Abstract
Calcium signaling is essential for the differentiation of many cell types, including skeletal muscle cells, but its mechanisms remain elusive. Here we demonstrate a crucial role for nicotinic acid adenine dinucleotide phosphate (NAADP) signaling in skeletal muscle differentiation. Although the inositol trisphosphate pathway may have a partial role to play in this process, the ryanodine signaling cascade is not involved. In both skeletal muscle precursors and C2C12, cells interfering with NAADP signaling prevented differentiation, whereas promoting NAADP signaling potentiated differentiation. Moreover, siRNA knockdown of two-pore channels, the target of NAADP, attenuated differentiation. The data presented here strongly suggest that in myoblasts, NAADP acts at acidic organelles on the recently discovered two-pore channels to promote differentiation.
Keywords: calcium signaling, myogenic differentiation, Ned-19
Cellular differentiation is a central process, not only in embryonic development, but also during regeneration in adult organisms, involving the complex activation and repression of different genes and resulting in morphological changes in numerous cell types. Greater understanding of the signaling pathways that link extracellular signals to transcriptional changes in these processes have been developing in recent years. Calcium is a ubiquitous intracellular messenger that modulates many aspects of cell physiology (1). More specifically, there are a number of calcium-sensitive transcription factors (2, 3). Differentiation can be inhibited in a number of cell types by the depletion of cytosolic calcium stores, suggesting a role for calcium in differentiation. However, there are several sources of calcium available and it remains unclear which sources contribute to the regulation of differentiation in a given cell type.
An increase in intracellular calcium concentration can be caused by a number of mechanisms. Calcium can enter the cell through the plasma membrane or can be released from intracellular stores controlled by second messengers or calcium itself (1). There are three second messengers that can trigger calcium release from intracellular stores: inositol 1,4,5-trisphosphate (InsP3), cyclic ADP ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (4). InsP3 and cADPR act at the endoplasmic and sarcoplasmic reticulum via InsP3 and ryanodine receptors (RyRs), respectively. In contrast, the NAADP signaling cascade is more controversial, with action reported at both RyRs on the endo- or sarcoplasmic reticulum (5–8), and two-pore channels (TPCs) on acidic stores having been described (9–11).
Skeletal muscle tissue has a remarkable capacity to regenerate because of the existence of myoblasts, so-called “satellite cells,” which reside within muscle tissue and undergo myogenic differentiation following injury or exercise (12). The role of calcium in this process has been investigated, demonstrating a clear impact on skeletal muscle differentiation following manipulation of a number of the aforementioned pathways. First, blocking of L-type calcium channels prevents myogenic differentiation of C2C12 cells, a model for muscle differentiation. Second, inhibiting reuptake of calcium into the sarcoplasmic reticulum with thapsigargin interferes with myogenic differentiation, suggesting a role of intracellular calcium signaling in this process (13). In skeletal muscle differentiation calcium elicits its action through calmodulin-dependent kinase and calcineurin, a calcium-dependent phosphatase, to regulate the expression of the myogenic regulatory factor myogenin, an essential part of the differentiation program (14, 15).
Despite the fundamental role of calcium levels in skeletal muscle differentiation, the mechanisms controlling its release in this process are only partly understood. In particular, the role of the second messenger NAADP in this process has never been analyzed. The impact of NAADP on differentiation has been demonstrated in PC12 cells (16), a common model to study neuronal differentiation. Moreover, a recent study shows that NAADP-mediated calcium signaling is involved in T-cell activation (5, 17).
We found that undifferentiated muscle cells have NAADP-sensitive stores. NAADP-acetoxymethylester (NAADP-AM), a recently developed cell-permeant form of NAADP (18), caused a calcium release in these cells and stimulated early and late events of terminal differentiation. Ned-19, a specific inhibitor of the NAADP signaling pathway (19), prevented skeletal muscle differentiation. In contrast, inhibitors of the RyR interfered only weakly with differentiation events. Undifferentiated myoblasts do not express the skeletal muscle-specific isoform of the RyR (RyR1). On the other hand, the NAADP receptor TPC2 and its isoform TPC1 are expressed in these cells and TPC2 is down-regulated in differentiating C2C12 cells, as well as during skeletal muscle development. Down-regulation of both TPC1 and TPC2 inhibits skeletal muscle differentiation. Taken together, these results show that NAADP is an essential second messenger in skeletal muscle differentiation and development, more likely acting via the TPC than the RyR1.
Results
Undifferentiated C2C12 Cells Release Calcium to NAADP but Not Ryanodine.
