Abstract
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and reading disability (RD) are complex childhood disorders that frequently co-occur, but the etiology of this comorbidity remains unknown.
Method
Participants were 457 twin pairs from the Colorado Learning Disabilities Research Center (CLDRC) twin study, an ongoing study of the etiology of RD, ADHD, and related disorders. Phenotypic analyses compared groups with and without RD and ADHD on composite measures of six cognitive domains. Twin analyses were then used to test the etiology of the relations between the disorders and any cognitive weaknesses.
Results
Phenotypic analyses supported the hypothesis that both RD and ADHD arise from multiple cognitive deficits rather than a single primary cognitive deficit. RD was associated independently with weaknesses on measures of phoneme awareness, verbal reasoning, and working memory, whereas ADHD was independently associated with a heritable weakness in inhibitory control. RD and ADHD share a common cognitive deficit in processing speed, and twin analyses indicated that this shared weakness is primarily due to common genetic influences that increase susceptibility to both disorders.
Conclusions
Individual differences in processing speed are influenced by genes that also increase risk for RD, ADHD, and their comorbidity. These results suggest that processing speed measures may be useful for future molecular genetic studies of the etiology of comorbidity between RD and ADHD.
Keywords: ADHD, reading disability, executive functions, comorbidity, genetics
1. Introduction
During the latter half of the twentieth century, conceptual models of complex disorders such as reading disability (RD) and attention-deficit / hyperactivity disorder (ADHD) typically implicated linear causal pathways in which a single genetic or environmental risk factor led to a single neurocognitive deficit that provided a necessary and sufficient explanation of all of the symptoms of the disorder. Models that proposed a 1:1 relation between a specific etiology, a specific neuropsychological dysfunction, and a categorical disorder worked well for conditions that were caused by a single gene, such as Huntington's Disease and phenylketonuria (although even in these examples the etiology is far more complex than was initially understood). In contrast, an increasing literature suggests that these models do not provide a satisfactory explanation for most complex disorders.
Pennington (2006) recently summarized the arguments against single-deficit models for complex disorders. Molecular genetic risk factors first identified for RD or ADHD have replicated in some studies but not others, and the observed effect sizes of these risk factors are too small to be a single risk factor that is sufficient to account for all cases of the disorder by itself (e.g., Bates, Luciano, Castles, Coltheart, Wright, and Martin 2007; Cardon, Smith, Fulker, Kimberling, Pennington, and DeFries 1994; Cardon, Smith, Fulker, Kimberling, Pennington, and DeFries 1995; Curran et al. 2001; Fisher and DeFries 2002; McGrath, Smith, and Pennington 2006). In addition, subsequent studies also identified several other genetic and neuropsychological risk factors for each disorder (e.g., Fisher and DeFries 2002; Gizer, Ficks, and Waldman 2009; Willcutt 2008; Zhou et al. 2008), providing additional evidence against single-deficit models for RD or ADHD.
Another important criticism of single-deficit models is especially germane to the current paper. Models that propose a single cognitive dysfunction that is unique to each disorder cannot easily account for the pervasive comorbidity between different disorders. For example, although RD and ADHD each occur in approximately 5% of children in the population, 25-40% of children with either RD or ADHD also meet criteria for the other disorder (e.g., August and Garfinkel 1990; Semrud-Clikeman, Biederman, Sprich-Buckminster, Lehman, Faraone, and Norman 1992; Willcutt and Pennington 2000). Similarly, studies of dimensional measures of reading and ADHD symptoms report significant correlations that are low to moderate in magnitude (r = .2 - .5; Bauermeister et al. 2005; Nigg, Hinshaw, Carte, and Treuting 1998; Willcutt et al. 2001; Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005).
Taken together, these converging results have precipitated a major reconceptualization of theoretical models of RD, ADHD, and other complex disorders. Rather than attempting to identify a single necessary and sufficient cause that is specific to each disorder, more recent theoretical models explicitly hypothesize that complex disorders are heterogeneous conditions that arise from the additive and interactive effects of multiple genetic and environmental risk factors to lead to weaknesses in multiple cognitive domains (Pennington 2006; Sonuga-Barke, Sergeant, Nigg, and Willcutt 2008; Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008). In this paper we use neuropsychological and behavior genetic methods to test multiple-deficit models of RD and ADHD, then test if a subset of cognitive weaknesses may increase susceptibility to both disorders, leading to comorbidity.
1.1. Causes of Comorbidity between RD and ADHD
Over 20 different theoretical models have been proposed to explain why comorbidity occurs between complex disorders (e.g., Angold, Costello, and Erkanli 1999; Neale and Kendler 1995), and a number of these hypotheses have been tested as explanations for comorbidity of RD and ADHD. Before attempting to understand the etiology of comorbidity between disorders, it is important to rule out the possibility that the observed comorbidity is an artifact caused by a biased sampling procedure or measurement problem. For example, artifactual comorbidity could occur due to ascertainment biases in clinic-referred samples, rater biases or shared method variance in the measures used to define the disorders, or symptom overlap between the disorders.
Most artifactual hypotheses can be rejected as explanations for comorbidity between RD and ADHD based on existing data. RD and ADHD co-occur more frequently than expected by chance in samples ascertained from clinics (e.g., Semrud-Clikeman, Biederman, Sprich-Buckminster, Lehman, Faraone, and Norman 1992) and non-referred samples recruited from the community (e.g., Fergusson and Horwood 1992; Willcutt and Pennington 2000), indicating that this comorbidity is not restricted to clinic-referred samples. The relation between RD and ADHD cannot be explained by shared method variance because RD is assessed by cognitive tests whereas ADHD is assessed by behavioral ratings, and the symptoms that define RD and ADHD do not overlap (American Psychiatric Association 2000).
The rater-bias hypothesis is somewhat more difficult to test, and the possibility remains that parents or teachers may be more likely to endorse ADHD symptoms if they know that the child is experiencing difficulty with reading. However, two of our results argue against this possibility. In unselected samples of twins attending preschool in the United States, Australia, and Scandinavia, parent and teacher ratings of ADHD symptoms were significantly correlated with pre-reading skills prior to the initiation of formal reading instruction, suggesting that these ratings were not biased by any overt reading difficulties exhibited by the child (Willcutt et al. 2007). The second study examined ratings of attention problems by parents, teachers, and children with RD themselves, and found that all three raters reported that children with RD experienced greater difficulties with attention than children without RD (Willcutt 2008). Although the rater-bias hypothesis cannot be conclusively rejected based on these results, these data suggest that it is not likely to explain most cases of comorbidity between RD and ADHD. Therefore, we turn next to behavior genetic methods that have been used to test if comorbidity between RD and ADHD is due to shared genetic or environmental influences that increase risk for both disorders.
1.2. Behavior genetic approaches to comorbidity
Behavior genetic studies provide a versatile and powerful approach to examine the etiology of individual disorders and their comorbidity. Although the specific etiological mechanisms that lead to RD and ADHD are still unknown, significant advances have been made in understanding the extent to which these difficulties are attributable to genetic or environmental influences. In this section we briefly describe behavioral genetic methods and summarize studies that have applied these approaches to understand the etiology of RD, ADHD, and their co-occurrence.
1.2.1. Family Studies
Previous studies clearly demonstrate that both RD and ADHD are familial (DeFries, Singer, Foch, and Lewitter 1978; Faraone, Biederman, and Friedman 2000; Finucci and Childs 1983; Friedman, Chhabildas, Budhiraja, W illcutt, and Pennington 2003). The relative risk for RD is 4 - 8 times higher in first-degree relatives of probands with RD than in relatives of individuals without RD, and the relative risk for ADHD is 6 - 8 times higher in biological family members of probands with ADHD. Similarly, studies of unselected samples indicate that correlations between biological family members are moderate to high for measures of reading (r = .40 - .70; e.g., Bates, Castles, Luciano, Wright, Coltheart, and Martin 2007; Byrne et al. 2002; Byrne et al. 2007; Harlaar, Spinath, Dale, and Plomin 2005; Petrill, Deater-Deckard, Thompson, Schatschneider, Dethorne, and Vandenbergh 2007; Wadsworth, Corley, Hewitt, Plomin, and DeFries 2002) and moderate for measures of individual differences in attention and activity level (r = .20 -.50; McLoughlin, Ronald, Kuntsi, Asherson, and Plomin 2007; Rietveld, Hudziak, Bartels, van Beijsterveldt, and Boomsma 2003; Willcutt et al. 2007).
Family studies also provide important information regarding the etiology of comorbidity. If common familial risk factors increase risk for both disorders, family members of probands with RD should be more likely to meet criteria for ADHD, and vice versa. Although not all studies find evidence of shared familial influences on learning disabilities and ADHD (Doyle, Faraone, DuPre, and Biederman 2001; Faraone et al. 1993), results from the sample used for the current analyses suggest that family members of probands with RD or ADHD alone are 2 - 3 times more likely to meet criteria for the other disorder than family members of comparison probands without either disorder (Friedman, Chhabildas, Budhiraja, Willcutt, and Pennington 2003).
Taken together, these studies provide tentative support for the hypothesis that shared familial influences may contribute to comorbidity between these disorders. Significant co-familiality suggests that RD and ADHD may be attributable to common genetic influences, but family studies cannot provide conclusive evidence. Because members of biological families living in the same home share both genetic and family environmental influences, other approaches such as twin studies are necessary to disentangle the relative contributions of genes and environment.
1.2.2. Twin studies
1.2.2.1. Concordance rates
By comparing the similarity of monozygotic (MZ) twins, who share all of their genes, to dizygotic (DZ) twins, who share half of their segregating genes on average, twin analyses provide estimates of the extent to which a trait or disorder is due to genetic or environmental influences (e.g., Plomin, DeFries, McClearn, and McGuffin 2008). The most straightforward test for genetic influences on a categorical disorder is a comparison of the rate of concordance in pairs of MZ versus DZ twins. If the disorder is influenced by genes, the proportion of pairs in which both twins meet criteria for the disorder will be higher in MZ pairs than DZ pairs. Virtually all previous twin studies reported higher concordance in MZ twin pairs versus DZ twin pairs for both RD and ADHD (Bakwin 1973; Goodman and Stevenson 1989; Harlaar, Spinath, Dale, and Plomin 2005; Hawke, Wadsworth, and DeFries 2006; Levy, Hay, McStephen, Wood, and Waldman 1997; Sherman, McGue, and Iacono 1997; e.g., Thapar, Harrington, and McGuffin 2001; Todd et al. 2001; Willcutt, Pennington, and DeFries 2000a).
