Abstract
Objective
A subset of drivers with type 1 diabetes mellitus (T1DM) may be at significant risk of hypoglycemia-related driving collisions and moving vehicle violations due to acute and chronic neurocognitive impairment. The present study compared drivers with T1DM with and without a recent history of multiple driving mishaps on a neurocognitive battery during euglycemia, progressive mild hypoglycemia, and recovery from hypoglycemia, to determine whether neurocognitive measures differentiate the two risk groups. We hypothesized that drivers with a history of multiple recent hypoglycemia-related driving mishaps would demonstrate greater psychomotor slowing, both during hypoglycemia and euglycemia.
Study design
Partcipants were 42 adults with T1DM and were assigned to one of two groups: those reporting no driving mishaps in the last year (−History) and those reporting two or more (+History).Neurocognitive testing was conducted before and repeated during a hyper-insulinemic clamping procedure.
Results
Not surprisingly, all drivers demonstrated a decrease in functioning across all neurocognitive tasks during hypoglycemia. However, in contrast to the common belief that neurocognitive functions return slowly and gradually following hypoglycemia, baseline neurocognitive functioning immediately recovered upon return of BG to euglycemia for all subjects. Between-group analyses revealed that subjects with a recent history of driving mishaps consistently demonstrated poorer performance on tasks measuring working memory.
Conclusion
Working memory is a potential neurocognitive indicator that may help differentiate adults with T1DM with and without a history of driving mishaps, predict future risk for driving mishaps, and provide targeted intervention programs to address this critical public health issue.
Keywords: type 1 diabetes mellitus, driving, neuropsychology, hypoglycemia
Introduction
Worldwide, driving collisions account for 1.2 million fatalities and 50 million injuries annually (1). Drivers with type 1 diabetes mellitus (T1DM) in both Europe and the United States have been found to have 138% more collisions and 50% more moving vehicle violations compared to their non-diabetic spouses (2). In addition to general collisions and moving vehicle violations, drivers with T1DM can have driving “mishaps” due to hypoglycemia that include collisions, moving vehicle violations, impaired driving resulting in someone else taking over control of the vehicle before a collision, and “automatic driving” during which a person drives from point A to point B only to “awake” with no recollection of the trip. When 415 drivers with T1DM were followed prospectively for 12 months, half of the sample reported at least one hypoglycemia-related driving mishap and half reported no such events (3). Just as some individuals with T1DM are more vulnerable to experiencing episodes of severe hypoglycemia, there is likely to be a subgroup of individuals who are at relatively higher risk of hypoglycemia-related driving mishaps (4). A prospective study of 98 drivers with T1DM demonstrated that those who reported two or more driving mishaps in the previous six months were most likely to experience driving mishaps in the next six months (5). In this study, drivers who reported a history of driving mishaps were found to have greater carbohydrate utilization when confronted with a standard insulin challenge, less epinephrine counter-regulation, and demonstrated worse driving performance during hypoglycemia when compared to those with no history of driving mishaps.
While severe hypoglycemia has a more obvious impact on driving and contributes directly to fatal car collisions (6), mild hypoglycemia has also been implicated as a potential driving risk factor. In an international survey study of over 340 T1DM drivers, the occurrence of mild symptomatic hypoglycemia while driving differentiated drivers with and without a history of driving mishaps (2). Mild hypoglycemia has been shown to disrupt cognitive-motor skills relevant to driving and can impair judgment regarding the decision to drive (7,8,9). It is possible that these acute neurocognitive deficits resulting from neuroglycopenia are further compounded by the chronic neurocognitive impairments associated with microvascular complications of T1DM (e.g., psychomotor slowing; diminished cognitive flexibility) in some individuals, making this subgroup more vulnerable to driving mishaps (10).
The present study compared drivers with T1DM with and without a recent history of multiple driving mishaps on a neurocognitive battery during euglycemia, progressive moderate hypoglycemia, and recovery from hypoglycemia, to determine whether neurocognitive measures differentiate the two groups. We specifically hypothesized that drivers with a history of multiple recent hypoglycemia-related driving mishaps would demonstrate greater impairment across neurocognitive domains, both during hypoglycemia and euglycemia.