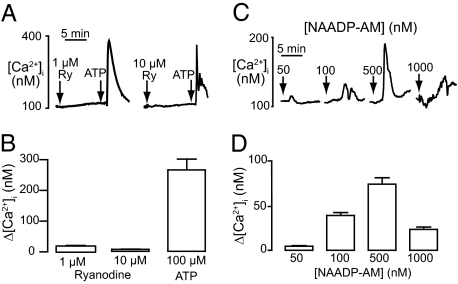
Previous studies have suggested a role for calcium signaling in skeletal muscle differentiation (13–15). To determine second-messenger responsiveness of myogenin-negative C2C12 myoblasts, we measured calcium release. These cells did not respond to 10 μM ryanodine, although they were able to respond to a subsequent addition of ATP (Fig. 1 A and B). In comparison, an addition of the cell-permeant form of NAADP (NAADP-AM) produced a clear increase in calcium with a typical bell-shaped concentration-response curve with a maximal response at 500 nM (Fig. 1 C and D).
Fig. 1.
NAADP induces calcium release in undifferentiated C2C12 cells. (A) Example traces of calcium release induced by 1 or 10 μM ryanodine (first arrow) followed by 100 μM ATP (second arrow). (B) Bar graph (mean with SEM; n = 25–40 cells) representing the change in cytosolic calcium following addition of ryanodine or ATP. (C) Example traces of calcium release induced by 50, 100, 500, and 1 μM NAADP-AM. Arrows indicate addition of NAADP-AM. (D) Bar graph (mean with SEM; n = 12–16 cells) representing the change in cytosolic calcium following addition of NAADP-AM.
Established Endosplasmic Reticulum-Linked Messengers Are Not Essential for Differentiation.
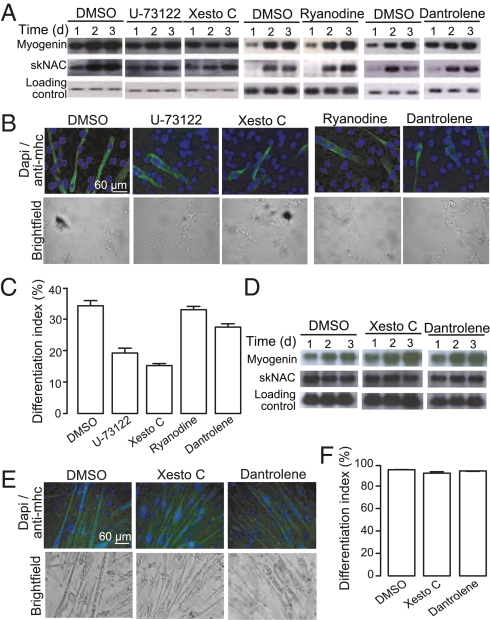
To determine the role for the established calcium stores and channels known to be present in skeletal muscle cells, we investigated the effect of different pharmacological agents on differentiation. Northern blots were carried out using specific probes to monitor the expression of the myogenic regulatory factor myogenin, as well as of the skeletal and heart muscle-specific transcription factor skNAC. Moreover, the expression of myosin heavy chain, a marker for late terminal differentiation, was used for calculating the differentiation index following 4 d of treatment. Thapsigargin, an agent that inhibits calcium reuptake into the endoplasmic reticulum, has been shown to interfere with C2C12 muscle differentiation (13). However, incubation with 0.1 nM (10-fold less than was used in these experiments) leads to cell death 2 to 4 d after induction of differentiation. Nonetheless, xestospongin C, a specific inhibitor of the InsP3 receptor (20), led to a slight deceleration in differentiation after 1 d. This effect was mimicked by U-73122 [phospholipase C inhibitor (21)], with a comparable increase in myogenin and skNAC expression (Fig. 2A), as well as on the presence of nuclei in myosin heavy chain-positive cells (Fig. 2 B and C). Inhibition of the RyR with dantrolene, or a high concentration of ryanodine, did not affect the expression of myogenin and skNAC (Fig. 2A) or the presence of nuclei in myosin heavy chain-positive cells (Fig. 2 B and C). To investigate a role for NAADP in differentiation in a more physiological setting, we used primary murine myoblasts. Inhibition of the InsP3 receptor, with Xestospongin C, or the RyR, with dantrolene, did not affect the expression of myogenin and skNAC (Fig. 2D) or the presence of nuclei in myosin heavy chain-positive cells (Fig. 2 E and F). We therefore went on to consider a role for the NAADP-mediated calcium release demonstrated earlier.
Fig. 2.
Neither the InsP3 or ryanodine signaling pathway is essential for skeletal muscle differentiation. C2C12 cells were induced to differentiate in the presence of 1 μM xestospongin C, 5 μM U-73122, 100 μM ryanodine, or 100 μM dantrolene. (A) RNA was harvested at the indicated time points and analyzed for expression of myogenin and skNAC by Northern blot analysis. (B) Following 4 d of differentiation, cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (C) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with DMSO, or an inhibitor of the IP3 or ryanodine signaling pathway. Primary murine myoblast were induced to differentiate in the presence of 1 μM xestospongin C, 5 μM U-73122, 100 μM dantrolene, or 100 μM ryanodine. (D) RNA was harvested at the indicated time points and analyzed for expression of myogenin and skNAC by Northern blot analysis. (E) Following 4 d of differentiation, cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (F) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with DMSO, or an inhibitor of the IP3 or ryanodine signaling pathway.