1.2.2.2. Dimensional approaches
Although the simplicity of a comparison of concordance rates is appealing, increasing evidence suggests that RD, ADHD, and most other complex disorders are defined by a diagnostic threshold imposed upon a continuous distribution of liability (Shaywitz, Escobar, Shaywitz, Fletcher, and Makuch 1992; e.g., Willcutt, Pennington, and DeFries 2000a). Transformation of a continuous measure such as reading performance or ADHD symptoms into a categorical variable (e.g., RD or ADHD versus unaffected) results in the loss of important information regarding both severity differences among individuals with the disorder and variability in subthreshold symptomatology. This methodological issue is especially critical for ADHD because the DSM-IV diagnostic criteria for ADHD include two separate symptom dimensions characterized by inattention versus hyperactivity and impulsivity, and correlations with reading achievement and more general academic difficulties are significantly higher with the inattention symptom dimension than the hyperactivity-impulsivity symptom dimension (Chhabildas, Pennington, and Willcutt 2001; Lahey and W Pelham 2001; Willcutt and Pennington 2000; W Dillcutt 2002; Molina, Smith, and olraich, Feurer, Hannah, Baumgaertel, and Pinnock 1998).
To facilitate the use of dimensional measures in twin studies, quantitative genetic methods were developed for analyses of the etiology of individual differences in the population (Neale, Boker, Xie, and Maes 2002) and clinically significant extreme scores on a continuous dimension of liability (e.g., DeFries and Fulker 1985; DeFries and Fulker 1988). Basic twin models estimate three parameters. Heritability is the proportion of the total phenotypic variance in a trait that is attributable to genetic influences. Shared environmental influences family in comparison to unrelated individuals in the population. These effects may potentially include environmental influences are environmental factors that increase the similarity of individuals within a family in comparison to unrelated individuals in the population. These effects may potentially include environmental influences within the home or any other shared experiences such as mutual friends or shared teachers. In contrast, nonshared environmental influences are environmental factors that that are independent or unique for members of twin pairs. These risk factors could include a head injury or other accident, a traumatic event, or exposure to physical or sexual abuse (if the other twin was not similarly exposed).
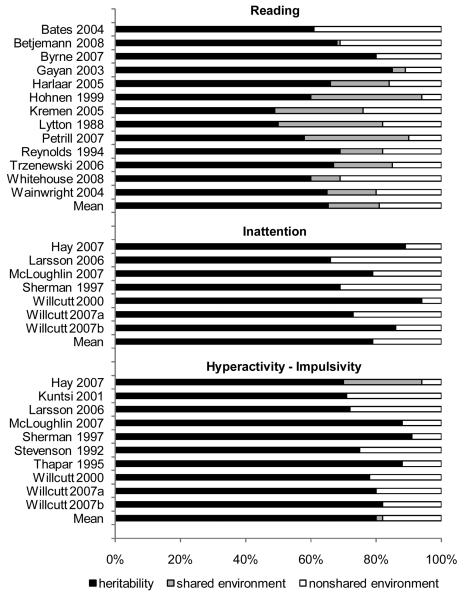
Figure 1 summarizes previous twin studies of dimensional measures of reading, inattention, and hyperactivity-impulsivity. Estimates of heritability are moderate to high for individual differences in single-word reading, inattention, and hyperactivity-impulsivity, and extreme scores on each of these measures are also significantly heritable (e.g., Harlaar, Spinath, Dale, and Plomin 2005; Levy, Hay, McStephen, Wood, and Waldman 1997; Stevenson 1992; Willcutt, Pennington, and DeFries 2000b; Willcutt, Pennington, Olson, and DeFries 2007). Shared environmental influences account for an additional 10 - 15% of the variance in reading, but are not significant in most studies of ADHD, and the remaining 20 - 25% of the variance in each phenotype is explained by nonshared environmental influences and measurement error.
Figure 1.
Twin studies of reading, inattention, and hyperactivity-impulsivity.
1.2.2.3. Twin studies of comorbidity between RD and ADHD
Based on the consistent finding that both individual differences and extreme scores on measures of ADHD and RD are highly heritable, several twin studies tested if comorbidity between RD and ADHD was explained by common genetic influences. Initial results were somewhat inconsistent, but generally suggested that comorbidity between RD and ADHD was at least partially explained by common genetic influences (e.g., Light, Pennington, Gilger, and DeFries 1995; Stevenson, Pennington, Gilger, DeFries, and Gillis 1993; Trzesniewski, Moffitt, Caspi, Taylor, and Maughan 2006). Subsequent studies clarified these results by conducting separate analyses of inattention and hyperactivity-impulsivity. These analyses indicated that common genetic influences accounted for most of the phenotypic covariance between reading difficulties and inattention, whereas common genetic influences were lower for reading and hyperactivity-impulsivity (Willcutt et al. 2007; Willcutt, Pennington, and DeFries 2000b; Willcutt, Pennington, Olson, and DeFries 2007). In addition to these shared genetic influences, individual differences in all three measures were also attributable to independent genetic and environmental influences.
1.3. Neuropsychological approaches to comorbidity
The partial genetic overlap between RD and ADHD suggests that there may also be partial overlap between the disorders at the cognitive level of analysis. For example, a shared genetic risk factor may lead to a specific neuropsychological weakness that increases susceptibility to both disorders. In this section we briefly summarize the extensive neuropsychological literatures on RD and ADHD, then describe results of studies that tested which cognitive risk factors are unique to RD or ADHD and which are plausible candidates for shared risk factors that affect both disorders.
1.3.1. Neuropsychology of RD
Studies of individuals with and without reading difficulties suggest that phonological decoding, defined as the ability to translate sequences of printed letters into the corresponding sounds, plays a central role in both normal and abnormal reading development (Pennington 2002; Vellutino, Fletcher, Snowling, and Scanlon 2004; Wagner 1986; Wagner et al. 1997). The unique contribution of phonological decoding (PD) to most cases of RD has been suggested by the presence of significant group deficits in PD when older children with RD are compared to younger readers without reading disability who are reading at the same level (Olson 1985; Rack, Snowling, and Olson 1992). Moreover, twin studies have shown that there are strong genetic influences on PD that also influence word reading (Bates, Castles, Luciano, Wright, Coltheart, and Martin 2007; Gayan and Olson 2001; Olson, Forsberg, and Wise 1994; Olson, Wise, Conners, Rack, and Fulker 1989; Petrill, Deater-Deckard, Thompson, Schatschneider, Dethorne, and Vandenbergh 2007). Deficits in PD and word reading are in turn linked to genetic influences on the oral language skill of phoneme awareness, defined as the ability to recognize and manipulate the phonemic constituents of speech (Gayan and Olson 2001; Olson, Forsberg, and Wise 1994). Problems with phoneme awareness are regarded by many as the most proximal cause of most cases of RD (c.f., Wagner, Torgesen, and Rashotte 1994).
In addition to the well-documented relation between reading difficulties and phonological processing, recent studies suggest that individuals with RD also have weaknesses in several other cognitive domains (Pennington 2006; Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008). These weaknesses include difficulty accessing the orthographic representation of words from the lexicon (Gayan and Olson 2001), weaknesses in other areas of speech and language processing (Olson 1994; e.g., Pisecco, Baker, Silva, and Brooke 2001), slower verbal naming speed and general processing speed (e.g., Compton, DeFries, and Olson 2001; Denckla and Rudel 1976; Shanahan et al. 2006; Tannock, Martinussen, and Frijters 2000), and weaknesses in executive domains such as verbal working memory, planning, and response inhibition (Klorman et al. 1999; Purvis and Tannock 2000; Roodenrys, Koloski, and Grainger 2001; Swanson, Mink, and Bocian 1999; Willcutt et al. 2001; Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005). Therefore, although phonological processing difficulties explain more variance in reading than any other cognitive dysfunction, these results provide support for a multiple-deficit cognitive model of RD in at least some cases.
1.3.2. Neuropsychology of ADHD
A large body of research suggests that the neuropsychology of ADHD may be even more complex. Groups with ADHD differ significantly from groups without ADHD on a wide range of measures, with the most consistent group differences on measures of processing speed, response variability, and executive functions such as working memory, response inhibition, and planning (see reviews by Barkley 1997; Nigg 2001; Nigg 2006; Pennington 2002; Pennington and Ozonoff 1996; Willcutt, Doyle, Nigg, Faraone, and Pennington 2005; Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008). Meta-analyses indicate that each of these weaknesses has a small to medium effect size, and none is necessary or sufficient to cause ADHD in isolation. These data suggest that a single core deficit in ADHD is unlikely to be found, and that ADHD is also best described by a multiple-deficit neuropsychological model (Pennington 2006; Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008).
1.3.3. Multivariate analyses to test cognitive explanations of comorbidity
We recently conducted a systematic meta-analysis of all published neuropsychological studies of childhood disorders to identify cognitive risk factors that might explain comorbidity between RD, ADHD, and other complex disorders (Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008).
The results of the review and a series of empirical studies all suggested that the strongest candidates for a shared cognitive weakness in RD and ADHD were processing speed, response variability, and verbal working memory (e.g., Rucklidge and Tannock 2002; Shanahan et al. 2006; Willcutt et al. 2001; Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005). In addition, several studies unexpectedly found deficits in response inhibition in groups with RD (e.g., Purvis and Tannock 2000; Willcutt et al. 2001), suggesting that additional research is needed to clarify the nature of this association.
Based on these results, McGrath et al. (under review) conducted multivariate analyses of the extensive battery of cognitive tests administered as part of the Colorado Learning Disabilities Research Center (CLDRC) twin study. The goal of these analyses was to test which neuropsychological processes were associated with both reading difficulties and ADHD symptoms, and which were specific to each disorder. Confirmatory factor analyses (CFA) supported a model that included 6 latent factors that were labeled verbal reasoning, phoneme awareness, processing speed, naming speed, working memory, and response inhibition. The authors then fitted a structural equation model in which the six cognitive factors predicted latent measures of word reading, inattention, and hyperactivity-impulsivity. The most parsimonious model included phoneme awareness and verbal reasoning as unique predictors of word reading, and response inhibition as a unique predictor of inattention and hyperactivity-impulsivity. Processing speed, naming speed, and working memory were modeled as potential shared cognitive deficits. Of these potential shared deficits, processing speed was the only measure to predict all three symptom dimensions.
