays an important role in reverse cholesterol transport by extracting cholesterol and phospholipids from peripheral cells and transferring it to the liver for excretion. In addition to its antiatherogenic properties, apoA-I also possesses anti-inflammatory and antioxidant properties (7), and apoA-I mimetic peptides, engineered to mimic anti-inflammatory and antioxidant functionalities of apoA-I, are effective for the treatment of atherosclerosis and a number of inflammatory disorders in mice, rats, and rabbits (8–11). More recently, apoA-I mimetic peptides were effective in vitro in stimulating HDL anti-inflammatory activity and inhibiting LDL proinflammatory activity in the plasma of patients with end-stage renal disease (12). Because lipid transport, inflammation, and oxidative stress are associated with the development and progression of cancer, we hypothesized that the reduced levels of apoA-I in ovarian cancer patients may have been causal in disease progression. We also hypothesized an antitumorigenic role for apoA-I in ovarian cancer.
A common mechanism of action for both apoA-I and apoA-I mimetic peptides is their ability to bind proinflammatory phospholipids (13). Interestingly, lysophospholipids are well-known activators of proliferation in cancer cells (14, 15) and have been suggested as viable biomarkers in ovarian and possibly other cancers. Lysophosphatidic acid (LPA), a bioactive lysophospholipid, is implicated in breast cancer progression (16, 17). Several reports identified altered fatty acid composition of lysophosphatidyl choline and elevated LPA levels in the ascites and malignant effusions of ovarian cancer patients (18, 19). LPA induces migration and invasion in both mouse and human epithelial ovarian cancer cells (20). Recent studies demonstrate that the mouse epithelial cancer cell line named ID8 is an ideal model system to test the effects of LPA and therapeutic agents of ovarian cancer in immunocompetent mouse models (21, 22).
In the present study, we demonstrate that overexpression of human apoA-I in transgenic mice inhibits tumor growth and improves survival in a mouse model of ovarian cancer. Moreover, we demonstrate that treatment with the apoA-I mimetic peptides, L-4F, D-4F (the peptide Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2 synthesized from all L- or all D-amino acids, respectively), or L-5F (Ac-D-W-L-K-A-F-Y-D-K-V-F-E-K-F-K-E-F-F-NH2, synthesized from all L-amino acids) decreases tumor burden in mice injected with ID8 cells. In contrast, a scrambled peptide (sc-4F) containing the same amino acids as in the 4F peptides but arranged in a sequence (Ac-D-W-F-A-K-D-Y-F-K-K-A-F-V-E-E-F-A-K-NH2) that prevents the formation of a class A amphipathic helix, and thus dramatically decreases lipid binding, did not reduce tumor burden in mice injected with ID8 cells. We further demonstrate that apoA-I mimetic peptides bind LPA with remarkable affinity and reduce serum LPA levels in mice. Our results are unique in demonstrating that apoA-I plays an important role in the progression of ovarian cancer, and apoA-I and apoA-I mimetic peptides may serve as a unique class of therapeutic agents for the treatment of ovarian cancer.
Results
Overexpression of Human apoA-I Improves Overall Survival in a Mouse Model of Ovarian Cancer.
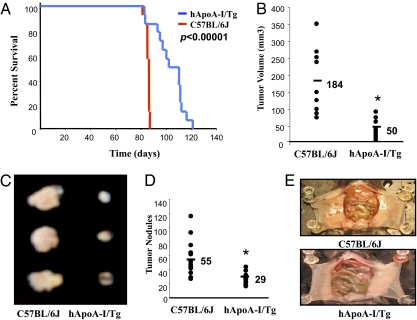
We first examined the effect of apoA-I overexpression on overall survival in a mouse (C57BL/6J) model of ovarian cancer (21–23). The survival studies were performed in wild-type C57BL/6J mice and hApoA-I/Tg mice on a C57BL/6J background. The Kaplan Meier survival curves shown in Fig. 1A demonstrate that following an intraperitoneal injection of ID8 cells, hApoA-I/Tg mice survived longer compared with C57BL/6J mice (hazard ratio 3.2, P < 0.0001). None of the C57BL/6J mice survived beyond 87 d. In contrast, all but one of the hApoA-I/Tg mice survived past 87 d. The median time to death was 86 d in the C57BL/6J mice and 106 d in the hApoA-I/Tg mice (Fig. 1A).
Fig. 1.