Materials and Methods
Subjects
Forty-two adults with T1DM (mean age = 42.5 ± 12, disease duration = 21.6 ± 9.4 years, HbA1c = 7.4 ± 0.8%) were recruited through regional advertisements. Inclusion criteria required that subjects: 1) had T1DM for at least one year, 2) measured their blood glucose ≥ 3 times a day, 3) were between the ages of 21 and 70, 4) drove a minimum of 6,000 miles year, and 5) either reported no driving mishaps (− History group) or reported having two or more driving mishaps in the past year (+ History group). Driving mishaps were defined as collisions, citations, “automatic” driving, or required someone to take control of their vehicle due to hypoglycemia. Further, because we planned to induce progressive hypoglycemia (approximately 2.5 mmol/L) through insulin infusion and to draw frequent blood samples during the protocol, exclusionary criteria included 1) hematocrit < 38% for males or <36% for females, 2) pregnancy, and 3) presence of an electronic pacemaker or more than 5% atrial or ventricular ectopy. Four subjects prematurely discontinued prior to study completion; three subjects had insufficient I.V. access for the hyperinsulinemic clamp procedure, and one subject experienced a lower extremity muscle twitch resulting from acute or chronic hypomagnesemia. As illustrated in Table 1, the + and − History groups did not differ on any demographic (i.e., age, sex, year of education), diabetes, or driving parameters with the exception of the number of driving mishaps and episodes of severe hypoglycemia in the previous 12 months.
Table 1.
Pre-study parameters of participants with and without a history of driving mishaps
| Variables | −HX | +Hx | p |
|---|---|---|---|
| N | 22 | 16 | |
| Age | 42 ±12.9 | 42 ±12.8 | ns |
| % Female | 34% | 62% | ns |
| Education/yrs | 15 ±2.6 | 16 ±2.2 | ns |
| HbA1c | 7.1 ±0.8 | 7.5 ±0.9 | ns |
| Yrs. with diabetes | 21 ±9.4 | 21 ±10.8 | ns |
| Insulin Units/day | 42 ±15.5 | 42 ±32.3 | ns |
| BMI | 27 ±5.2 | 26 ±4.2 | Ns |
| %Hypoglycemia Unawareness (N) | 82% (18) | 75% (12) | ns |
| Severe hypoglycemia in past 12 month | 0.5 ±0.7 | 1.6 ±2.2 | <.03 |
| % Subjective neuropathy (N) | 23% (5) | 44% (7) | ns |
| % Objective Neuropathy (N) | 9% (2) | 19% (3) | ns |
| % Retinopathy (N) | 41% (9) | 25% (4) | ns |
| % Laser eye therapy (N) | 4% (1) | 12% (2) | ns |
| Years Driving experience | 27 | 27 | ns |
| Miles driven/yr | 18.5714 ±12.040 | 17.7308 ±16.133 | ns |
| SMBG before driving* | 1 | 1.7 | ns |
| Fast acting sugar in car* | 2 | 2.9 | ns |
| # Mild hypo while driving in past 6 months | 0.7 | 1.1 | ns |
| # Driving mishaps in past year | 0 | 2.8 | .0001 |
| Hypoglycemic nadir (clamp) | 2.74±.89 mmol/l | 2.64 ±.28 mmol/l | ns |
| Peak epinephrine during hypoglycemia (clamp) | 1883.01+ pmol/l | 1184.38 pmol/l | =.05 |
Mean ratings on 0 (Never) to 4 (Always) scales
Procedure
The current study was part of a larger study examining the impact of progressive hypoglycemia on driving simulation performance (8). After acquiring approval from our institutional review board (IRB) and obtaining informed consent, participants completed an outpatient screening evaluation including a medical history, physical examination, 12 lead EKG, and laboratory tests for HbA1c, complete blood count, and comprehensive metabolic panel. All procedures were in accordance with the ethical standards of the IRB and with the Helsinki Declaration.
For the 48 hours prior to admission, the subjects were encouraged to avoid hypoglycemia. Their total insulin was reduced by 10%, routine blood glucose (BG) testing was increased to 5 times a day, and the subjects were instructed to eat 10g of glucose prophylactically whenever blood glucose (BG) fell below 5.5 mmol/L. Intermediate and long-acting insulins were discontinued 24 and 36 hours prior to admission, respectively. During this pre-admission period and also during hospital admission, only short and rapid-acting insulins were used.