NAADP Promotes Skeletal Muscle Differentiation.
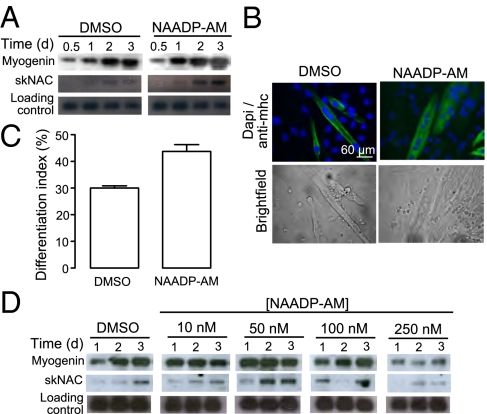
C2C12 cells were incubated with differentiation medium in the presence or absence of NAADP-AM. Preliminary data demonstrated that 50 nM NAADP-AM was sufficient to promote differentiation in terms of myogenin expression, but 250 nM caused inhibition. C2C12 cells were therefore treated over 72 h with 50 nM NAADP-AM. These cells showed an increased expression level of both transcripts compared with control cells (Fig. 3A). Therefore, C2C12 cells were differentiated in the presence of 50 nM NAADP-AM for 4 d and late-terminal differentiation was considered. In control cells treated with differentiation medium alone, about 30% of the nuclei were typically located in myosin heavy chain-positive cells. In comparison, up to 50% of the nuclei of NAADP-AM treated cells were found in myosin heavy chain-positive cells (Fig. 3 B and C). Similarly, in primary murine myoblasts, low concentrations (10 and 50 nM) of NAADP-AM promotes the expression of skeletal muscle differentiation markers (Fig. 3D). On the other hand, differentiation of these cells in the presence of a high concentration of NAADP-AM (250 nM) inhibited the expression myogenin and skNAC in primary myoblasts. Finally, in both C2C12 cells and primary murine myoblasts, the expression of differentiation markers was not altered by treatment with a range of concentrations of cell impermeant NAADP (20, 50, 100, and 500 nM), clearly demonstrating that NAADP must act intracelullarly to effect differentiation.
Fig. 3.
NAADP-AM promotes skeletal muscle differentiation. C2C12 cells were induced to differentiate in the presence or absence of 50 nM NAADP-AM. (A) RNA was harvested at the indicated time points and analyzed for expression of myogenin and skNAC by Northern blot using specific probes. (B) Following 4 d of differentiation, cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (C) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with either DMSO or NAADP-AM. (D) Primary murine myoblast were induced to differentiate in the presence or absence of 10, 50, 100, or 250 nM NAADP-AM. RNA was harvested at the indicated time points and analyzed for the expression of myogenin and skNAC by Northern blot analysis.
NAADP Signaling via Acidic Organelles Is Essential for Differentiation.
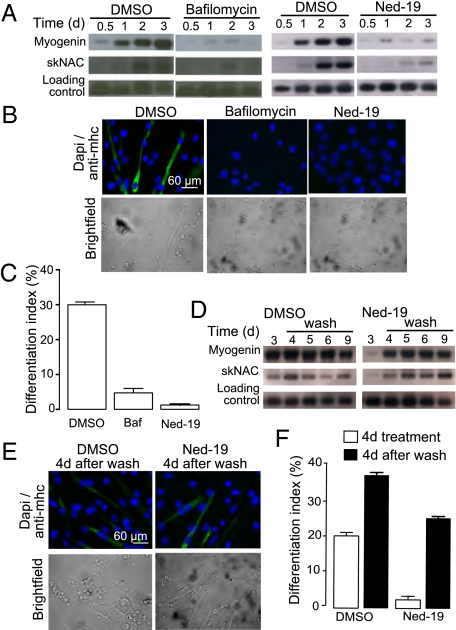
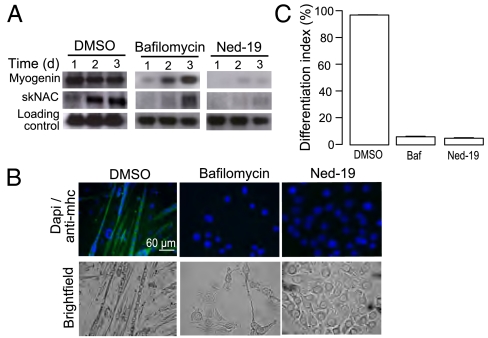
Given that promoting NAADP signaling clearly caused an increase in differentiation, we investigated the effect of inhibiting NAADP signaling on differentiation. Initially, we used bafilomycin (inhibitor of lysosomal H+-ATPase) to determine if calcium stored in acidic organelles was required. Calcium loading of these stores is dependent on the proton gradient and, as such, bafilomycin is able to deplete lysosomal stores of calcium content (22). Treatment with bafilomycin resulted in a decrease in myogenin and skNAC expression (Fig. 4A), as well as a strongly reduced differentiation index (Fig. 4 B and C). To inhibit NAADP signaling more selectively, we used the NAADP antagonist, Ned-19 (19). Expression levels of myogenin and skNAC were markedly decreased in Ned-19–treated cells compared with control cells (Fig. 4A). Moreover, virtually none of the nuclei of the Ned-19-treated cells were located in myosin heavy chain-positive cells (Fig. 4 B and C). To verify whether Ned-19 specifically inhibits a pathway essential for skeletal muscle differentiation rather then causing an irreversible damage to C2C12 cells, we investigated whether cells would recover following removal of Ned-19. Indeed, the expression levels of myogenin and skNAC after the medium change increased to levels comparable to those seen in control cells (Fig. 4D). Moreover, the amount of nuclei found in myosin heavy chain-positive cells increased markedly after the medium switch (Fig. 4 E and F). Consistent with these results in C2C12 cells, treatment of primary myoblasts with either bafilmoycin or Ned-19 prevented differentiation in terms of up-regulation of myogenin and skNAC after treatment with differentiation medium (Fig. 5A). Indeed, under these conditions very little expression of myosin heavy chain is evident, even after 4 d (Fig. 5 B and C), once again demonstrating an absolute requirement for NAADP signaling in differentiation.