Improved survival and significantly decreased tumor size in hApoA-I/Tg mice after injection of ID8 cells. (A) Wild-type C57BL/6J and hApoA-I/Tg mice on C57BL/6J background (n = 14 per group, 9 wk of age) were given an intraperitoneal injection of ID8 cells (8 × 106 cells per mouse) on day 1. The curves represent the percent of live mice on the days indicated. (B) Flank tumors were established in wild-type C57BL/6J (n = 9) and hApoA-I/Tg female mice on C57BL/6J background (n = 10), as described in Materials and Methods. Mice were killed 5 wk after flank injection and tumor volumes were calculated by the formula V = 1/2(LXW2), where L is length (longest dimension) and W is width (shortest dimension). (C) Representative tumors from the two groups are shown. (D) Wild-type C57BL/6J and hApoA-I/Tg mice on a C57BL/6J background (n = 13, 8 wk of age) were injected with ID8 cells by intraperitoneal injection (8 × 106 cells per mouse) and tumor burden was analyzed after 9 wk. Average tumor nodules on liver, spleen, kidney, diaphragm, and intestines, were counted for each mouse and data from each group was combined and analyzed. (E) Representative mice from the two groups showing the tumor nodules on the peritoneal membranes. *P < 0.01.
Tumor Burden Following ID8 Cell Injection Is Significantly Decreased in hApoA-I/Tg Mice Compared with Wild-Type C57BL/6J mice.
To determine whether improved survival in hApoA-I/Tg mice was a result of reduced tumor burden, hApoA-I/Tg and C57BL/6J mice were injected with ID8 cells by subcutaneous injection (5 × 106 cells per mouse; n = 9 for C57BL/6J and n = 10 for hApoA-I/Tg) or intraperitoneal injection (8 × 106 cells per mouse; n = 13 per group), and tumor burden was analyzed after 5 and 9 wk, respectively. The size of flank tumors 5 wk after subcutaneous injection of ID8 cells was significantly larger in C57BL/6J mice compared with hApoA-I/Tg mice (184 mm3 vs. 50 mm3, P < 0.01) (Fig.1 B and C). Moreover, following intraperitoneal injection of ID8 cells, tumor load was markedly greater in C57BL/6J mice when compared with hApoA-I/Tg mice (average number of tumor nodules on liver, kidney, spleen, diaphragm, and intestines, collectively, were 55 in C57BL/6J mice and only 29 in the hApoA-I/Tg mice, P < 0.01) (Fig.1 D and E).
Tumor Development Following ID8 Cell Injection Is Significantly Decreased in Mice Injected with apoA-I Mimetic Peptides, L-5F and L-4F.
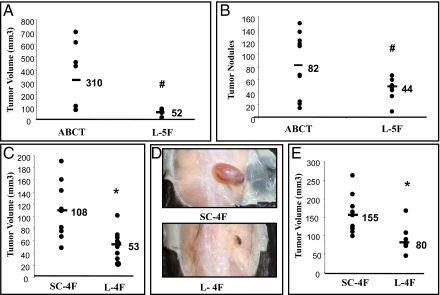
To determine whether apoA-I mimetic peptides could reduce tumor development similar to human apoA-I, wild-type C57BL/6J mice were first injected with ID8 cells subuctaneously in the flank or intraperitoneally. The mice received L-5F (10 mg/kg) or vehicle ABCT buffer (50 mM ammonium bicarbonate, pH 7.0, containing 0.1 mg/mL Tween-20) by subuctaneous injection at a site distant from the site where the ID8 cells were injected daily for 5 wk (for flank tumors) and 9 wk (for intraperitoneal tumors). The size of the flank tumors was significantly larger in C57BL/6J mice treated with ABCT buffer compared with mice treated with L-5F (310 mm3 vs. 52 mm3, P < 0.05) (Fig. 2A). Following intraperitoneal injection, the number of tumor nodules was greater in C57BL/6J mice treated with ABCT buffer compared with mice treated with L-5F (average number of tumor nodules on liver, kidney, spleen, diaphragm, and intestines, collectively, 82 vs. 44, P < 0.05) (Fig. 2B). L-4F treated mice also developed significantly smaller flank tumors when compared with mice injected with a control peptide, scrambled-4F (sc-4F) (108 mm3 vs. 53 mm3, P < 0.01) (Fig. 2 C and D). Moreover, when L-4F and sc-4F were given 2 wk after the injection of ID8 cells, the mice receiving L-4F developed significantly smaller flank tumors when compared with sc-4F treated mice (155 mm3 vs. 80 mm3, P < 0.01) (Fig. 2E).
Fig. 2.