Subjects were admitted to the University of Virginia General Clinical Research Center (GCRC) at 4 PM on the evening prior to the hyperinsulinemic clamping procedure. A neuropsychological test battery was then administered during euglycemia by a trained examiner to evaluate chronic neurocognitive functioning (see Table 2 for a list of the neuropsychological measures). Subjects were then provided with a standardized (50% carbohydrate, 20% protein and 30% fat), eucaloric, caffeine-free evening meal at 6 PM and a bedtime snack at 9 PM. Subjects were allowed to drink glucose-free caffeine-free drinks throughout the evening, and were asked to go to bed around 11 PM. Subjects were not allowed to eat any additional food during the hospitalization other than that provided by the GCRC or that required to treat BG < 5.5 mmol/L. Two IV lines were placed in the non-dominant hand and arm area for overnight infusion of insulin and hourly blood sampling, to maintain the glucose between 5.6 and 8.3 mmol/L.
Table 2.
Battery of neuropsychological tests administered before GCRC admission testing, respective group means and contrast p levels
| Test | Outcome variable | − History | + History | p |
|---|---|---|---|---|
| BG level pre testing | mmol/L | 9.4 | 10.2 | .56 |
| BG level post testing | mmol/L | 9.2 | 9.1 | .94 |
| PEG BOARD Time | Sec. | 79.6 | 82.1 | .74 |
| Drops | # pins | 0.41 | 0.37 | .92 |
| WAIS-R Block Design | Raw Score | 34.9 | 33.9 | .74 |
| Digit symbol sub | Raw Score, # correct | 58.2 | 56.4 | .65 |
| Digit Vigilance RED T | Sec. | 193.1 | 198.4 | .66 |
| BLUE T | Sec. | 200.0 | 207.1 | .57 |
| RED E | Sec. | 2.0 | 2.7 | .43 |
| BLUE E | Sec. | 2.2 | 3.6 | .18 |
| TMT Trails A | Sec. | 29.0 | 30.8 | .62 |
| TMT Trails B | Sec. | 62.3 | 65.0 | .70 |
| Serial subtraction 1 (327) | # correct | 24.4 | 18.9 | .06 † |
| 2 (325) | # correct | 25.1 | 19.1 | .03 * |
| Verbal Fluency A | # words | 8.9 | 9.3 | .75 |
| S | # words | 11.5 | 11.4 | .92 |
| PASAT 4 sec, out of 49 | # correct/49 | 40.3 | 35.8 | .13 |
| PASAT 2 sec | # correct | 37.2 | 29.3 | .02 * |
| Stroop- word | age corrected | 94.5 | 89.0 | .28 |
| Stroop- color | age corrected | 71.7 | 67.7 | .37 |
| Stroop-CW conflict | age corrected | 39.5 | 39.4 | .98 |
p < .05
p < 1.0 (approaching significance)
Note: Tests in bold were re-administered as part of the abbreviated battery repeated 6 times during GCRC admission
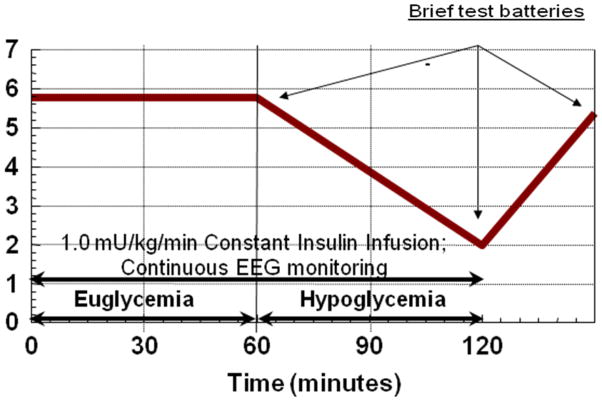
On the morning of testing, subjects were awakened at approximately 7AM and given time to perform basic hygiene. They continued to fast until after the study procedures were completed. An additional retrograde hand IV was inserted and activated charcoal packets were affixed to the fingers and hand areas for arterialized sampling of BG every 5 minutes and epinephrine every 10 minutes. Euglycemia, with a plasma glucose goal of 6.1 mmol/L, was achieved and maintained using variable dextrose infusion. After glucose and insulin stabilization, the subjects performed the first brief 30 minutes of neurocognitive testing. This was a rehearsal/practice trial not used for data analysis. Subsequently, dextrose infusion was slowed or discontinued to ensure a steady descent into hypoglycemia at a BG rate of fall of .056 mmol/dl/min. At a BG of 5 to 5.6 mmol/L, the subject was asked to complete a second brief cognitive battery (Figure 1). Progressive hypoglycemia testing occurred when BG reached 3.9 mmol/L and ended at a BG nadir or 2.5 mmol/L. Once the BG nadir was achieved, BG was returned to euglycemia (5.6 mmol/L and then the final testing occurred.