1.4. The present study
The present study builds on the results of McGrath et al. (under review) by testing the genetic and environmental etiology of scores on composite measures of word reading, inattention, hyperactivity-impulsivity, and the six cognitive composites. The primary goals of the study were as follows:
Zero-order correlations were calculated between all pairs of composites to provide an overview of the phenotypic associations among the variables. Multiple logistic regression analyses were then conducted to test which neuropsychological composites independently predicted the diagnoses of RD and ADHD, and whether any of the cognitive variables significantly predicted both disorders.
Univariate twin analyses were conducted to test the etiology of individual differences and extreme scores on each of the diagnostic measures and neuropsychological composites. We hypothesized that all measures would be significantly heritable, but that shared environmental influences would only be significant for the measures of reading and the six cognitive domains.
Multivariate twin analyses were used to estimate the extent to which RD, ADHD, and the neurocognitive composites are attributable to common or unique genetic and environmental influences. In the final step of the multivariate analyses, Cholesky decomposition models were fitted to test if genetic or environmental influences on one of the cognitive composites could account for comorbidity between RD and ADHD. Based on our previous results (e.g., Shanahan et al., 2006; McGrath et al., under review), we predicted that these analyses would reveal that genetic influences on processing speed explain at least a portion of the phenotypic covariance between RD and ADHD.
2. Method
2.1. Participants
Participants were 244 MZ twin pairs (112 male, 132 female) and 213 same-sex DZ twin pairs (104 male, 109 female) from the Colorado Learning Disabilities Research Center (CLDRC) twin study, an ongoing study of the etiology of reading disabilities, ADHD, and related disorders (e.g., DeFries et al. 1997). Because recruitment and testing procedures for the study are described in detail elsewhere (e.g., Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005), we provide an abbreviated summary here.
2.1.1. Screening procedures
Without regard to reading or ADHD status, permission was sought from parents of all twin pairs between 8 and 18 years of age in 22 local school districts to review the school records of both members of each pair for evidence of reading problems. In addition, parents and teachers were asked to complete ratings of DSM-IV ADHD symptoms on the Disruptive Behavior Rating Scale (Barkley and Murphy 1998). If either member of a twin pair had a history of reading difficulties or met screening criteria for ADHD, the pair and any siblings between 8 and 18 years of age were invited to participate in the full study. A comparison group of control twins were selected from the overall sample of pairs who did not meet the screening criteria for RD or ADHD. Because the primary focus of the CLDRC is the etiology of RD and ADHD, pairs at risk for one or both disorders were oversampled (approximately 65% of the final tested sample) to increase statistical power for analyses of these extreme groups.
2.1.2. Exclusion criteria
CLDRC staff conducted a telephone screening interview prior to any testing. Potential participants with a documented brain injury, significant hearing or visual impairment, or other rare genetic or environmental etiology (e.g., Fragile X syndrome, Down syndrome or other sex chromosome anomalies) were excluded from the sample. Pairs were also excluded if one of the twins had received a diagnosis of autism, psychosis, bipolar disorder, or pervasive developmental disorder, and three participants were excluded from analyses due to a Full Scale IQ score below 75 on the Wechsler Intelligence Scale for Children, Revised (Wechsler 1974).
2.1.3. Final sample
Because the goal of this paper is to examine the etiology and neuropsychology of comorbidity between reading difficulties and DSM-IV ADHD, analyses were restricted to same-sex twin pairs for whom a measure of DSM-IV ADHD was completed. The zygosity of each pair was determined based on selected items from the Nichols and Bilbro (1966) questionnaire, and cases with ambiguous zygosity were confirmed by analysis of a panel of DNA markers.
2.2. Procedures
Measures of general cognitive ability, processing speed, and component reading and language skills were administered in two initial testing sessions at the University of Colorado Department of Psychology and Institute for Behavioral Genetics. EF tasks were completed during a third session scheduled approximately one month later at the University of Denver Department of Psychology. The order of the tests was counterbalanced in each of the testing sessions. Each session lasted approximately two and one-half hours, and frequent breaks were provided to minimize fatigue and maximize motivation.
Measures at all sites were administered by trained examiners who had previous experience working with children. All examiners were unaware of the diagnostic status of the child and the results of the testing conducted at the other sites. Parents of participants who were taking psychostimulant medication were asked to withhold medication for 24 hours prior to each session of the study.
2.3. Diagnostic measures
2.3.1. Word reading
Consistent with the procedures used by McGrath et al. (under review), a composite word reading measure was created by calculating the mean of standardized, age-regressed scores on the PIAT Reading Recognition and Spelling subtests (Dunn and Markwardt 1970) and a time-limited word recognition test (Olson, Forsberg, Wise, and Rack 1994). For categorical analyses RD was defined by a cutoff score 1.25 SD below the estimated population mean on the reading composite.
2.3.2. ADHD symptom dimensions
Parents and teachers completed the DBRS, a widely-used rating scale that asks the respondent to indicate on a four point scale (never or rarely, sometimes, often, and very often) the frequency that the child exhibits each DSM-IV ADHD symptom. Composite inattention and hyperactivity-impulsivity scores are the mean of standardized, age-regressed DBRS ratings by the mother, father, and teacher. To define ADHD status for categorical phenotypic analyses parent and teacher ratings were combined with the algorithm used in the DSM-IV field trials for the disruptive behavior disorders (Lahey et al. 1994) and our previous analyses of this sample (Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005; Willcutt, Pennington, Olson, and DeFries 2007).
2.4. Neuropsychological Measures
The extensive neuropsychological test battery used for these analyses included the 28 measures that loaded on the six latent traits identified by McGrath et al. (under review). Cognitive composite scores were created by calculating the mean of the age-regressed standardized scores on the measures that loaded on each latent trait. Due to space constraints it is not possible to describe all of the cognitive measures in detail. An abbreviated description of each task is provided in Table 1, along with the estimated reliability of the task and its primary loading in the CFA. The original source for each measure is provided in the notes for Table 1, and all measures are described in detail in previous papers by our group (Shanahan et al. 2006; e.g., Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005).
Table 1.
Measures
| Measurea | Reliabilityb | CFA Loadingc |
Brief Descriptiond |
|---|---|---|---|
| Single Word Reading | |||
| PIAT Reading Recognition6 | .89 | .92 | Read single words that increase in semantic and phonetic difficulty (untimed). |
| PIAT Spelling6 | .65 | .83 | Select the correct spelling of real words from four phonologically similar options. |
| Timed Oral Reading14 | .89 | .95 | Read aloud single words within two seconds of their presentation. |
| DSM-IV Inattention | |||
| DBRS mother and father report1 | .75 - .93d | .82 - .84 | Mother, father, and teacher ratings of DSM-IV hyperactivity - impulsivity symptoms on a |
| DBRS teacher report1 | .71 - .94d | .57 | 0 - 3 scale with anchors not at all, sometimes, often, and very often. |
| DSM-IV Hyperactivity - impulsivity | |||
| DBRS mother and father report1 | .77 - .91d | .75 - .84 | Mother, father, and teacher ratings of DSM-IV inattention symptoms on a 0 - 3 scale |
| DBRS teacher report1 | .66 - .93d | .46 | with anchors not at all, sometimes, often, and very often. |
| Phoneme Awareness | |||
| Phoneme Deletion13 | .80 | .81 - .90 | Remove a phoneme from a nonword or real word and say the resulting word. |
| Pig Latin14 | .78 | .80 | Move first phoneme of a spoken word to the end of the word, then add the sound “ay”. |
| Lindamood Auditory Concept.11 | .67 | .79 | Use colored blocks to represent phonemes in spoken sound sequences and nonwords. |
| Verbal Reasoning | |||
| WISC-R Information17 | .85 | .83 | Verbal responses to questions that assess general knowledge. |
| WISC-R Similarities17 | .81 | .73 | Explain verbally the conceptual similarity between pairs of words. |
| WISC-R Vocabulary17 | .86 | .85 | Provide verbal definition of words presented by the examiner. |
| WISC-R Comprehension17 | .77 | .68 | Provide verbal responses to questions about general principles and social situations. |
| Working memory | |||
| Nonword Repetition8 | .80 | .67 | Repeat pronouncable nonwords of increasing length presented on a recording. |
| WISC-R Digit Span17 | .78 | .59 - .60 | Repeat a series of numbers presented verbally in order or in reverse order. |
| Sentence Span16 | .71e | .59 | Provide the last word for a set of simple sentences read by the examiner, then reproduce these words in order after the set is completed. |
| Counting Span2 | .67e | .59 | Count aloud the number of yellow dots on a series of cards. At the end of each set state in order the number of yellow dots that appeared on each card in the set. |
| Response inhibition | |||
| CPT commission errors10 | .72 - .85 | .42 - .43 | Total responses to incorrect targets during an 18-minute continuous performance test. |
| Stop-signal Task12 | .90 - .96 | .66 | Computerized measure of stop-signal reaction time, a measure of inhibitory control. |
| Processing Speed | |||
| WISC-III Symbol Search18 | .74 - .85 | .65 | Determine if a symbol matches one of four target stimuli as quickly as possible. |
| WISC-R Coding17 | .72 | .67 | Rapidly copy symbols associated with numbers based on a key at the top of the page. |
| Colorado Perceptual Speed3,4 | .81 | .77 | Identify a target string of letters among three phonetically similar and dissimilar foils. |
| Identical Pictures7 | .82 | .71 | Fast and accurate recognition of a target picture among an array of pictures. |
| Trailmaking Test15 | .66 - .86 | .46 - .47 | Total time to connect a series of circles in ascending order. Part A includes only numbers, and Part B alternates between numbers and letters. |
| Naming Speed | |||
| RAN Color Naming5 | .82 | .74 | The rapid automatized naming task assesses the ability to rapidly produce verbal labels. |
| RAN Number Naming5 | .86 | .58 | On the four test trials the participant names a series of colors, numbers, letters, and |
| RAN Letter Naming5 | .86 | .67 | pictures as quickly as possible in 15 seconds. |
| RAN Picture Naming5 | .80 | .51 | |
| Stroop Color Naming9 | .82 | .77 | Name the color of patches of ink as quickly as possible in 45 seconds. |
Superscript numbers after each task indicate the initial reference for the task:
Olson et al. 1994
Estimated reliability obtained from the original citation unless otherwise noted.