ID8 cell-mediated tumor sizes are significantly decreased in C57BL/6J mice injected with apoA-I mimetic peptides. Flank tumors and intraperitoneal tumors were established in C57BL/6J female mice (n = 10 per group) as described in Materials and Methods. (A) Mice were killed 5 wk after ID8 cell subcutaneous injection and tumor volumes were calculated. The data shown are for mice receiving vehicle alone (ABCT) or vehicle containing L-5F starting on the day of injection of the ID8 cells. (B) Mice were killed 9 wk after intraperitoneal injection of ID8 cells and the average number of tumor nodules on liver, spleen, kidney, diaphragm, and intestines were counted for each mouse and data from each group were combined and analyzed. The data shown are for mice receiving vehicle alone (ABCT) or vehicle containing L-5F starting on the day of injection of the ID8 cells. (C) Mice were killed 5 wk after flank injection of ID8 cells and tumor volumes were calculated. The data shown are for mice receiving scrambled 4F (sc-4F) or L-4F starting on the day of injection of the ID8 cells. Both sc-4F and L-4F were administered subcutaneously at a site distant from the site where the ID8 cells were injected. (D) Representative tumors are shown from mice in C. (E) Experimental details are identical to C except that the injections of L-4F and sc-4F were started 2 wk after the injection of ID8 cells in the flank. #P < 0.05; *P < 0.01.
Tumor Development Following ID8 Injection Is Significantly Decreased in Mice Given the apoA-I Mimetic Peptide, D-4F, in Drinking Water.
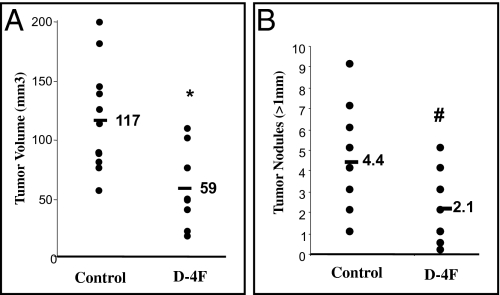
D-4F has the same sequence as L-4F but it is synthesized from all D-amino acids and can be administered orally (24, 25). We next examined whether D-4F given in drinking water is effective in reducing tumor development in wild-type C57BL/6J mice. Mice receiving D-4F in drinking water at a concentration of 300 μg/mL (129.8 μM) starting on the day of flank injection until being killed (5 wk), showed significant reduction in flank tumor size (117 mm3 vs. 59 mm3, P < 0.01) compared with C57BL/6J mice receiving regular drinking water (Fig. 3A). Furthermore, mice receiving D-4F in drinking water (300 μg/mL; 129.8 μM) starting on the day of intraperitoneal injection until being killed (9 wk), showed a significant reduction in the number of tumor nodules (average number of tumor nodules >1 mm, 4.4 vs. 2.1, P < 0.05) compared with C57BL/6J mice receiving drinking water without peptide (Fig. 3B).
Fig. 3.
ID8 cell-mediated tumor size is significantly decreased in C57BL/6J mice treated with apoA-I mimetic peptide, D-4F, in drinking water. Flank tumors and intraperitoneal tumors were established in C57BL/6J (n = 11 per group) as described in Materials and Methods. For each experiment, two groups of mice were used. One group received regular drinking water ad libitum (Control) and the other group received D-4F (D-4F) in drinking water at a concentration of 300 μg/mL (129.8 μM) starting on the day of flank or intraperitoneal injections. (A) Mice were killed 5 wk after flank injection and tumor volumes were calculated. (B) Tumor burden was analyzed at 9 wk following intraperitoneal injections. Average tumor nodules >1 mm on liver, spleen, kidney, diaphragm, and intestines, were counted for each mouse and data from each group were combined and analyzed. #P < 0.05; *P < 0.01.
Effect of apoA-I and apoA-I Mimetic Peptides on ID8 Cell Viability and Proliferation in Vitro.
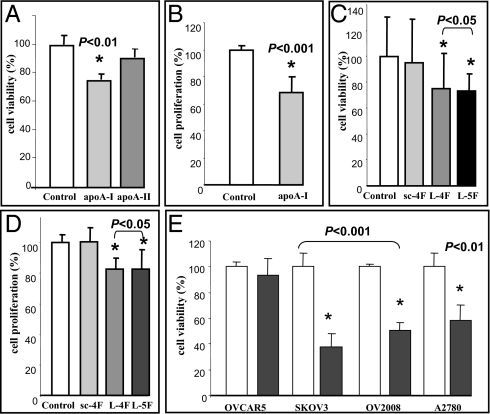
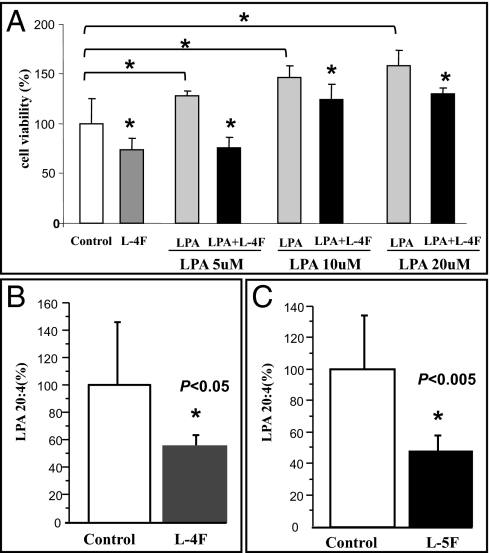
To examine the mechanisms by which apoA-I and apoA-I mimetic peptides inhibit ID8 cell-mediated tumor development in mice, the effect of apoA-I and apoA-I mimetic peptides on ID8 cell viability was determined in vitro. Cell viability was more than 20% lower (P < 0.01) in ID8 cells 48 h following treatment with human apoA-I (100 μg/mL; 3.57 μM) when compared with no treatment and compared with cells treated with human apoA-II (100 μg/mL; 5.75 μM), which is another protein in HDL (Fig. 4A). Moreover, apoA-I significantly inhibited proliferation of ID8 cells (P < 0.001) as measured by BrdU incorporation (Fig. 4B). Similarly, apoA-I mimetic peptides, L-5F and L-4F, but not sc-4F (all at 10 μg/mL; 4.11 μM for L-5F and 4.12 μM for L-4F and sc-4F) reduced ID8 cell viability (Fig. 4C) and proliferation (Fig. 4D). Furthermore, under identical experimental conditions, neither apoA-I nor apoA-I mimetic peptides affected the viability and proliferation of mouse primary ovarian epithelial cells.