Figure 1.
Study Design of Acute Neurocognitive Testing on Each Day of GCRC Admission
Neuropsychological Testing. The neurocognitive test battery administered prior to the two-day admission to the GCRC included the following measures: Grooved Pegboard (visual-motor coordination), Wechsler Adult Intelligence Scale-Revised (WAIS-R) Block Design subtest (visuospatial and constructive ability), WAIS-R Digit Symbol subtest (psychomotor speed), Digit Vigilance (rapid visual tracking), Trail Making Test (psychomotor speed, attention, and cognitive flexibility), Serial Subtraction (attention and working memory), Verbal Fluency (rapid word production), Paced Auditory Serial Addition Task (PASAT; divided attention, information processing speed, and working memory), and Stroop Test (selective attention). With the exception of the 2 WAIS-R subtests, all of the neuropsychological measures yield 2 or more scores. The abbreviated test battery administered during acute euglycemia, mild hypoglycemia, and immediately upon recovery from hypoglycemia during GCRC study included Serial Subtraction, Verbal Fluency, PASAT and Stroop Test (Table 2).
Data analysis
Independent samples t-tests were used to evaluate whether + History and −History subjects differed on the neurocognitive test battery administered pre-admission. A repeated measures 3 conditions (euglycemia 5.6 mmol/L vs. hypoglycemia 2.5 mmol/L vs. recovery euglycemia 5.6 mmol/L) X 2 groups (+ History vs. −History group) analysis of variance (ANOVA) was conducted for each of the repeated cognitive tests during GCRC admission to identify differences in subtest performance on the abbreviated neurocognitive test battery among the glycemic conditions and between the two driving risk groups. Given that no between-group differences in age or education were identified, raw scores on the neurocognitive measures were used in the analyses.
Results
Table 2 lists the mean raw scores for each test in the neurocognitive test battery administered the evening before BG manipulation and repeat testing at each glycemic level (euglycemia, hypoglycemia nadir, and recovery euglycemia). For testing the evening before BG manipulation, the +History group demonstrated significantly poorer performance on the second Serial Subtraction subtest (t = 2.22, p = .03) and PASAT 2 sec (t = 2.47, p = .02) than did the −History group. A marginally lower score was also found for the first Serial Subtraction subtest (t = 1.95, p = .06).
Next, ANOVAs were conducted for each of the repeated neurocognitive measures during GCRC admission. For Serial Subtraction, significant main effects were found for condition (F = 19.01, p < .01) and History group (F = 18.81, p < .01); however, the interaction was not significant. Specifically, Serial Subtraction performance was significantly lower for both groups during hypoglycemia, and significantly lower for the +History group across all three conditions when compared to the −History group. Similar results were found for PASAT, with significant main effects found for condition (F = 8.23, p < .01) and History group (F = 6.68, p = .01). PASAT performance was significantly lower for both groups during hypoglycemia, and lower at all conditions for the +History group when compared to the −History group. For Verbal Fluency, a significant main effect was found for condition only (F = 11.99, p < .01), with no significant main effect for History group or an interaction. Likewise, for Stroop, only a main effect was found for condition (F = 13.60, p < .01), with no main effect for History group or an interaction. For the Stroop Test, as well as Verbal Fluency, performance was significantly lower at hypoglycemia for both groups, but no significant between-groups difference was found. There was no significant difference between baseline and recovery performance on any of the repeated neurocognitive measures for either group.
Conclusions
On all four neurocognitive measures repeated at euglycemia, hypoglycemia, and recovery, performance decreased significantly during hypoglycemia, regardless of group. This finding is not surprising, given the numerous studies demonstrating that hypoglycemia is associated with neurocognitive deficits in children and adults with T1DM in the literature (11,12). One finding of the present study that was unique is that it demonstrated a return to baseline neurocognitive functioning immediately upon return of BG to euglycemia, regardless of driving history group. It is widely believed, with some empirical support in the literature, that recovery of neurocognitive functioning following hypoglycemia is a slow and gradual process, possibly taking up to 1.5 days (13, 14). The only other study to our knowledge that also demonstrates a return to baseline functioning upon return to euglycemia employed an admittedly simple cognitive task assessing selective attention (15). The present study provides preliminary evidence that individuals with type 1 diabetes may rapidly recover higher-level executive functions, such as cognitive flexibility and working memory, immediately after returning to euglycemia following hypoglycemia, when the hypoglycemia conditions is brief.