Factor loading on the primary construct in the confirmatory factor analysis described by McGrath et al. (under review).
range includes estimates of 1-year test-retest reliability (Willcutt, Chhabildas, and Pennington 2001) and Cronbach's α.
3. Data analyses
3.1. Data cleaning and transformations
As expected, correlational analyses revealed that performance on all neuropsychological variables improved as a linear function of age (p < .01 for all measures). Therefore, to control for the influence of age, an age-adjusted score was created for each measure by regressing the variable onto age and age-squared and saving the residual score. The distribution of each age-adjusted variable was then assessed for outliers prior to any additional analyses. Outliers were defined as scores that fell more than three standard deviations (SD) from the mean of the overall sample and more than 0.5 SD beyond the next most extreme score. After confirming that these outlying scores were entered correctly in the datafile, each outlier was adjusted to a score 0.5 SD units beyond the next highest score, with multiple outliers rescored to 0.1 SD apart. After these adjustments, the distribution of each variable was assessed for significant deviation from normality. A logarithmic transformation was implemented to approximate a normal distribution for variables with skewness or kurtosis greater than one (parent and teacher ADHD ratings, phoneme deletion, Pig Latin, Trails A and B, CPT commissions errors). After these transformations were completed skewness and kurtosis was less than one for all measures.
Due to the high number of statistical tests needed to examine the relations among the nine composite scores, an alpha of .01 was adopted as the threshold for statistical significance, and p-values between .05 and .01 are described as marginally significant.
3.2. Phenotypic analyses
Because the scores of twins in a pair are not fully independent observations, phenotypic analyses were conducted using the “CLUSTER” option inM-plus (Muthén and Muthén 2009) to obtain standard errors, test statistics, and p-values that are robust to nonindependence. A dummy code for zygosity was included in initial phenotypic models to control for any differences between participants from MZ and DZ pairs, but this code was dropped from the final models because it had no significant impact on any result. Because our sample is enriched for reading difficulties and ADHD, we compared results of phenotypic and behavior genetic analyses run in the control group only, the selected group only, the entire sample, and an estimated population sample in which a random sample was selected to approximate the proportions of each group in the original population prior to enrichment for RD and ADHD. Point estimates were similar across the four analyses, and the overall pattern of results was nearly identical. Therefore, to simplify interpretation we describe results based on analyses of the entire sample. A summary of the results for the other approaches is available from the lead author upon request.
As noted previously, there is ongoing debate regarding the strengths and weaknesses of dimensional versus categorical definitions of RD and ADHD. Therefore, in this paper we conducted both dimensional analyses of individual differences in the entire sample and analyses of extreme scores indicative of reading deficits or clinically significant elevations of ADHD symptoms. For phenotypic analyses we first calculated zero-order correlations between the reading, inattention, and hyperactivity-impulsivity composites and the six cognitive composite measures. We then compared the cognitive profile of categorically defined groups with RD only, ADHD only, RD+ADHD, or neither RD or ADHD, and conducted multiple logistic regression analyses to test which cognitive composites significantly predicted categorical diagnoses of RD, ADHD, or both disorders.
3.3. Etiology of individual differences in the entire sample
Behavior genetic analyses of the full sample were conducted with the Mx statistical modeling package (Neale, Boker, Xie, and Maes 2002). Raw data rather than covariance matrices were analyzed in Mx to allow the inclusion of cases with missing data. Initial univariate models were fitted to estimate the proportion of variance in each composite that is due to genetic influences (h2), shared environmental influences (c2), and nonshared environmental influences (e2). Multivariate analyses were then conducted to test the extent to which the same genetic or environmental influences contribute to covariance between measures. The genetic correlation (rg), shared environmental correlation (rc), and nonshared environmental correlation (re) indicate the extent to which the total genetic or environmental variance of each measure is shared between the two measures. For the final individual differences analysis Cholesky decomposition models were fitted to examine the extent to which shared genetic influences on processing speed could account for comorbidity between reading difficulties and ADHD symptoms (a detailed description of Cholesky analyses in another subset of this sample is provided by Betjemann, Willcutt, Olson, Keenan, DeFries, and Wadsworth 2008).
3.4. Etiology of extreme scores
Although variance components analyses are optimal for analyses of individual differences in unselected or minimally selected samples, this approach is not designed for analyses of extreme groups. In contrast, the multiple regression analysis described by DeFries and Fulker (DF analysis; 1985, 1988) was specifically developed to test the etiology of extreme group membership.
3.4.1. The basic DF model
DF analysis is based on the differential regression of MZ and DZ cotwin scores toward the population mean when probands are selected due to an extreme score on a phenotype of interest. Although scores of both MZ and DZ cotwins are expected to regress toward the population mean, scores of DZ cotwins should regress further than scores of MZ cotwins to the extent that extreme scores are influenced by genes. After appropriate standardization and transformation of scores, the magnitude of differential regression by zygosity provides a direct estimate of the heritability of the extreme group deficit (h2g). To test the etiology of extreme scores on the diagnostic and neuropsychological measures, separate models were fitted to data from each of the nine composite scores. Probands were selected separately for each analysis based on a cutoff score 1.25 SD below the estimated population mean on the measure (all composites were scaled so that lower scores indicated greater impairment).
3.4.2. Bivariate DF analysis
A simple generalization allows the univariate DF multiple regression model to be applied to bivariate twin data to test the etiology of comorbidity between diagnostic groups or the etiology of covariance between each diagnostic measure and other extreme scores (e.g., Light and DeFries 1995; Willcutt, Pennington, Olson, and DeFries 2007). Rather than comparing the relative similarity of MZ and DZ twins on the same trait, the bivariate model compares the relation between the proband's score on the selected trait and the cotwin's score on a second, unselected trait. For example, if common genetic influences contribute to the association between RD and ADHD, the ADHD score of the cotwins of MZ probands with RD would be expected to regress less toward the ADHD population mean than the ADHD score of DZ cotwins. The bivariate multiple regression model provides an estimate of bivariate h2g, an index of the extent to which the proband deficit on the selected measure is due to genetic influences that are also associated with deficits on the unselected measure. The estimates of univariate and bivariate h2g can then be used to estimate the genetic correlation in the selected group (rg[sel]), a measure of the extent to which the genetic influences on each extreme score are common to both scores (e.g., Gayan and Olson 2001).
4. Results
4.1. Phenotypic analyses
4.1.1. Zero-order correlations
All nine composite measures were significantly correlated with all other measures, but the magnitude of the correlations varied significantly (Table 2). As expected, word reading had the highest correlation with phoneme awareness (r = .71). Correlations between word reading and the other five cognitive composites were also significant and medium to large in magnitude (r = .35 - .61), although the correlation between reading and response inhibition was significantly smaller than the correlations with the other four composites. Analyses of the ADHD composites provided further support for the distinction between inattention and hyperactivity-impulsivity. Although both symptom dimensions were significantly correlated with all six cognitive composites, correlations with inattention were significantly higher than correlations with hyperactivity-impulsivity on all six cognitive composites.
Table 2.
Phenotypic correlations (95% confidence interval) between composite measures of reading, ADHD, and neuropsychological functioning
| Word Reading |
Inattention | Hyperactivity - Impulsivity |
Phoneme Awareness |
Verbal Reasoning |
Response Inhibition |
Naming Speed |
Processing Speed |
|
|---|---|---|---|---|---|---|---|---|
| Inattention | .35 (.30, .40) | -- | ||||||
| Hyperactivity - impulsivity | .18 (.13, .23) | .63 (.60, .66) | -- | |||||
| Phoneme Awareness | .71 (.68, .74) | .27 (.22, .32) | .18 (.13, .23) | -- | ||||
| Verbal Reasoning | .61 (.57, .65) | .24 (.19, .29) | .13 (.07, .19) | .53 (.49, .57) | -- | |||
| Response Inhibition | .35 (.30, .40) | .32 (.27, .37) | .23 (.18, .28) | .38 (.33, .43) | .32 (.27, .37) | -- | ||
| Naming Speed | .49 (.45, .53) | .30 (.25, .35) | .10 (.04, .16) | .45 (.40, .50) | .34 (.29, .39) | .30 (.25, .35) | -- | |
| Processing Speed | .56 (.52, .60) | .37 (.32, .42) | .19 (.14, .24) | .46 (.41, .51) | .47 (.43, .51) | .34 (.29, .39) . | 59 (.55, .63) | -- |
| Working Memory | .58 (.54, .62) | .28 (.23, .33) | .18 (.13, .23) | .64 (.61, .67) | .49 (.45, .53) | .33 (.28, .38) . | 42 (.37, .47) | .43 (.38, .48) |
Note. Scores are scaled so that negative scores indicate worse performance. All correlations are significant (P < .01)
4.1.2. Categorical analyses
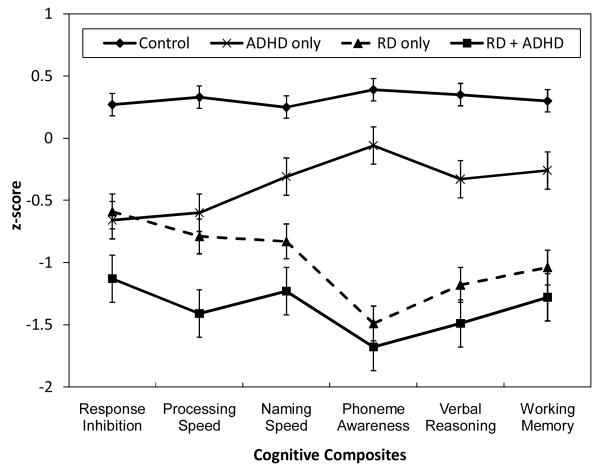
Figure 2 summarizes analyses that compared the performance of groups with RD, ADHD, both disorders, or neither disorder on the six neuropsychological composites. Groups with RD or ADHD exhibited significant weaknesses on all 6 neuropsychological composites compared to the control group without RD or ADHD. Both groups with RD were more impaired than the group with ADHD alone on measures of phoneme awareness, verbal reasoning, working memory, and naming speed, and the comorbid group was more impaired than the groups with either disorder alone on measures of response inhibition and processing speed.
Figure 2.
Performance of groups with and without RD and ADHD on the six cognitive composites.