Fig. 4.
ApoA-I and apoA-I mimetic peptides reduce viability and inhibit ID8 cell proliferation, and also reduce cell viability of cis-platinum–resistant human ovarian cancer cell lines in vitro. ID8 cells were cultured as described in Materials and Methods, and incubated with either apoA-I or apoA-II (100 μg/mL; 3.57 or 5.75 μM, respectively) or apoA-I mimetic peptides (L-5F, L-4F, or sc-4F at a concentration of 10 μg/mL; 4.11 μM for L-5F, and 4.12 μM for L-4F and sc-4F) for 48 h. (A, C, and E) Cells were assayed for viability using the MTS assay kit. (B and D) BrdU incorporation was analyzed as described in Materials and Methods. Data are represented as the mean ± SD of the percent of control cells. All experiments were performed in triplicate and each assay was carried out in quadruplicates. (E) OVCAR5, SKOV3, OV2008, and A2780 cell lines were cultured as described in Materials and Methods and were either untreated (open bars) or treated with 10 μg/mL (4.12 μM) of L-4F (closed bars).
ApoA-I Mimetic Peptides Inhibit Viability of Human Ovarian Cancer Cell Lines.
To examine whether apoA-I peptides are effective in human ovarian cancer cell lines, we analyzed cell viability following apoA-I mimetic peptide addition in four cis-platinum–resistant cell lines. L-4F at 10 μg/mL (4.12 μM) significantly reduced cell viability in three out of four human ovarian cancer cell lines that are known to be cis-platinum–resistant (Fig. 4E).
ApoA-I Mimetic Peptides Inhibit LPA-Induced Proliferation of ID8 Cells and Reduce Serum LPA Levels in Mice Injected with ID8 Cells.
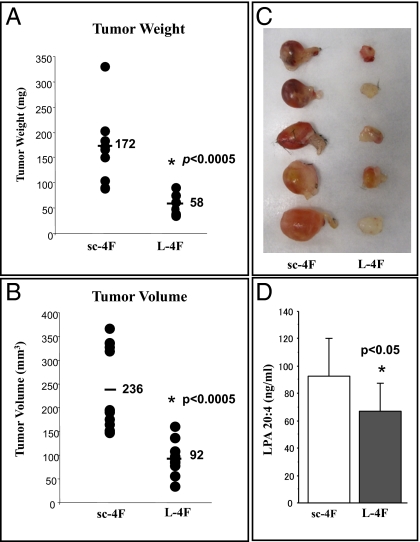
LPA has been identified as an important mediator of tumor development, progression, and metastases in humans (18, 19). Li et al. recently demonstrated that LPA stimulates cell migration, invasion, and colony formation, as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice (20). We have previously demonstrated that apoA-I and apoA-I mimetic peptides bind lipids with high affinity, and apoA-I mimetic peptides are several to four to six orders of magnitude better than apoA-I in binding oxidized lipids (26).We first examined whether apoA-I and L-4F can bind LPA using surface plasmon resonance, as described previously (26). Binding affinity is defined by the equation KD = kd/ka, where kd represents dissociation rate constant and ka represents association rate constant. Thus, the larger KD is the weaker binding, and the smaller KD is the stronger binding. Both apoA-I and L-4F bind LPA, although similar to the binding of other oxidized lipids, L-4F binds LPA better than apoA-I by six orders of magnitude (Table 1). Moreover, as expected, LPA (5–20 μM) significantly improved ID8 cell growth (Fig. 5A) and L-4F significantly reduced LPA-induced viability at all doses tested (Fig. 5A). Furthermore, in the mouse experiments shown from Fig. 2, serum LPA levels were significantly reduced in mice receiving L-4F (Fig. 5B) and L-5F (Fig. 5C) compared with their corresponding control mice. The data in Fig. 6 demonstrate that incorporation of L-4F into mouse chow at 100 mg/kg per day significantly reduced tumor burden and LPA plasma levels compared with providing the same dose of sc-4F in the chow.