Fig. 4.
NAADP signaling is essential for differentiation of C2C12 cells. C2C12 cells were induced to differentiate in the presence of DMSO, 200 nM bafilomycin, or 100 μM Ned-19. (A) RNA was harvested at the indicated time points and analyzed for expression of myogenin and skNAC by Northern blot analysis. (B) Following 4 d of differentiation, cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (C) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with control, bafilomycin, or Ned-19. Recovery of C2C12 differentiation following removal of Ned-19 was demonstrated by (D) Northern blot, (E) myosin heavy chain and DAPI staining, and (F) calculation of the differentiation index.
Fig. 5.
NAADP signaling is essential for differentiation of primary murine myoblasts. Primary murine myoblast were differentiated in the presence of with 200 nM bafilomycin or 100 μM Ned-19. (A) RNA was harvested at the indicated time points and analyzed for the expression of myogenin and skNAC by Northern blot analysis. (B) Following 4 d of differentiation, cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (C) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with control, bafilomycin, or Ned-19.
Calcium Channel Expression in Skeletal Muscle Cells.
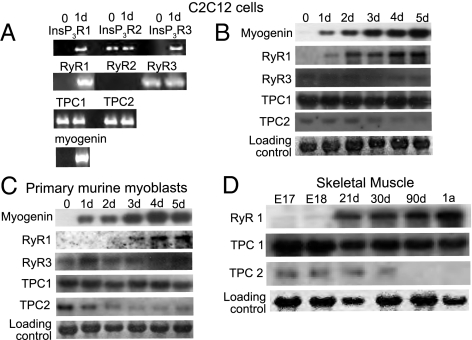
It has been suggested that NAADP acts at either the TPCs (9–11) or at the RyR (23). To determine which of these is responsible for the NAADP-mediated effects, we decided to clarify which are expressed in C2C12 cells and how they change during differentiation. We performed RT-PCR experiments with specific primers against each receptor type along with specific primers for the early differentiation marker myogenin as a control (Fig. 6A). As expected, myogenin was not detectable in undifferentiated C2C12 cells but was present in 24-h differentiated cells (Fig. 6A). The InsP3 receptor subtypes 1 and 3 were absent in undifferentiated cells, whereas the InsP3 receptor subtype 2 was detectable. All three InsP3 receptor subtypes were detectable in 24-h differentiated cells. Similarly, the RyR subtypes 1 and 2 (RyR1 and -2) were absent but RyR3 was detectable in undifferentiated cells. Although the skeletal muscle-specific RyR1 was expressed in 24-h differentiated cells, the heart-specific RyR2 could not be detected. Additionally, the two recently identified NAADP receptors, TPC1 and TPC2, were present in both undifferentiated, as well as 24-h differentiated C2C12 cells (Fig. 6A). For a more extensive comparison, we performed Northern blot experiments using specific probes on RNAs from C2C12 cells that had been placed in differentiation medium for 1 to 5 d (Fig. 6B). As expected, C2C12 cells do not express RyR2 (heart-specific isoform) at any time during differentiation. Comparatively, there is a clear induction in the expression of RyR1, consistent with its role in skeletal muscle function; the expression of RyR3 stays constant during the differentiation process. Finally, although TPC1 is expressed during the whole differentiation process, TPC2 is down-regulated during the first 3 d after the induction of differentiation (Fig. 6B). The primary murine skeletal muscle cells we used for our experiments showed the same expression behavior that was observed in C2C12 cells. The RyR1 was not present in undifferentiated cells and was strongly induced after 24 h of differentiation induction. The RyR3 was present in undifferentiated as well as differentiated cells. Both TPC isoforms were present in undifferentiated primary myoblasts. Although the TPC1 was still present to the same extent after 5 d of expression, the TPC2 was strongly down-regulated after the first day of differentiation (Fig. 6C). To determine the relevance of the findings to in vivo development, we decided to look at the calcium channel expression in skeletal muscle during embryonic and postnatal development in mice. Previous findings have suggested that TPCs are not