Because RD and ADHD were associated with significant weaknesses on each of the cognitive composites, separate multiple logistic regression analyses were conducted to test which cognitive constructs independently predicted RD or ADHD. In the first model the six cognitive constructs were included as simultaneous predictors of RD status, and the model was then repeated with ADHD as the dependent measure (Table 3). RD was independently predicted by lower scores on all cognitive composites except response inhibition. In contrast, overall ADHD status was predicted by response inhibition and processing speed only, and results were similar when the inattentive and combined subtypes were analyzed separately. Taken together, these results suggest that processing speed is the most promising candidate for a shared cognitive weakness in RD and ADHD.
Table 3.
Multiple logistic regression models predicting RD and ADHD status from scores on the six cognitive composites
| Cognitive composite scores predicting RD or ADHD status |
||||||
|---|---|---|---|---|---|---|
| Phoneme Awareness |
Verbal Reasoning |
Response Inhibition |
Naming Speed |
Processing Speed |
Working Memory |
|
| Dependent measure | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| RD | −1.303 (.142)*** | −.774 (.131)*** | −.011 (.097) | −.351 (.141)* | −.272 (.123)* | −.382 (.149)* |
| Total ADHD | .164 (.094) | −.204 (.089)^ | −.304 (.072)*** | .006 (.098) | −.638 (.104)*** | −.084 (.107) |
| Inattentive Type | .214 (.116) | −.214 (.111) | −.277 (.091)** | .089 (.120) | −.931 (.133)*** | −.174 (.137) |
| Combined Type | .065 (.140) | −.215 (.131) | −.274 (.097)* | .046 (.145) | −.552 (.160)** | .030 (.163) |
Note. All scores are scaled so that a negative B indicates that a poor cognitive score predicts the diagnosis.
= p < .05
= p < .01
= p < .001
= p < .0001.
4.2. Univariate twin analyses
The first set of behavior genetic analyses tested the univariate etiology of individual differences and extreme scores on the three diagnostic measures and six cognitive composites. MZ correlations were significantly higher than DZ correlations for all nine composites, suggesting that individual differences on all measures are influenced by genes (Table 4). Similarly, in the DF analyses of extreme scores the mean of the MZ cotwins regressed less toward the population mean than the mean of the DZ cotwins on all measures (Table 5). Indeed, high heritability estimates were obtained for both individual differences and extreme scores on the measures of inattention and hyperactivity-impulsivity (h2 = .72 - .74; h2g = .84 - .86), and moderate heritability estimates were obtained for the word reading composite and the six neurocognitive composites (h2 = .41 - .67; h2g = .44 - .65; Tables 4 and 5). Shared environmental influences were significant for all measures except the ADHD and response inhibition composites, but the point estimates for shared environment influences were smaller in magnitude than the estimates of genetic influences (c2 = .01 - .40; c2g = .00 - .35).
Table 4.
Univariate twin analyses of individual differences on the nine composite measures in the entire sample
| Intraclass Correlations |
Genetic and Environmental Parameter Estimates (95% CI) |
||||
|---|---|---|---|---|---|
| Measure | MZ (244 pairs) |
DZ (213 pairs) |
Heritability | Shared Environment |
Nonshared Environment |
| Measures of RD and ADHD | |||||
| Single word reading | .86 | .55 | .61 (.48, .75)* | .27 (.13, .40)* | .12 (.10, .15)* |
| DSM-IV inattention | .76 | .16 | .74 (.66, .79)* | .01 (.00, .07) | .25 (.20, .31)* |
| DSM-IV hyperactivity - impulsivity | .78 | .24 | .72 (.60, .79)* | .03 (.00, .14) | .24 (.20, .30)* |
| Cognitive composite scores | |||||
| Phoneme Awareness | .81 | .45 | .67 (.53, .79)* | .15 (.04, .29)* | .17 (.14, .22)* |
| Verbal Reasoning | .84 | .61 | .46 (.33, .61)* | .40 (.25, .52)* | .14 (.12, .18)* |
| Response Inhibition | .49 | .27 | .41 (.22, .50)* | .03 (.00, .18) | .56 (.48, .65)* |
| Naming Speed | .69 | .38 | .51 (.31, .68)* | .15 (.01, .32)* | .34 (.28, .41)* |
| Processing Speed | .74 | .41 | .59 (.42, .74)* | .15 (.01, .31)* | .25 (.21, .31)* |
| Working Memory | .71 | .45 | .50 (.32, .69)* | .21 (.03, .38)* | .29 (.24, .35)* |
= p < .01
Table 5.
Univariate twin analyses of extreme scores
| MZ pairs |
DZ pairs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Nb | Proband M (SD) |
Cotwin M (SD) |
Nb | Proband M (SD) |
Cotwin M (SD) |
h2g (SE) | c2g (SE) | |
| Diagnostic measures | ||||||||
| Single word reading | 110 | −2.00 (0.57) | −1.75 (0.75) | 94 | −2.06 (0.68) | −1.14 (1.22) | 0.64 (.13)*** | 0.23 (.15) |
| DSM-IV inattention | 99 | −1.95 (0.39) | −1.41 (0.68) | 87 | −1.95 (0.42) | −0.57 (1.26) | 0.86 (.14)*** | − 0.14 (.16) |
| DSM-IV hyperactivity - impulsivity | 90 | −1.96 (0.53) | −1.48 (0.76) | 78 | −1.98 (0.50) | −0.66 (1.09) | 0.84 (.15)*** | − 0.09 (.17) |
| Cognitive composite scores | ||||||||
| Phoneme Awareness | 100 | −2.01 (0.57) | −1.65 (0.83) | 88 | −2.04 (0.47) | −1.01 (1.25) | 0.65 (.14)*** | 0.17 (.16) |
| Verbal Reasoning | 92 | −1.96 (0.72) | −1.58 (0.91) | 82 | −1.92 (0.50) | −1.11 (0.98) | 0.46 (.15)*** | 0.35 (.16)* |
| Response Inhibition | 84 | −2.15 (1.06) | −1.13 (1.21) | 78 | −2.18 (0.98) | −0.67 (1.13) | 0.44 (.15)** | 0.09 (.21) |
| Naming Speed | 88 | −1.84 (0.44) | −1.29 (0.82) | 76 | −1.95 (0.59) | −0.77 (1.14) | 0.61 (.15)*** | 0.09 (.17) |
| Motor Speed | 92 | −1.93 (0.74) | −1.46 (1.01) | 80 | −1.99 (0.61) | −1.03 (1.02) | 0.48 (.14)*** | 0.28 (.18) |
| Working Memory | 92 | −1.85 (0.45) | −1.42 (0.70) | 78 | −1.84 (0.41) | −0.83 (0.96) | 0.63 (.14)*** | 0.13 (.17) |
Note.
P < .05
P < .01
P < .001.
Scores are expressed as standard deviations from the estimated population mean. All scores are scaled so that lower scores indicate greater impairment on all measures.
Total number of pairs in which at least one twin met the criteria for the proband group (score at least 1.25 SD below the population mean).
Similar estimates of genetic and shared environment influences were obtained in the DF analyses of extreme scores and the Mx analyses of individual differences (mean difference between h2g and h2 = .05; mean difference between c2g and c2 = -.03). Although these analyses cannot test definitively whether the same etiological influences act on extreme scores and individual differences, the similarity of these and results is consistent with this hypothesis.
4.3. Multivariate twin anayses
After testing the univariate etiology of each composite measure, we next examined the genetic and environmental associations among the nine constructs. Genetic correlations were significant between measures of reading, inattention, and hyperactivity-impulsivity (Tables 6 and 7), confirming previous results that suggested that the association between reading difficulties and ADHD is due in part to common genetic influences. On the other hand, the confidence intervals for the genetic correlations do not include unity, indicating that unique genetic influences contribute to each of the three diagnostic phenotypes.
Table 6.
Genetic and shared environmental correlations among the nine composite measures in the overall sample
| Word Reading |
Inattention | Hyperactivity - Impulsivity |
Phoneme Awareness |
Verbal Reasoning |
Response Inhibition |
Naming Speed |
Processing Speed |
Working Memory |
|
|---|---|---|---|---|---|---|---|---|---|
| Word reading | -- | .46* (.34, .58) |
.24* (.09, .39) |
.78* (.71, .85) |
.62* (.50, .73) |
.71* (.45, .95) |
.62* (.45, .77) |
.67* (.54, .80) |
.69* (.55, .82) |
| Inattention | .49 (−1.0, 1.0) |
-- | .73* (.65, .81) |
.31* (.21, .43) |
.40* (.24, .56) |
.48* (.27, .73) |
.40* (.24, .58) |
.55* (.42, .70) |
.40* (.23, .56) |
| Hyperactivity - Impulsivity | .36 (−1.0, 1.0) |
.81 (−1.0, 1.0) |
-- | .14 (−.01, .29) |
.08 (−.11, .27) |
.36* (.11, .66) |
.18 (−.01,. .37) |
.25* (.08, .41) |
.25* (.05, .46) |
| Phoneme Awareness | .93* (.68, 1.0) |
.72 (−1.0, 1.0) |
.45 (−.42, 1.0) |
-- | .51* (.36, .65) |
.59* (.36, .83) |
.46* (.35, .62) |
.48* (.34, .63) |
.76* (.64, .88) |
| Verbal Reasoning | .93* (.77, 1.0) |
.61 (−1.0, 1.0) |
.54 (−.97, 1.0) |
.84* (.53, 1.0) |
-- | .66* (.34, .95) |
.44* (.21, .69) |
.60* (.40, .78) |
.78* (.57, .94) |
| Response Inhibition | .28 (−.56, .99) |
.89 (−1.0, 1.0) |
.51 (−.86, 1.0) |
.61 (−.56, .98) |
.29* (.05, .97) |
-- | .34 (−.02, .69) |
.69* (.40, .93) |
.79* (.48, .97) |
| Naming Speed | .57* (.03, 1.0) |
.75 (−1.0, 1.0) |
.47 (−.99, 1.0) |
.81* (.11, 1.0) |
.41 (−.11, .95) |
.83* (.36, 1.0) |
-- | .67* (.52, .83) |
.52* (.29, .73) |
| Processing Speed | .79* (.35, 1.0) |
.68 (−1.0, 1.0) |
.63 (−1.0, 1.0) |
.87* (.35, 1.0) |
.67* (.24, 1.0) |
.55 (−.40, 1.0) |
.88 (−.05, 1.0) |
-- | .46* (.24, .66) |
| Working Memory | .75* (.35, .96) |
.15 (−.94, 1.0) |
−.17 (−1.0, 1.0) |
.76* (.14, .97) |
.46* (.02, .86) |
.24 (−.24, .78) |
.63 (−.43, 1.0) |
.64 (−.17, 1.0) |
-- |
Note. Genetic correlations (rg) are above the diagonal, and shared environmental correlations (rc) are below the diagonal.