Table 1.
Comparison of the binding affinity (KD) of LPA for L-4F and apoA-I
| Protein or peptide | KD |
| ApoA-I | 1,330 ± 131 nM |
| L-4F | 0.000523 ± 0.0015 nM |
The data shown are the Mean ± SD.
Fig. 5.
ApoA-I mimetic peptides inhibit LPA induced proliferation of ID8 cells and reduce serum LPA levels in mice injected with ID8 cells. (A) ID8 cells were cultured as described in Materials and Methods, and incubated with either apoA-I mimetic peptides L-4F (10 μg/mL; 4.12 μM) or LPA at a concentration 5, 10, or 20 μM, or cells treated with both L-4F and LPA for 48 h. Data are represented as the mean ± SD of the percent of control cells. All experiments were performed in triplicate and each assay was carried out in quadruplicates. (B and C) Serum LPA levels from the mice described in Fig. 2 B and C were determined as described in Materials and Methods. The values shown are the Mean ± SD. *P < 0.05.
Fig. 6.
ID8 cell-mediated tumor size and LPA plasma levels are significantly decreased in C57BL/6J mice treated with L-4F added to mouse chow. Flank tumors were established in C57BL/6J (n = 10 per group) as described in Materials and Methods. The mice received mouse chow containing scrambled L-4F (sc-4F) or L-4F (L-4F) at a dose of 100 mg/kg per day. (A) Mice were killed 5 wk after flank injection and tumor weight (A) and volumes (B) were determined with representative tumors shown in C. The plasma levels of LPA were determined as described in Materials and Methods and the data are shown (Mean ± SD) for LPA20:4 (D).
Discussion
Inflammation and oxidative stress contribute to the etiology of almost every known disease. Reactive oxygen species generated by enzymatic and nonenzymatic systems modify lipids and sterols, producing oxidized lipids and oxidized sterols that, if unchecked, produce undesirable inflammation and more oxidative stress. Under normal physiological conditions, antioxidant enzyme systems and HDL-associated proteins and enzymes reduce oxidative stress. However, under a variety of inflammatory conditions these antioxidant defense mechanisms are often reduced, probably as an evolutionary mechanism to promote oxidative stress to kill invading microbes.
Oxidative stress has long been associated with the pathophysiology of cancer. Highly metastatic and invasive tumors appear to flourish under conditions of maximum oxidative stress (27). Reactive oxygen species may be conducive to the vitality of cancer cells and drive signaling transduction pathways, which leads to activation of redox-sensitive transcription factors and genes involved in cancer cell growth, proliferation, and survival (28, 29). Because oxidized lipid mediated inflammation appears to be common (30, 31), it is not surprising that lipoproteins may be a central modulator/regulator of diseases, including cancer and atherosclerosis, in which inflammation is an important component.
Indeed, in a large cohort of early-stage breast cancer survivors, statin therapy was associated with improved prognosis and decreased risk of recurrence (32). The Health Professionals Follow-up Study identified statin users to have a significantly lower risk of metastatic and fatal disease in prostate cancer patients (33). In women with advanced-stage epithelial ovarian cancers, a statistically significant longer time to progression and improved overall survival was observed for those patients also on statin therapy (34) and, more recently, LDL was demonstrated to be a significant predictor of clinical outcome in advanced epithelial ovarian cancer patients (35). In another study, paraoxonase activity (a key anti-oxidant protein associated with HDL) correlated inversely with stage, grade, and CA-125 level of ovarian cancer (36). Decreased paraoxonase activity and increased lipid hydroperoxide levels are suggested to play a role in the initiation and progression of epithelial ovarian cancer (36). All of these studies allude to the importance of circulating lipoproteins and their metabolism in the regulation of tumorigenesis.
apoA-I is the major protein constituent of HDL. Decreased apoA-I levels have been reported in the serum of patients with pancreatic cancer, gastric cancer, and ovarian cancer (4, 37, 38). Serum apoA-I levels are also down-regulated in patients with lymphoblastic leukemia (39). Scribano et al. (40) showed that patients with acute lymphoblastic leukemia who achieved remission after receiving chemotherapy showed significant increases in apoA-I levels. In this article, we demonstrate that apoA-I plays an important role in ovarian tumorigenesis. We are unique in demonstrating that overexpression of human apoA-I is associated with an improved overall survival in a mouse model of ovarian cancer (Fig. 1). The improved survival in hApoA-I/Tg mice was associated with a significant decrease in overall tumor burden (Fig. 2) following injection of a murine-derived ovarian adenocarcinoma cell line (ID8), which is histologically similar to human ovarian adenocarcinoma.