expressed in adult skeletal muscle tissues (9, 24). As these channels appear to be essential for differentiation, we wanted to determine at which time point during skeletal muscle development their expression is decreased. We therefore tested RNAs from skeletal muscle of different stages of mouse development. In contrast to a previous report (24), we found TPC1 is expressed in the earliest embryonic stage we tested, E17, and is not down-regulated in adult tissue (Fig. 6D). TPC2 on the other hand is expressed in embryonic stage E17, E18, and up to 3 wk after birth, and as reported before, is absent in adult tissue. As a comparison, there was no detectable expression of RyR1 until 3 wk after birth, followed by increased expression in later stages of skeletal muscle development (Fig. 6D).
Fig. 6.
Calcium channel expression during skeletal muscle differentiation. C2C12 cells were induced to differentiate. (A) Before and after 24 h of differentiation the RNA was harvested and analyzed for expression of InsP3R, RyR, and TPC subtypes by RT-PCR with specific primers. Differentiation was monitored by detection of myogenin. (B) RNA was harvested at the indicated time points and analyzed using Northern blot for expression of RyR and TPC subtypes, using specific probes. Differentiation was monitored by detection of myogenin. (C) Primary murine myoblasts were induced to differentiate. RNA was harvested at the indicated time points and analyzed for the expression of RyR and TPC subtypes, using specific probes. Differentiation was monitored by detection of myogenin. (D) RNA from mouse skeletal muscle at different stages of development was analyzed using Northern blot for the expression of TPC1, TPC2, and RyR1 using specific probes.
C2C12 Differentiation Is Altered by Down-Regulation of the TPCs on Acidic Organelles.
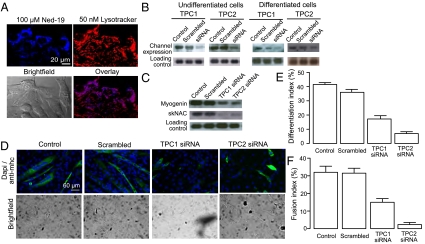
Ned-19, the selective antagonist of NAADP signaling, being a derivative of tryptophan, can also act as a fluorescent marker of the receptor (19), allowing us to determine at which calcium store NAADP is acting. Given that both Ned-19 and bafilomycin demonstrated a similar level of inhibition, and the change in TPC channels during differentiation, the most likely store was the acidic organelles. Indeed, incubation with 100 μM Ned-19 for 1 h resulted in clear labeling of undifferentiated C2C12 cells that colocalized with lysotracker (Fig. 7A), a marker for acidic organelles. To determine whether the inhibition of differentiation caused by Ned-19 and bafilomycin is consistent with the action at TPC receptors, we used siRNA to decrease the expression of these receptors and measure the effects of the down-regulation on the differentiation of C2C12 cells. Therefore, C2C12 cells were transfected with siRNA against each receptor isoform. In both cases the treatment caused a decrease in expression of the respective receptor type (Fig. 7B). To measure the impact of the siRNA treatment on the differentiation process, C2C12 cells were transfected with siRNA against TPC1 or TPC2, differentiated for 24 h, and the myogenin and skNAC expression was monitored. Down-regulation of both TPC1 and TPC2 caused a decrease in myogenin and skNAC expression (Fig. 7C) compared with cells that were transfected with scrambled siRNA or differentiation medium only. Next, siRNA-treated C2C12 cells were differentiated for 4 d, stained for myosin heavy chain, and the differentiation index was calculated (Fig. 7 D and E). TPC1 siRNA-treated C2C12 cells differentiated into multinucleated myotubes, although to a lesser extent than control siRNA-treated cells. The differentiation index was 18% compared with 35% of the control siRNA-treated cells. The differentiation index of the TPC2 siRNA-treated C2C12 was also reduced to 8%. Moreover, these cells did not seem to differentiate into multinucleated myotubes. A calculation of the fusion index, the percentage of nuclei in myosin heavy chain-positive cells with at least three nuclei revealed that the TPC2 siRNA-treated cells had a strongly reduced the fusion index of 2% compared with 32% of the control siRNA-treated cells (Fig. 7F).