Correlations with confidence intervals that do not include zero are significant.
Table 7.
Bivariate heritabilities and genetic correlations of extreme scores on the diagnostic measures and the cognitive composites
| Measure selected in the proband |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Readinga |
Inattentionb |
Hyperactivity - Impulsivityc |
||||||||||
| Unselected Cotwin measure |
Bivariate h2g (SE) |
t | pd | rg[sel] | Bivariate h2g (SE) |
t | pd | rg[sel] | Bivariate h2g (SE) |
t | pd | rg[sel] |
| Diagnostic measures | . | |||||||||||
| Reading | -- | -- | -- | -- | .57 (.14) | 4.09 | .00003 | .74 | .31 (.14) | 2.21 | .02 | .41 |
| Inattention | .56 (.13) | 4.31 | .00001 | .73 | -- | -- | -- | -- | .60 (.14) | 4.29 | .00002 | .69 |
| Hyperactivity - impulsivity | .30 (.13) | 2.31 | .01 | .39 | .71 (.14) | 5.07 | 5 × 10−7 | .81 | -- | -- | ||
| Cognitive composite scores | . | |||||||||||
| Phoneme Awareness | .50 (.13) | 3.85 | .00008 | .76 | .35 (.14) | 2.50 | .007 | .46 | .21 (.15) | 1.40 | .08 | .28 |
| Verbal Reasoning | .43 (.13) | 3.31 | .0006 | .73 | .37 (.14) | 2.64 | .005 | .58 | .25 (.14) | 1.79 | .04 | .40 |
| Response Inhibition | .31 (.14) | 2.21 | .01 | .57 | .35 (.14) | 2.50 | .007 | .56 | .24 (.14) | 1.71 | .04 | .39 |
| Naming Speed | .41 (.13) | 3.15 | .0009 | .63 | .47 (.15) | 3.13 | .001 | .64 | .28 (.14) | 2.00 | .03 | .39 |
| Processing Speed | .50 (.13) | 3.85 | .00008 | .88 | .56 (.14) | 4.01 | .00005 | .86 | .38 (.15) | 2.53 | .006 | .59 |
| Working Memory | .38 (.14) | 2.71 | .004 | .58 | .32 (.15) | 2.13 | .04 | .43 | .22 (.15) | 1.47 | .08 | .30 |
110 MZ pairs, 94 DZ pairs.
99 MZ pairs, 87 DZ pairs.
90 MZ pairs, 78 DZ pairs.
One-tailed test.
Genetic correlations were significant and moderate to high between reading and the six cognitive measures (rg above the diagonal in Table 6, and rg[sel] is included in Table 7). Although the largest genetic correlations with reading were with phoneme awareness and processing speed, the significance and magnitude of the genetic correlations with the other cognitive composites are consistent with a multiple deficit model of RD.
Moderate and significant genetic correlations were also observed between inattention and all of the cognitive composites, with the strongest genetic associations with processing speed and response inhibition. Genetic correlations with hyperactivity-impulsivity were lower and only marginally significant for several measures, although once again the highest genetic correlation were with processing speed and response inhibition.
Shared environmental correlations with reading were significant and large for phoneme awareness, working memory, processing speed, and verbal reasoning, suggesting that the majority of the shared environmental influences on each of these measures act on all of the measures (below the diagonal in Table 6). In contrast, although the point estimates for shared environmental correlations with inattention or hyperactivity-impulsivity were often high, none were significant due to the negligible effect of shared environmental influences on ADHD symptoms (Table 6).
4.4. Slow processing speed as an explanation for comorbidity between RD and ADHD
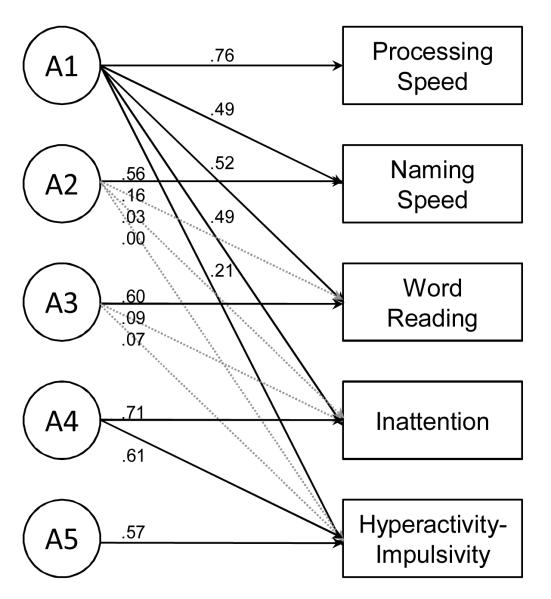
Based on our previous and current results, we conducted a final targeted analysis to test if common genetic influences on processing and naming speed accounted for comorbidity between RD and ADHD. A genetic Cholesky decomposition analysis was used to estimate the shared and independent genetic influences on reading, inattention, hyperactivity-impulsivity, and the processing and naming speed composites (Figure 3).
Figure 3.
Genetic Cholesky decomposition analysis of ADHD, reading, and processing and naming speed. Solid paths are significant (p < .01).
The significant path loadings on the first genetic factor (A1) indicate that common genetic influences account for significant covariance among the five measures. Because the Cholesky model is hierarchical, paths from genetic factors A2 - A5 test for additional genetic influences that are independent of those included in A1. A separate genetic factor contributes significantly to covariance between inattention and hyperactivity-impulsivity that is independent of word reading (A4), and there were unique genetic influences on naming speed (A2), word reading (A3), and hyperactivity-impulsivity (A5) that were not significantly associated with any of the other composites. Finally, the most important result for the primary question in this paper is the absence of any additional shared genetic influences on reading and either ADHD composite after accounting for the genetic influences that are shared with processing speed.
These results suggest that comorbidity between reading difficulties and ADHD is primarily attributable to common genetic influences that lead to slow processing and naming speed. To test the specificity of this result, additional analyses were run in which working memory or inhibition was entered first in the Cholesky model rather than processing and naming speed. In each model there were significant shared genetic influences between reading and inattention that were independent of the genetic influences that are shared with inhibition or working memory.
5. Discussion
This study examined the etiology and neuropsychology of comorbidity between RD and ADHD in a sample of twins overselected for RD and ADHD. The primary goal of the study was to clarify the nature of the relation between RD and ADHD by testing which neuropsychological functions are associated with RD, ADHD, or both disorders, and which cognitive weaknesses may be due to the common genetic influences that lead to comorbidity between RD and ADHD.
5.1. Univariate etiologies of RD, ADHD, and neuropsychological functioning
Consistent with the previous studies summarized in the introduction, univariate twin analyses indicated that individual differences and extreme scores on measures of single-word reading, inattention, and hyperactivity-impulsivity are highly heritable. Shared environmental influences were also significant for reading difficulties but not ADHD.
In contrast to the extensive literatures on the etiology of RD and ADHD, the current analyses are among the first to test the etiology of several of the cognitive constructs. Individual differences and extreme scores on all six cognitive composites were moderately heritable, and shared environmental influences were significant for all cognitive scores except response inhibition.
5.2. Cognitive models of RD and ADHD
Although weak phoneme awareness was the strongest predictor of RD, reading difficulties were also independently predicted by verbal reasoning, naming speed, processing speed, and working memory. Individual differences in each of these cognitive domains were due to both genetic and shared environmental influences, and genetic and shared environmental correlations were large and significant between reading and all five cognitive composites. Therefore, in contrast to theoretical models that suggested that RD is caused by a single primary deficit in phoneme awareness, these results suggest that RD is a complex disorder with a multifactorial etiology that leads to multiple correlated cognitive weaknesses.
Analyses of ADHD revealed a similar pattern. The present results support the hypothesis that ADHD is associated with a significant weakness in response inhibition (e.g., Barkley 1997; Nigg 2001), and demonstrate for the first time that the relation between inhibitory control and ADHD is due to common genetic influences. Other shared genes appear to influence ADHD and slow processing speed, suggesting that both response inhibition and processing speed must be included in a comprehensive cognitive model of ADHD.
5.3. Etiology of comorbidity between RD and ADHD
Twin analyses of individual differences and extreme scores confirmed our previous results suggesting that comorbidity between RD and ADHD may be primarily attributable to common genetic influences (e.g., Willcutt, Pennington, Olson, and DeFries 2007). The most parsimonious model of a common genetic etiology for RD and ADHD includes shared genetic risk factors that influence pathophysiological pathways that increase susceptibility to both disorders. In phenotypic analyses the only cognitive composite that predicted both RD and ADHD was slow processing speed, and subsequent behavior genetic analyses indicated that the relation between reading, ADHD, and processing speed was explained primarily by common genetic influences. Moreover, the correlation between reading and ADHD symptoms was no longer significant after accounting for the genetic influences that were shared with processing speed. These results replicate and extend previous analyses by our group and others (Rucklidge and Tannock 2002; Semrud-Clikeman, Guy, Griffin, and Hynd 2000; Shanahan et al. 2006; Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005), and provide strong support for the hypothesis that slow processing speed may account for comorbidity between RD and ADHD.
5.4. Limitations and future directions
The present results should be interpreted in light of several limitations. Because the CLDRC twin project has been ongoing for nearly twenty years, older versions of the WISC and the PIAT have been retained to allow comparisons to be made across the entire sample. In addition, due to the time constraints of the overall study, DSM-IV ADHD was defined by parent and teacher ratings on the DBRS rather than a full structured diagnostic interview. To test the potential impact of this decision we used data from another ongoing study in our laboratory to examine the concordance between the DBRS and the DSM-IV version of the Diagnostic Interview for Children and Adolescents (Reich, Welner, and Herjanic 1997). The concordance between diagnoses derived from the DBRS and the DICA-IV was extremely high (97% agreement; Kappa = .93), suggesting that these methods are likely to yield similar results.