Over the last 8 y, peptide mimetics of apoA-I have been tested in animal models for their ability to confer the anti-inflammatory and antioxidant properties associated with apoA-I. ApoA-I mimetic peptides markedly reduce atherosclerosis in animal models (11, 41). Furthermore, several published studies of apolipoprotein mimetic peptides in models of inflammatory disorders other than atherosclerosis suggest that they have efficacy in a wide range of inflammatory conditions (10, 13), including, in animal models of dyslipidemia (10, 11, 42, 43), diabetes and vascular inflammation (44–48), renal disease (12, 49), sepsis (50), and Alzheimer's disease (51). Our studies demonstrate that apoA-I mimetic peptides are very effective in preventing the development of tumors (Figs. 2 and 3) in immunocompetent mice injected with ID8 cells.
It has been recently demonstrated that apoA-I mimetic peptides exert their anti-inflammatory properties, in large part, by their ability to bind proinflammatory lipids. LPA, a proinflammatory lysophospholipid, is implicated in the etiology of a number of human cancers, including ovarian cancer (14). LPA has been reported to enhance tumor growth of ID8 cells and ascites formation in female C57BL/6 mice (20). We demonstrated that apoA-I and apoA-I mimetic peptides bind LPA, but the apoA-I mimetic peptides bind LPA with an affinity that is six orders of magnitude greater than apoA-I. In vitro, LPA-induced cell growth is prevented by L-4F (Fig. 5A), suggesting that the apoA-I mimetic peptides are able to inhibit LPA action on ID8 cells. Importantly in vivo, LPA levels were significantly reduced in mice that received apoA-I mimetic peptides when compared with control mice (Figs. 5 and 6), and the relative serum LPA levels correlated with the tumor burden in these two groups of mice (Figs. 2 and 6). Taken together, our results suggest that a plausible mechanism of action for apoA-I mimetic peptides in this model is to bind and aid in reducing the levels of pro-tumorigenic lipids, such as LPA. In these studies, we did not directly test apoA-I's ability to prevent LPA-induced cell growth in vitro or apoA-I's ability to reduce LPA plasma levels following injection or oral administration of apoA-I, compared with the mimetic peptides (Figs. 5 and 6). However, based on the relative binding affinities of apoA-I and L-4F for LPA (Table 1), we would suspect that much higher amounts of apoA-I would be required to achieve the same results.
We previously reported that L-4F administered orally was ineffective because of degradation in the gastrointestinal tract, which was avoided by using D-4F (24). The maximal dose of L-4F tested in those experiments (24) was a dose of 5 mg/kg administered in saline as a single bolus by stomach tube. The data in Fig. 6 demonstrate efficacy of oral L-4F when administered in mouse chow daily at a dose 20-fold higher for 5 wk.
Our current findings suggest that inhibition of cell growth is the dominant mechanism. We did not see differences in apoptosis but did see significant differences in BrdU incorporation in cells following peptide treatment. In future studies we will explore the mechanisms by which cell growth is inhibited and we will determine if there is cell-cycle arrest and, if so, we will determine where in the cell cycle the arrest occurs. In vitro the apoA-I mimetic peptide, L-4F, reduced the viability of human papillary serous adenocarcinoma cell lines resistant to cis-platinum: namely, SKOV3, OV2008, and A2780 (Fig. 4E). Some human ovarian cancer cell lines appear to have high levels of the dual specificity MAP kinase phosphatase, MKP-1, which is necessary for their resistance to cis-platinum (52). We previously reported that MKP-1 is markedly induced by oxidized phospholipids (53, 54) of the kind bound by L-4F with very high affinity. It will be interesting in future studies to determine if L-4F treatment decreased MKP-1 in these cis-platinum–resistant cell lines. These data have potentially important clinical implications because the vast majority of patients treated for advanced stage ovarian cancer will succumb to their disease secondary to the development of cis-platinum–resistant recurrent disease (55). The data in Figs. 3 and 6 suggest that it might be possible to administer apoA-I mimetic peptides orally in ovarian cancer and monitor LPA plasma levels as a biomarker.
In conclusion, our data suggest that apoA-I is not only a biomarker for the detection of early-stage ovarian cancer, but apoA-I and apoA-I mimetic peptides may also be promising therapeutic agents for the treatment of ovarian cancer.
Materials and Methods
Mice.
The Animal Research Committee at the Universtity of California at Los Angeles approved all mouse protocols. C57BL/6-Tg (APOA-I) 1Rub/J female mice (hApoA-I/Tg) carrying the human apoA-I transgene and the C57BL/6J female littermates were purchased from The Jackson Laboratory.
ID8 cell line (a mouse ovarian epithelial papillary serous adenocarcinoma cell line) was a generous gift from K. F. Roby (Center for Reproductive Sciences, University of Kansas Medical Center, Kansas City, KS).