Fig. 7.
C2C12 differentiation is altered by down-regulation of TPCs on acidic organelles. (A) Confocal microscopy of undifferentiated C2C12 cells. (Upper Left) Labeling of NAADP receptor with 100 μM Ned-19. (Upper Right) Labeling of acidic organelles with 50 nM lysotracker. (Lower Left) Bright field image of C2C12 cells. (Lower Right) Overlay of Ned-19 and lysotracker labeling showing colocalization. (B) C2C12 cells were transfected with siRNA against TPC1 or TPC2, respectively. RNA was harvested either 24 h after transfection (undifferentiated cells) or 24 h after differentiation was induced (differentiated cells). The RNA was analyzed for expression of TPC1 or TPC2, respectively, by Northern blot analysis. (C) C2C12 cells were transfected with siRNA against TPC1 or TPC2 for 24 h and differentiated for 24 h. RNA was harvested and analyzed for expression of myogenin and skNAC by Northern blot analysis. (D) C2C12 cells were transfected with siRNA against TPC1 or TPC2 for 24 h and differentiated for 4 d. The cells were stained for myosin heavy chain and DAPI to determine the differentiation index (percent nuclei in myosin heavy chain-positive cells). (E) Bar chart (mean with SEM; n = 4) representing the differentiation index following treatment for 4 d with control, scrambled siRNA, TPC1 siRNA, or TPC2 siRNA. (F) Bar chart (mean with SEM; n = 4) representing the fusion index (percent nuclei in myosin heavy chain-positive cells with at least three nuclei) following treatment for 4 d with control, scrambled siRNA, TPC1 siRNA, or TPC2 siRNA.
Discussion
Myogenin is one of the major transcription factors of the myogenic differentiation program and is expressed early during the terminal differentiation process (25). It is well known that in mouse myoblasts the expression of myogenin, as well as other transcription factors, such as the myocyte enhancer factor-2, are regulated by calcium-dependent signal transduction pathways (e.g., the calcium/calmodulin-dependent kinase pathway or the calcineurin pathway) (14, 15). Therefore, it is clear that mechanisms that control the cytoplasmic calcium concentration are essential for induction of the myogenic differentiation program.
NAADP has recently been evolving as a new universal second messenger, with a role in a variety of signaling cascades from fertilization (26–28), insulin secretion (26, 29, 30), T-cell activation (5, 17), neurotransmitter secretion (31) and, most pertinently, neurite outgrowth (16, 32). Treatment of undifferentiated C2C12 cells with the cell permeant derivative of NAADP (NAADP-AM) induces calcium release, clearly demonstrating the existence of the machinery required for NAADP signaling in these cells. Both, C2C12 myoblasts and primary murine myoblasts show an increased expression of transcripts that are part of the myogenic differentiation program, such as myogenin and skNAC, when differentiated in the presence of NAADP-AM. On the other hand, both bafilomycin, an inhibitor of the lysosomal H+-ATPase, which is known to inhibit NAADP-dependent calcium release in a number of cell types (33–35), and Ned-19, a highly selective inhibitor of the NAADP signaling pathway (19, 36, 37), prevent expression of these transcripts and inhibits the formation of myotubes positive for myosin heavy chain. Taken together, these results are unique in showing that calcium release triggered by NAADP is essential of skeletal muscle differentiation.
In our study, we confirm previous reports that C2C12 myoblasts possess InsP3-sensitive calcium stores and express InsP3 receptor subtypes (38, 39). Additionally, the InsP3 signaling pathway can be linked to differentiation of C2C12 myoblasts (13), but not to differentiation of primary murine myoblasts. In comparison, although skeletal muscle cells express RyR subtypes, they do not appear to be functional in myogenin-negative cells and the RyR signaling pathway proves to be unnecessary for early events of differentiation in both C2C12 cells and primary murine skeletal muscle cells.
According to earlier reports, where Northern blot analyses had been performed, the two mammalian TPC isoforms are not expressed in adult skeletal muscle cells (9, 24). The results of our RT-PCR experiments clearly show that both isoforms are expressed in undifferentiated C2C12 cells. Because NAADP-AM caused a calcium release in these cells, NAADP is likely acting via the TPCs rather than the absent RyR1 in myoblasts. The expression of TPC1 does not change significantly during the differentiation process of C2C12 cells or primary myoblasts. TPC2, on the other hand, is down-regulated within 2 d after the induction of the differentiation. These data suggest that signaling via TPC2 is mainly required during early differentiation events rather than being part of the signaling required for differentiated skeletal muscle cells. We also investigated whether signaling via TPC2 might be mainly required in the first month of skeletal muscle development. Our results from RNAs taken from mice at different stages of development support this suggestion, as TPC2 is strongly down-regulated during development. In comparison, RyR1 only appears 3 wk postnatally, with increased levels up to adulthood, but TPC1 is present throughout all stages of development into adult mice.