With regard to between-group differences and implications for driving behavior, subjects with T1DM who were considered to be at high-risk of driving mishaps (+ History) consistently demonstrated poorer performance compared to the lower risk group (− History) on the Serial Subtractions and PASAT, regardless of their glycemic condition (i.e., at pre-admission and during euglycemia, nadir, and recovery from hypoglycemia). None of the other neurocognitive tests showed significant group differences pre-admission or during BG manipulation. What distinguishes the Serial Subtraction and PASAT tests from the remainder of the battery is that these tasks assess working memory, the ability to temporarily store and mentally manipulate information. In these neurocognitive tasks, participants were required to remember auditorily-presented information and quickly perform simple mental arithmetic problems based on temporarily stored bits of numeric information. While hypoglycemia has been associated with working memory impairment, (12), to our knowledge, this study is the first to identify a potential neurocognitive indicator that may differentiate adults with T1DM with and without a history of driving mishaps.
It is unclear why individuals with T1DM who have a history of two or more driving mishaps over the last two years demonstrate poorer working memory, even during euglycemia. The groups did not differ on any demographic, diabetes or driving variables, except for a recent history of severe hypoglycemia and hypoglycemia driving mishaps. One possible explanation is that the +History group has a greater absolute deficit in working memory based on the fact that they demonstrated poorer performance on the above-mentioned measures before the hyper-insulinemic clamping procedure when compared to the −History group. If this is the case, it may be that this absolute deficit in working memory exceeds a threshold during hypoglycemia that is essential for safe driving. The findings do not suggest a general deficit that could be attributable to neuropathy, retinopathy or other complications of diabetes, given that the + and − History groups did not differ on other neurocognitive measures.
While the role of working memory in driving performance has not been studied extensively, one study examining left turn performance at intersections in a simulated driving task found that working memory is associated with the ability to successfully judge and choose gaps in oncoming traffic prior to making a left turn (16). Interestingly, greater working memory performance was associated with longer decision time in this study, which the authors suggest may reflect the tendency of individuals with better working memory ability to allow more time to gather relevant information before deciding to proceed through an intersection. In contrast, the authors speculate that individuals with poorer working memory are less able to hold and process all relevant information, and therefore may make more hurried decisions to execute a left turn. This study highlights the process by which working memory may mediate driving performance, as well as a specific driving domain (left turn performance) on which to focus in future research examining working memory and driving performance in T1DM individuals with and without driving mishaps. Future studies should also attempt to replicate and extend the present study findings to identify specific neurocognitive indicators of driving performance aimed at identifying T1DM individuals at high risk for future driving mishaps.
While the present study provides only preliminary evidence that performance on working memory measures may be used to identify T1DM drivers at higher risk for future driving mishaps, the relevance of this area of study to clinical practice and public health is readily apparent. If specific neurocognitive predictors of driving risk in individuals with T1DM can be established, brief evidence-based neurocognitive evaluations can be used to identify those at risk of driving mishaps, possibly even early on in their driving careers, and provide targeted interventions aimed at reducing future driving risk. Given that increasingly more evidence is indicating that only a subgroup of individuals with T1DM is at higher risk for driving mishaps, the existing social stigma of driving with diabetes may be reduced, and existing driving restrictions for individuals with T1DM may be refined.
Table 3.
Brief neuropsychological test results during euglycemia, hypoglycemia and recovery
| Hx | Euglycemia | Hypoglycemia | Recovery | BG* p | Group** p | |
|---|---|---|---|---|---|---|
| Pre-Post BG (mmol/L) | − | 5.6-±0.5 | 2.8±0.3 | 5.7±0.4 | .001 | .89 |
| + | 5.5±0.3 | 3.0–0.9 | 5.6±0.5 | .001 | Ns.91 | |
| Tests | Hx | Euglycemia | Hypoglycemia | Recovery | BG* p | Group** p |
| Serial subtraction | − | 24.7±8.7 | 18.3±5.2 | 26.1±7.9 | .000 | .000 |
| + | 20.3±9.6 | 14.2 ±6.7 | 21.1±9.0 | |||
| Verbal Fluency | − | 10.6±3.2 | 8.4±2.4 | 10.3±3.3 | .000 | .27 |
| + | 10.4±2.2 | 8.3±3.3 | 10.3±3.3 | |||
| PASAT, 2 | − | 14.4±3.1 | 12.7±4.1 | 14.8±2.8 | .000 | .009 |
| + | 13.6±3.3 | 10.8±4.1 | 14.8±3.3 | |||
| Stroop | − | 67.2±12.9 | 55.5±16.3 | 68.4±15.1 | .000 | .2 |
| + | 63.7±13.4 | 55.5±13.0 | 64.6±14.8 |
p-values of ANOVA examining the change in neurocognitive performance across the three glycemic conditions during the clamping procedure
p-values of between-group differences on the neurocognitive tests across the three glycemic conditions during the clamping procedure.