The extensive battery of cognitive measures is a strength of the current study. Nonetheless, our current analyses did not include measures of response variability, delay aversion, planning, or motor output, all of which have been shown to be significantly associated with ADHD or RD in previous studies (e.g., Aman, Roberts, Jr., and Pennington 1998; Castellanos, Sonuga-Barke, Scheres, Di, Hyde, and Walters 2005; Nigg, Hinshaw, Carte, and Treuting 1998; Solanto et al. 2001; Sonuga-Barke, Taylor, Sembi, and Smith 1992; see review by Willcutt, Sonuga-Barke, Nigg, and Sergeant 2008). Future studies could provide a useful extension of the current results by administering measures of these additional domains in an etiologically-informative study of RD and ADHD.
Although factor analyses support the construct validity of processing speed (e.g., Shanahan et al., 2006), current theoretical models are inadequate. This problem is exacerbated by the fact that many previous studies, including our own, administered both verbal and non-verbal processing speed measures that varied widely in the degree to which the task required strong executive control or sustained attention (e.g., Willcutt, Pennington, Olson, Chhabildas, and Hulslander 2005). Additional research is needed with tasks in which these potential confounds are carefully measured or controlled. In addition, studies that incorporate related methodologies such as functional neuroimaging or event-related potentials are likely to provide important converging evidence to understand the nature of the processing speed deficit in RD, ADHD, and other developmental disorders.
5.5. Conclusions
The current results suggest that RD and ADHD are each associated with weaknesses in multiple neuropsychological domains. Deficits in phonological processing, verbal reasoning, and naming speed are primarily associated with RD, whereas weak response inhibition may be independently associated with ADHD. Twin analyses suggest that comorbidity between RD and ADHD is primarily due to common genetic influences that lead to slow processing speed, suggesting that measures of processing efficiency may be useful endophenotypes for future molecular genetic studies of RD and ADHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aman CJ, Roberts RJ, Jr., Pennington BF. A neuropsychological examination of the underlying deficit in attention deficit hyperactivity disorder: frontal lobe versus right parietal lobe theories. Developmental Psychology. 1998;34:956–969. doi: 10.1037/0012-1649.34.5.956. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- August GJ, Garfinkel BD. Comorbidity of ADHD and reading disability among clinic-referred children. Journal of Abnormal Child Psychology. 1990;18:29–45. doi: 10.1007/BF00919454. [DOI] [PubMed] [Google Scholar]
- Bakwin H. Reading disability in twins. Developmental Medicine and Child Neurology. 1973;15:184–187. doi: 10.1111/j.1469-8749.1973.tb15158.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy K. Attention-deficit hyperactivity disorder: A clinical workbook. Guilford Press; New York, NY: 1998. [Google Scholar]
- Bates TC, Castles A, Coltheart M, Gillespie N, Wright M, Martin NG. Behaviour genetic analyses of reading and spelling: A component processes approach. Australian Journal of Psychology. 2004;56:115–126. [Google Scholar]
- Bates TC, Castles A, Luciano M, Wright MJ, Coltheart M, Martin NG. Genetic and environmental bases of reading and spelling: A unified genetic dual route model. Reading and Writing. 2007;20:147–171. [Google Scholar]
- Bates TC, Luciano M, Castles A, Coltheart M, Wright MJ, Martin NG. Replication of reported linkages for dyslexia and spelling and suggestive evidence for novel regions on chromosomes 4 and 17. European Journal of Human Genetics. 2007;15:194–203. doi: 10.1038/sj.ejhg.5201739. [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Matos M, Reina G, Salas CC, Martinez JV, Cumba E, Barkley RA. Comparison of the DSM-IV combined and inattentive types of ADHD in a school-based sample of Latino/Hispanic children. Journal of Child Psychology and Psychiatry. 2005;46:166–179. doi: 10.1111/j.1469-7610.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- Betjemann RS, Willcutt EG, Olson RK, Keenan JM, DeFries JC, Wadsworth SJ. Word reading and reading comprehension: stability, overlap and independence. Reading and Writing. 2008;21:539–558. [Google Scholar]
- Byrne B, Delaland C, Fielding-Barnsley R, Quain P, Samuelsson S, Hoien T, Corley R, DeFries JC, Wadsworth S, Willcutt E, Olson RK. Longitudinal twin study of early reading development in three countries: Preliminary results. Annals of Dyslexia. 2002;52:49–73. [Google Scholar]
- Byrne B, Samuelsson S, Wadsworth SJ, Hulslander J, Corley R, DeFries JC, Quain P, Willcutt EG, Olson RK. Longitudinal twin study of early literacy development: preschool through grade 1. Reading and Writing. 2007;20:77–102. [Google Scholar]
- Cardon LR, Smith SD, Fulker DW, Kimberling WJ, Pennington BF, DeFries JC. Quantitative trait locus for reading disability on chromosome 6. Science. 1994;266:276–279. doi: 10.1126/science.7939663. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Smith SD, Fulker DW, Kimberling WJ, Pennington BF, DeFries JC. Quantitative trait locus for reading disability: correction. Science. 1995;268:1553. doi: 10.1126/science.7777847. [DOI] [PubMed] [Google Scholar]
- Case R, Kurland M, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology. 1982;33:386–404. [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di MA, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Compton DL, DeFries JC, Olson RK. Are RAN- and phonological awareness-deficits additive in children with reading disabilities? Dyslexia. 2001;7:125–149. doi: 10.1002/dys.198. [DOI] [PubMed] [Google Scholar]
- Curran S, Mill J, Tahir E, Kent L, Richards S, Gould A, Huckett L, Sharp J, Batten C, Fernando S, Ozbay F, Yazgan Y, Simonoff E, Thompson M, Taylor E, Asherson P. Association study of a dopamine transporter polymorphism and attention deficit hyperactivity disorder in UK and Turkish samples. Molecular Psychiatry. 2001;6:425–428. doi: 10.1038/sj.mp.4000914. [DOI] [PubMed] [Google Scholar]
- Decker SN. Cognitive processing rates among disabled and normal reading young adults: A nine year follow-up study. Reading and Writing: An Interdisciplinary Journal. 1989;1:123–134. [Google Scholar]
- DeFries JC, Filipek PA, Fulker DW, Olson RK, Pennington BF, Smith SD, Wise BW. Colorado Learning Disabilities Research Center. Learning Disabilities: A Multidisciplinary Journal. 1997;8:7–19. [Google Scholar]
- DeFries JC, Fulker DW. Multiple regression analysis of twin data. Behavior Genetics. 1985;15:467–473. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Fulker DW. Multiple regression analysis of twin data: etiology of deviant scores versus individual differences. Acta Geneticae Medicae et Gemellologiae. 1988;37:205–216. doi: 10.1017/s0001566000003810. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Singer SM, Foch TT, Lewitter FI. Familial nature of reading disability. British Journal of Psychiatry. 1978;132:361–367. doi: 10.1192/bjp.132.4.361. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Naming of object-drawings by dyslexic and other learning disabled children. Brain and Language. 1976;3:1–15. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, DuPre EP, Biederman J. Separating attention deficit hyperactivity disorder and learning disabilities in girls: a familial risk analysis. American Journal of Psychiatry. 2001;158:1666–1672. doi: 10.1176/appi.ajp.158.10.1666. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Markwardt FC. Examiner's Manual: Peabody Individual Achievement Test. American Guidance Service; Circle Pines, MN: 1970. [Google Scholar]
- Faraone SV, Biederman J, Friedman D. Validity of DSM-IV subtypes of attention-deficit/hyperactivity disorder: a family study perspective. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:300–307. doi: 10.1097/00004583-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Lehman BK, Keenan K, Norman D, Seidman LJ, Kolodny R, Kraus I, Perrin J, Chen WJ. Evidence for the independent familial transmission of attention deficit hyperactivity disorder and learning disabilities: results from a family genetic study. American Journal of Psychiatry. 1993;150:891–895. doi: 10.1176/ajp.150.6.891. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Attention deficit and reading achievement. Journal of Child Psychology and Psychiatry. 1992;33:375–385. doi: 10.1111/j.1469-7610.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Finucci JM, Childs B. Dyslexia: Family Studies. In: Ludlow CL, Cooper JA, editors. Genetic aspects of speech and language disorders. Academic Press; New York, NY: 1983. [Google Scholar]
- Fisher SE, DeFries JC. Developmental dyslexia: genetic dissection of a complex cognitive trait. Nature Reviews: Neuroscience. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- French JW, Ekstrom RG, Price LA. Manual for a kit of reference tests for cognitive factors. Educational Testing Service; Princeton, NJ: 1963. [Google Scholar]
- Friedman MC, Chhabildas N, Budhiraja N, Willcutt EG, Pennington BF. Etiology of the comorbidity between RD and ADHD: Exploration of the non-random mating hypothesis. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2003;120B:109–115. doi: 10.1002/ajmg.b.20029. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Willis CS, Baddeley AD, Emslie H. The children's test of nonword repetition: A test of phonological working memory. Memory. 1994;2:103–107. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- Gayan J, Olson RK. Genetic and environmental influences on orthographic and phonological skills in children with reading disabilities. Developmental Neuropsychology. 2001;20:483–507. doi: 10.1207/S15326942DN2002_3. [DOI] [PubMed] [Google Scholar]
- Gayan J, Olson RK. Genetic and environmental influences on individual differences in printed word recognition. Journal of Experimental Child Psychology. 2003;84:97–123. doi: 10.1016/s0022-0965(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Golden JC. Stroop Color and Word Test. Stoelting Company; Chicago,IL: 1978. [Google Scholar]
- Goldstein G, Watson JR. Test-retest reliability of the Halstead-Reitan battery and the WAIS in a neuropsychiatric population. The Clinical Neuropsychologist. 1989;3:265–273. [Google Scholar]
- Goodman R, Stevenson J. A twin study of hyperactivity--II. The aetiological role of genes, family relationships and perinatal adversity. Journal of Child Psychology and Psychiatry. 1989;30:691–709. doi: 10.1111/j.1469-7610.1989.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Gordon M. The Gordon Diagnostic System. Gordon Systems; DeWitt, NY: 1983. [Google Scholar]
- Harlaar N, Spinath FM, Dale PS, Plomin R. Genetic influences on early word recognition abilities and disabilities: a study of 7-year-old twins. Journal of Child Psychology and Psychiatry. 2005;46:373–384. doi: 10.1111/j.1469-7610.2004.00358.x. [DOI] [PubMed] [Google Scholar]
- Hawke JL, Wadsworth SJ, DeFries JC. Genetic influences on reading difficulties in boys and girls: the Colorado twin study. Dyslexia. 2006;12:21–29. doi: 10.1002/dys.301. [DOI] [PubMed] [Google Scholar]