The results we obtained from siRNA studies strongly support a role of the TPCs in the differentiation process. First, treatment of C2C12 cells with TPC2 siRNA resulted in a decreased differentiation and fusion index. Second, TPC1 siRNA treated C2C12 cells show a decreased number of multinucleated myotubes, although the number of nuclei per myotube did not differ from control cells, suggesting a role for this receptor type in C2C12 differentiation but not in the fusion processes. Additionally, Ned-19, which we have shown to also interfere with the differentiation of C2C12 cells, is shown to bind to a protein on acidic organelles, and bafilomycin, which also prevented myogenic differentiation in our studies, depletes calcium in these same stores. There has yet been little evidence for the presence of RyRs on the lysosomes but the evidence for TPCs at this location is growing (9–11, 40).
To bring our results in line with earlier reports, it is conceivable that NAADP has two different modes of action in skeletal muscle cells: first, it is part of the initiation of the differentiation process that leads to the formation of new skeletal muscle cells from precursors by releasing calcium from acidic stores via the TPCs. Second, NAADP might contribute to the contraction process in mature skeletal muscle by releasing calcium from the sarcoplasmic reticulum via the RyR type 1 (8).
In conclusion the work presented here, uniquely demonstrates an absolute requirement for the NAADP signaling cascade in skeletal muscle differentiation. The discrepancy between the levels of inhibition demonstrated by manipulation of the NAADP signaling cascade compared with manipulation of the InsP3 signaling cascade clearly demonstrates there is not an absolute requirement for InsP3. It is known in other systems that NAADP is the beginning of a calcium-releasing cascade, where NAADP-induced calcium release “triggers” further release from the endoplasmic reticulum by sensitization of the InsP3 or RyRs (41, 42). This might also be the case when the myogenic differentiation program is started. Finally, given the lack of functional RyRs in myoblasts and the results of siRNA experiments, it is clear the action of NAADP is via TPCs, most likely TPC2, located on acidic organelles.
Materials and Methods
C2C12 cells were grown in DMEM with 1 g/L glucose supplemented with 20% FBS until 90% to 100% confluence. C3H Primary myoblasts were isolated as described previously (43). Cells were grown on collagen-coated plates in 80% nutrient mixture F-10 Ham 20% FBS, 2.5 ng/mL basic fibroblast growth factor at 37 °C, and 5% CO2. Differentiation was induced with 2% horse serum. NAADP-AM was synthesized in house (18). Ned-19 was obtained from IBScreen (19). All other chemicals were from Calbiochem or Sigma. A detailed description is provided in SI Materials and Methods.
Cells were loaded with fura-2 via the acetoxymetyl ester (45 min) and imaged on an inverted microscope using alternating 340- and 380-nm excitation and >520-nm emission (long-pass) detected with a charged-coupled device camera controlled with MetaFluor software. For immunofluorescence, cells were fixed with ice-cold methanol (20 min) at −20 °C, blocked with 10% FCS, and incubated with the myosin heavy chain antibody in 10% FCS (30 min), washed three times with PBS, and then incubated with FITC conjugated goat anti-mouse antibody in 10% FCS (25 min). Nuclei were stained with DAPI. NAADP receptors were visualized with Ned-19 (100 μM, 1 h) using 355-nm excitation and 415-nm band-pass emission on a confocal microscope. Lysosomes were visualized with Lysotracker Red (50 nM, 15 min) using 568-nm excitation and 590-nm long-pass emission.
Total RNA from skeletal muscle mouse tissue was obtained from Zyagen. Complementary DNA was synthesized using the cDNA first-strand synthesis kit and 3 μg RNA from C2C12 cells as a template. Amplification of DNA fragments was carried out with PCR using specific primers. PCR products were cloned into the pBluescript SK vector using the BamHI and EcoRI cloning sites. Inserts were verified by sequencing. Transfection with specific siRNA was carried out using predesigned, TPC1-specific siRNA. For Northern blots, total cellular RNA was isolated using the RNAeasy mini isolation kit, run on an agarose gel and transferred to a nylon membrane and detected with phosphatase-coupled antidogoxigenine antibody and developed with CDP-Star as a chemoluminescent substrate revealed with X-ray film.