Acknowledgments
This research was supported by NIH grants DK28288 and RR00847.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peden M, Scurfield R, Sleet D, Mohan D, Hyder AA, Jarawan E, et al. World report on road traffic injury prevention. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 2.Cox DJ, Penberthy JK, Zrebiec J, Weinger K, Aikens J, Stetson B, et al. Diabetes and driving: International survey of frequency and correlates. Diabetes Care. 2003;26:2329–2334. doi: 10.2337/diacare.26.8.2329. [DOI] [PubMed] [Google Scholar]
- 3.Cox DJ, Ford D, Gonder-Frederick LA, Clark WL, Mazze R, Weinger K, et al. Driving Mishaps Among Individuals with Type 1 Diabetes Mellitus: A Prospective Study. Diabetes Care. 32:2177–2180. doi: 10.2337/dc08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Assessment of risk for severe hypoglycemia among adults with IDDM. Diabetes Care. 1998;21:1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 5.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Clarke WL. Physiological and performance differences between drivers with Type 1 Diabetes Mellitus (T1DM) with and without a recent history of driving mishaps: An exploratory study. Can J Diabetes. 2003;27:23–29. [Google Scholar]
- 6.Cox DJ, Kovatchev BK, Vandecar K, Gonder-Frederick L, Ritterband R, Clarke W. Hypoglycemia Preceding Fatal Car Collisions. Diabetes Care. 2006;29:467–468. doi: 10.2337/diacare.29.02.06.dc05-1836. [DOI] [PubMed] [Google Scholar]
- 7.Cox DJ, Gonder-Frederick LA, Clarke WL. Driving decrements in type I diabetes during moderate hypoglycemia. Diabetes. 1993;42:239–243. doi: 10.2337/diab.42.2.239. [DOI] [PubMed] [Google Scholar]
- 8.Cox DJ, Kovatchev BP, Anderson SM, Clarke WL, Gonder-Frederick LA. Type 1 diabetic drivers with and without a history of recurrent hypoglycemia-related driving mishaps: Physiological and performance differences during euglycemia and the induction of hypoglycemia. Diabetes Care. doi: 10.2337/dc09-2130. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D. To drive or not to drive: That is the decision. J Amer Med Assoc. 1999;282:750–754. doi: 10.1001/jama.282.8.750. [DOI] [PubMed] [Google Scholar]
- 10.Brands AMA, Biessels GJ, De Haan EHF, Kappelle LJ, Kessels RPC. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 11.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles in children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 12.Sommerfield AJ, Deary IJ, McAuley V, Frier BM. Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2003;26:390–396. doi: 10.2337/diacare.26.2.390. [DOI] [PubMed] [Google Scholar]
- 13.Strachan MWJ, Deary IJ, Ewing FME, Frier BM. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23:305–312. doi: 10.2337/diacare.23.3.305. [DOI] [PubMed] [Google Scholar]
- 14.Zammitt NN, Warren RE, Deary IJ, Frier BM. Delayed recovery of cognitive function following hypoglycemia in adults with type 1 diabetes. Diabetes. 2008;57:732–736. doi: 10.2337/db07-0695. [DOI] [PubMed] [Google Scholar]
- 15.Lobmann R, Smid HGOM, Pottag D, Wagner K, Heinze HJ, Lehnert H. Impairment and recovery of elementary cognitive function induced by hypoglycemia in type 1 diabetic patients and healthy controls. Journal of Clinical Endocrinology and Metabolism. 2000;85:2758–2766. doi: 10.1210/jcem.85.8.6737. [DOI] [PubMed] [Google Scholar]
- 16.Guerrier JH, Manivannan P, Nair SN. The role of working memory, field dependence, visual search, and reaction time in the left turn performance of older female drivers. Applied Ergonomics. 1999;30:109–119. doi: 10.1016/s0003-6870(98)00017-9. [DOI] [PubMed] [Google Scholar]