Survival Studies.
Nine-week-old hApoA-I/Tg mice and C57BL6/J mice were given an intraperitoneal injection containing 8 × 106 ID8 cells in a total volume of 0.8 mL of DMEM (without supplements). The mice were monitored with weekly weight and abdominal girth measurements until death.
Tumor-Load Studies.
For subcutaneous studies, hApoA-I/Tg mice or C57BL/6J mice (9-wk-old), were given a 0.5 mL subcutaneous injection of 5 × 106 ID8 cells prepared as a single cell suspension in PBS mixed with an equal volume of the cold Matrigel (10 mg/mL of protein). The mice were killed 5 wk after injection and tumor volumes were measured using the formula V = 1/2 (L × W2). For intraperitoneal studies, hApoA-I/Tg mice or C57BL/6J mice (9-wk-old) were given an intraperitoneal injection containing 8 × 106 ID8 cells in a total volume of 0.8 mL of DMEM (without supplements). Nine weeks after the injection, the mice were killed and tumor loads were assessed by counting the number of tumor nodules on the parietal peritoneal surfaces and the visceral peritoneal surfaces of the intestine, liver, kidney, and spleen.
Cell-Culture Experiments.
ID8, OV2008, and A2780 cells (2,000 cells per well) were first cultured in complete medium in 96-well culture plates, and 24 h later the medium was replaced with serum and growth factor-free medium. Following an overnight incubation, the cells were either left untreated (no treatment) or treated with 100 μg/mL of human apoA-I or apoA-II (3.57 or 5.75 μM, respectively), or treated with 10 μg/mL of apoA-I mimetic peptides, L-4F or sc-4F (4.12 μM for both L-4F and sc-4F) or L-5F (4.11 μM). Cells were incubated for an additional 48 h and assayed for viability using the MTS assays kit (Promega) according to the manufacturer's protocol. For proliferation assay, cells were labeled with BrdU for the last 4 h of the 48-h incubation. Cells were subsequently washed, fixed, and incubated with mouse anti-BrdU antibody for 1 h at room temperature and detected by a peroxidase-coupled goat anti-mouse secondary antibody (Calbiochem). Absorbance was measured using dual wavelengths 450 and 540 nm.
LPA Binding Affinity and Serum LPA Levels.
LPA (20:4) was purchased from Avanti Polar Lipids. Binding affinity of LPA for apoA-I and L-4F was determined as described previously (26). Serum LPA levels were determined as described previously (56).
Statistical Analyses.
The data are shown as means ± SD for each group. We performed statistical analyses by unpaired t test. All results were considered statistically significant at P < 0.05.
Acknowledgments
We thank Susan Hama, David Meriwether, Arnab Chatopadhyay, and Ekambaram Ganapathy for their technical support, and Dr. Oliver Dorigo's laboratory in the Department of OB/GYN, University of California Los Angeles, for providing the human ovarian cancer cell lines. This work was supported by funds from the Womens Endowment, the Carl and Roberta Deutsch Family Foundation, the Joan English Fund for Women's Cancer Research, the VA Merit I Award (to R.F.-E.), the Ovarian Cancer Coalition, the Helen Beller Foundation, Wendy Stark Foundation, Sue and Mel Geleibter Family Foundation, US Public Health Service Grants HL-30568 (to A.M.F., S.T.R., M.N.) and HL-082823 (to S.T.R.), and the Laubsich and M. K. Grey funds at the Univerisity of California, Los Angeles.
Footnotes
Conflict of interest statement: M.N., A.M.F., G.M.A., and S.T.R. are principals in Bruin Pharma. A.M.F. is an officer in Bruin Pharma.