Supplementary Material
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BB/D012694/1) and the Charité Rahel Hirsch research fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007381107/-/DCSupplemental.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Ikura M, Osawa M, Ames JB. The role of calcium-binding proteins in the control of transcription: structure to function. Bioessays. 2002;24:625–636. doi: 10.1002/bies.10105. [DOI] [PubMed] [Google Scholar]
- 3.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 4.Galione A, Ruas M. NAADP receptors. Cell Calcium. 2005;38:273–280. doi: 10.1016/j.ceca.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Dammermann W, et al. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc Natl Acad Sci USA. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dammermann W, Guse AH. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J Biol Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- 7.Langhorst MF, Schwarzmann N, Guse AH. Ca2+ release via ryanodine receptors and Ca2+ entry: Major mechanisms in NAADP-mediated Ca2+ signaling in T-lymphocytes. Cell Signal. 2004;16:1283–1289. doi: 10.1016/j.cellsig.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Hohenegger M, Suko J, Gscheidlinger R, Drobny H, Zidar A. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem J. 2002;367:423–431. doi: 10.1042/BJ20020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brailoiu E, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zong X, et al. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter GA, Jr., Makuck RF, Rivkees SA. Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem. 2002;277:28942–28947. doi: 10.1074/jbc.M203961200. [DOI] [PubMed] [Google Scholar]
- 14.Armand AS, et al. Cooperative synergy between NFAT and MyoD regulates myogenin expression and myogenesis. J Biol Chem. 2008;283:29004–29010. doi: 10.1074/jbc.M801297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q, et al. p38 Mitogen-activated protein kinase-, calcium-calmodulin-dependent protein kinase-, and calcineurin-mediated signaling pathways transcriptionally regulate myogenin expression. Mol Biol Cell. 2002;13:1940–1952. doi: 10.1091/mbc.02-02-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brailoiu E, et al. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 17.Berg I, Potter BV, Mayr GW, Guse AH. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca(2+)-signaling. J Cell Biol. 2000;150:581–588. doi: 10.1083/jcb.150.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkesh R, et al. Cell-permeant NAADP: A novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell Calcium. 2008;43:531–538. doi: 10.1016/j.ceca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Naylor E, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gafni J, et al. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 21.Bleasdale JE, et al. Inhibition of phospholipase C dependent processes by U-73, 122. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:590–593. [PubMed] [Google Scholar]
- 22.Rodrigues CO, Scott DA, Docampo R. Characterization of a vacuolar pyrophosphatase in Trypanosoma brucei and its localization to acidocalcisomes. Mol Cell Biol. 1999;19:7712–7723. doi: 10.1128/mcb.19.11.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guse AH. Second messenger signaling: Multiple receptors for NAADP. Curr Biol. 2009;19:R521–R523. doi: 10.1016/j.cub.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 24.Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 25.Pownall ME, Gustafsson MK, Emerson CP., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 26.Galione A, et al. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflugers Arch. 2009;458:869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deguchi R. Fertilization causes a single Ca2+ increase that fully depends on Ca2+ influx in oocytes of limpets (Phylum Mollusca, Class Gastropoda) Dev Biol. 2007;304:652–663. doi: 10.1016/j.ydbio.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Churchill GC, et al. Sperm deliver a new second messenger: NAADP. Curr Biol. 2003;13(2):125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 29.Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, Galione A. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr Biol. 2003;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proc Natl Acad Sci USA. 2002;99:14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brailoiu E, Patel S, Dun NJ. Modulation of spontaneous transmitter release from the frog neuromuscular junction by interacting intracellular Ca2+ stores: Critical role for nicotinic acid-adenine dinucleotide phosphate (NAADP) Biochem J. 2003;373:313–318. doi: 10.1042/BJ20030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brailoiu E, et al. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki M, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 34.Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- 35.Macgregor A, et al. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J Biol Chem. 2007;282:15302–15311. doi: 10.1074/jbc.M611167200. [DOI] [PubMed] [Google Scholar]
- 36.Thai TL, Churchill GC, Arendshorst WJ. NAADP receptors mediate calcium signaling stimulated by endothelin-1 and norepinephrine in renal afferent arterioles. Am J Physiol Renal Physiol. 2009;297:F510–F516. doi: 10.1152/ajprenal.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen D, et al. Analogues of the nicotinic acid adenine dinucleotide phosphate (NAADP) antagonist Ned-19 indicate two binding sites on the NAADP receptor. J Biol Chem. 2009;284:34930–34934. doi: 10.1074/jbc.M109.016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell JA, et al. IP3 receptors and associated Ca2+ signals localize to satellite cells and to components of the neuromuscular junction in skeletal muscle. J Neurosci. 2003;23:8185–8192. doi: 10.1523/JNEUROSCI.23-23-08185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdés JA, et al. NFAT activation by membrane potential follows a calcium pathway distinct from other activity-related transcription factors in skeletal muscle cells. Am J Physiol Cell Physiol. 2008;294:C715–C725. doi: 10.1152/ajpcell.00195.2007. [DOI] [PubMed] [Google Scholar]
- 40.Zhu MX, Ma J, Parrington J, Galione A, Evans AM. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010;584:1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel S, Churchill GC, Galione A. Coordination of Ca2+ signalling by NAADP. Trends Biochem Sci. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- 42.Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 2001;20:2666–2671. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.