References
- 1.Edwards BK, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nossov V, et al. The early detection of ovarian cancer: From traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. 2008;199:215–223. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Kozak KR, et al. Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: Potential use in diagnosis and prognosis. Proc Natl Acad Sci USA. 2003;100:12343–12348. doi: 10.1073/pnas.2033602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozak KR, et al. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 5.Su F, et al. Validation of candidate serum ovarian cancer biomarkers for early detection. Biomark Insights. 2007;2:369–375. [PMC free article] [PubMed] [Google Scholar]
- 6.Nosov V, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009;200:639.e1–639.e5. doi: 10.1016/j.ajog.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Tardif JC, Heinonen T, Noble S. High-density lipoprotein/apolipoprotein A-I infusion therapy. Curr Atheroscler Rep. 2009;11(1):58–63. doi: 10.1007/s11883-009-0009-7. [DOI] [PubMed] [Google Scholar]
- 8.Navab M, et al. Oral small peptides render HDL antiinflammatory in mice and monkeys and reduce atherosclerosis in ApoE null mice. Circ Res. 2005;97:524–532. doi: 10.1161/01.RES.0000181229.69508.2f. [DOI] [PubMed] [Google Scholar]
- 9.Reddy ST, et al. Oral amphipathic peptides as therapeutic agents. Expert Opin Investig Drugs. 2006;15(1):13–21. doi: 10.1517/13543784.15.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Van Lenten BJ, et al. Multiple indications for anti-inflammatory apolipoprotein mimetic peptides. Curr Opin Investig Drugs. 2008;9:1157–1162. [PMC free article] [PubMed] [Google Scholar]
- 11.Navab M, et al. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J Lipid Res. 2009;50:1538–1547. doi: 10.1194/jlr.M800539-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76:437–444. doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Lenten BJ, et al. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11(1):52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao YJ, et al. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: Comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem. 2001;290:302–313. doi: 10.1006/abio.2001.5000. [DOI] [PubMed] [Google Scholar]
- 16.Boucharaba A, et al. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci USA. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitayama J, et al. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–R646. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DL, et al. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 19.Sutphen R, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–1191. [PubMed] [Google Scholar]
- 20.Li H, et al. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol Cancer Ther. 2009;8:1692–1701. doi: 10.1158/1535-7163.MCT-08-1106. [DOI] [PubMed] [Google Scholar]
- 21.Roby KF, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 22.Greenaway J, Henkin J, Lawler J, Moorehead R, Petrik J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol Cancer Ther. 2009;8:64–74. doi: 10.1158/1535-7163.MCT-08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun. 2003;309:377–383. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Navab M, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 25.Navab M, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 26.Van Lenten BJ, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicotera TM, Privalle C, Wang TC, Oshimura M, Barrett JC. Differential proliferative responses of Syrian hamster embryo fibroblasts to paraquat-generated superoxide radicals depending on tumor suppressor gene function. Cancer Res. 1994;54:3884–3888. [PubMed] [Google Scholar]
- 28.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review) J Nutr Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, et al. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 30.Bochkov VN, et al. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, et al. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platz EA, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 34.Elmore RG, Ioffe YI, Scoles DR, Karlan BY, Li AJ. Impact of statin therapy on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111:102–105. doi: 10.1016/j.ygyno.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Li AJ, Elmore RG, Chen IY, Karlan BY. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol Oncol. 2010;116:78–81. doi: 10.1016/j.ygyno.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camuzcuoglu H, et al. Serum paraoxonase and arylesterase activities in patients with epithelial ovarian cancer. Gynecol Oncol. 2009;112:481–485. doi: 10.1016/j.ygyno.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Ehmann M, et al. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205–214. doi: 10.1097/01.mpa.0000250128.57026.b2. [DOI] [PubMed] [Google Scholar]
- 38.Takaishi S, Wang TC. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007;98:284–293. doi: 10.1111/j.1349-7006.2007.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998;83:379–384. [PubMed] [Google Scholar]
- 40.Scribano D, et al. Return to normal values of lipid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica. 1996;81:343–345. [PubMed] [Google Scholar]
- 41.Navab M, et al. Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol. 2010;30:164–168. doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Lenten BJ, et al. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007;48:2344–2353. doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Mahdavi H, Kim JB, Safarpour S, Tien DA, Navab M. Dyslipidemia and cardiovascular diseases. Curr Opin Lipidol. 2009;20:157–158. doi: 10.1097/MOL.0b013e32832956ed. [DOI] [PubMed] [Google Scholar]
- 44.Van Lenten BJ, et al. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106:1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe J, et al. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–18301. doi: 10.1074/jbc.M109.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou J, et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res. 2005;97:1190–1197. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson SJ, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson SJ, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaysen GA. Potential restoration of HDL function with apolipoprotein A-I mimetic peptide in end-stage renal disease. Kidney Int. 2009;76:359–361. doi: 10.1038/ki.2009.205. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, et al. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol. 2009;297:H866–H873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handattu SP, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer's disease. Neurobiol Dis. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Zhou JY, Zhang L, Wu GS. Involvement of MKP-1 and Bcl-2 in acquired cisplatin resistance in ovarian cancer cells. Cell Cycle. 2009;8:3191–3198. doi: 10.4161/cc.8.19.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy ST, et al. Identification of genes induced by oxidized phospholipids in human aortic endothelial cells. Vascul Pharmacol. 2002;38:211–218. doi: 10.1016/s1537-1891(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 54.Reddy ST, et al. Potential role for mitogen-activated protein kinase phosphatase-1 in the development of atherosclerotic lesions in mouse models. Arterioscler Thromb Vasc Biol. 2004;24:1676–1681. doi: 10.1161/01.ATV.0000138342.94314.64. [DOI] [PubMed] [Google Scholar]
- 55.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 56.Murph M, et al. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: Potential biomarkers for cancer diagnosis. Methods Enzymol. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]