- Hay DA, Bennett KS, Levy F, Sergeant J, Swanson J. A twin study of attention-deficit/hyperactivity disorder dimensions rated by the strengths and weaknesses of ADHD-symptoms and normal-behavior (SWAN) scale. Biological Psychiatry. 2007;61:700–705. doi: 10.1016/j.biopsych.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Hohnen B, Stevenson J. The structure of genetic influences on general cognitive, language, phonological, and reading abilities. Developmental Psychology. 1999;35:590–603. doi: 10.1037//0012-1649.35.2.590. [DOI] [PubMed] [Google Scholar]
- Klorman R, Hazel-Fernandez LA, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM, Stuebing KK, Shaywitz BA. Executive functioning deficits in attention-deficit/hyperactivity disorder are independent of oppositional defiant or reading disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1148–1155. doi: 10.1097/00004583-199909000-00020. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Jacobson KC, Xian H, Eisen SA, Waterman B, Toomey R, Neale MC, Tsuang MT, Lyons MJ. Heritability of word recognition in middle-aged men varies as a function of parental education. Behavior Genetics. 2005;35:417–433. doi: 10.1007/s10519-004-3876-2. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, Barkley RA, Newcorn J, Jensen P, Richters J. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. American Journal of Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt EG. Validity of the diagnosis and dimensions of attention deficit hyperactivity disorder. In: Jensen PJ, Cooper JR, editors. Attention Deficit Hyperactivity Disorder: State of the Science. Civic Research Institute; New York: 2002. pp. 1-1–1-23. [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Light JG, DeFries JC. Comorbidity of reading and mathematics disabilities: genetic and environmental etiologies. Journal of Learning Disabilities. 1995;28:96–106. doi: 10.1177/002221949502800204. [DOI] [PubMed] [Google Scholar]
- Light JG, Pennington BF, Gilger JW, DeFries JC. Reading-Disability and Hyperactivity Disorder -Evidence for A Common Genetic Etiology. Developmental Neuropsychology. 1995;11:323–335. [Google Scholar]
- Lindamood C, Lindamood P. Lindamood auditory conceptualization test. Reading Resources Corporation; Boston, MA: 1971. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Lytton H, Watts D, Dunn BE. Stability of genetic determination from age 2 to 9: a longitudinal twin study. Social Biology. 1988;35:62–73. doi: 10.1080/19485565.1988.9988688. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Shanahan MA, Santerre-Lemmon LE, Barnard HD, Willcutt EG, Olson RK, DeFries JC, Pennington BF. A multiple deficit model of reading disability and attention-deficit/hyperactivity disorder. Processing speed is a shared cognitive deficit. 2010 doi: 10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Smith SD, Pennington BF. Breakthroughs in the search for dyslexia candidate genes. Trends in Molecular Medicine. 2006;12:333–341. doi: 10.1016/j.molmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of Abnormal Child Psychology. 2007;35:999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Smith BH, Pelham WE. Factor structure and criterion validity of secondary school teacher ratings of ADHD and ODD. Journal of Abnormal Child Psychology. 2001;29:71–82. doi: 10.1023/a:1005203629968. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide: Fourth Edition. Muthen and Muthen; Los Angeles, CA: 1998-2009. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Virginia Commonwealth University; Richmond, VA: 2002. [Google Scholar]
- Neale MC, Kendler KS. Behavior Genetics. Vol. 25. 1995. Multifactorial Models of Comorbidity; p. 281. [PMC free article] [PubMed] [Google Scholar]
- Nichols R, Bilbro W. The diagnosis of twin zygosity. Acta Genetica et Statistica Medica. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? Understanding what goes wrong and why. Guilford; New York, NY: 2006. [Google Scholar]
- Nigg JT, Hinshaw SP, Carte ET, Treuting JJ. Neuropsychological correlates of childhood attention-deficit/hyperactivity disorder: explainable by comorbid disruptive behavior or reading problems? Journal of Abnormal Psychology. 1998;107:468–480. doi: 10.1037/0021-843X.107.3.468. [DOI] [PubMed] [Google Scholar]
- Olson RK. Disabled reading processes and cognitive profiles. In: Gray DB, Kavanagh JF, editors. Biobehavioral Measures of Dyslexia. York Press; Parkton, MD: 1985. [Google Scholar]
- Olson RK. Language deficits in “specific” reading disability. In: Gernsbacher M, editor. Handbook of Psycholinguistics. Academic Press; New York, NY: 1994. pp. 895–916. [Google Scholar]
- Olson RK, Forsberg H, Wise B. Genes, environment, and the development of orthographic skills. In: Berninger VW, editor. The varieties of orthographic knowledge I: Theoretical and developmental issues. Kluwer Academic Publishers; Dordrecht, Netherlands: 1994. pp. 27–72. [Google Scholar]
- Olson RK, Forsberg H, Wise B, Rack J. Measurement of word recognition, orthographic, and phonological skills. In: Lyon GR, editor. Frames of reference for the assessment of learning disabilities: New views on measurement issues. Paul H. Brookes Publishing Company; Baltimore, MD: 1994. pp. 243–277. [Google Scholar]
- Olson RK, Wise B, Conners F, Rack J, Fulker DW. Specific deficits in component reading and language skills: Genetic and environmental influences. Journal of Learning Disabilities. 1989;22:339–348. doi: 10.1177/002221948902200604. [DOI] [PubMed] [Google Scholar]
- Pennington BF. The Development of Psychopathology. Guilford Press; New York, NY: 2002. [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101:385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Petrill SA, Deater-Deckard K, Thompson LA, Schatschneider C, Dethorne LS, Vandenbergh DJ. Longitudinal genetic analysis of early reading: The Western Reserve Reading Project. Reading and Writing. 2007;20:127–146. doi: 10.1007/s11145-006-9021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisecco S, Baker DB, Silva PA, Brooke M. Boys with reading disabilities and/or ADHD: distinctions in early childhood. Journal of Learning Disabilities. 2001;34:98–106. doi: 10.1177/002221940103400201. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. Worth Publishers; New York: 2008. [Google Scholar]
- Purvis KL, Tannock R. Phonological processing, not inhibitory control, differentiates ADHD and reading disability. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:485–494. doi: 10.1097/00004583-200004000-00018. [DOI] [PubMed] [Google Scholar]
- Rack JP, Snowling MJ, Olson RK. The Nonword Reading Deficit in Developmental Dyslexia - A Review. Reading Research Quarterly. 1992;27:28–53. [Google Scholar]
- Reich W, Welner Z, Herjanic B. Diagnostic Interview for Children and Adolescents - IV. Multi-Health System, Inc.; North Towanda Falls, NY: 1997. [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reaitn Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Reynolds CA, Hewitt JK, Erickson MT, Silberg JL, Rutter M, Simonoff E, Meyer J, Eaves LJ. The genetics of children's oral reading performance. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:425–434. doi: 10.1111/j.1469-7610.1996.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, Hudziak JJ, Bartels M, van Beijsterveldt CE, Boomsma DI. Heritability of attention problems in children: I. cross-sectional results from a study of twins, age 3-12 years. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2003;117B:102–113. doi: 10.1002/ajmg.b.10024. [DOI] [PubMed] [Google Scholar]
- Roodenrys S, Koloski N, Grainger J. Working memory function in attention deficit hyperactivity disordered and reading disabled children. British Journal of Developmental Psychology. 2001;19:325–337. [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Biederman J, Sprich-Buckminster S, Lehman BK, Faraone SV, Norman D. Comorbidity between ADDH and learning disability: a review and report in a clinically referred sample. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:439–448. doi: 10.1097/00004583-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Guy K, Griffin JD, Hynd GW. Rapid naming deficits in children and adolescents with reading disabilities and attention deficit hyperactivity disorder. Brain and Language. 2000;74:70–83. doi: 10.1006/brln.2000.2337. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt EG, Olson RK, DeFries JC. Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. Journal of Abnormal Child Psychology. 2006;34:585–602. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, Makuch R. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. New England Journal of Medicine. 1992;326:145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Iacono WG, McGue MK. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Sherman DK, McGue MK, Iacono WG. Twin concordance for attention deficit hyperactivity disorder: a comparison of teachers' and mothers' reports. American Journal of Psychiatry. 1997;154:532–535. doi: 10.1176/ajp.154.4.532. [DOI] [PubMed] [Google Scholar]
- Siegel LS, Ryan EB. The development of working memory in normally achieving and subtypes of learning disabled children. Child Development. 1989;60:973–980. doi: 10.1111/j.1467-8624.1989.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America. 2008;17:367–84, ix. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. Journal of Child Psychology and Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Stevenson J. Evidence for a genetic etiology in hyperactivity in children. Behavior Genetics. 1992;22:337–344. doi: 10.1007/BF01066665. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Pennington BF, Gilger JW, DeFries JC, Gillis JJ. Hyperactivity and spelling disability: testing for shared genetic aetiology. Journal of Child Psychology and Psychiatry. 1993;34:1137–1152. doi: 10.1111/j.1469-7610.1993.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Swanson HL, Mink J, Bocian KM. Cognitive processing deficits in poor readers with symptoms of reading disabilities and ADHD: More alike than different? Journal of Educational Psychology. 1999;91:321–333. [Google Scholar]
- Tannock R, Martinussen R, Frijters J. Naming speed performance and stimulant effects indicate effortful, semantic processing deficits in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:237–252. doi: 10.1023/a:1005192220001. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harrington R, McGuffin P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. British Journal of Psychiatry. 2001;179:224–229. doi: 10.1192/bjp.179.3.224. [DOI] [PubMed] [Google Scholar]
- Todd RD, Rasmussen ER, Neuman RJ, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Heath A. Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. American Journal of Psychiatry. 2001;158:1891–1898. doi: 10.1176/appi.ajp.158.11.1891. [DOI] [PubMed] [Google Scholar]
- Trzesniewski KH, Moffitt TE, Caspi A, Taylor A, Maughan B. Revisiting the association between reading achievement and antisocial behavior: new evidence of an environmental explanation from a twin study. Child Development. 2006;77:72–88. doi: 10.1111/j.1467-8624.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Corley RP, Hewitt JK, Plomin R, DeFries JC. Parent-offspring resemblance for reading performance at 7, 12 and 16 years of age in the Colorado Adoption Project. Journal of Child Psychology and Psychiatry. 2002;43:769–774. doi: 10.1111/1469-7610.00085. [DOI] [PubMed] [Google Scholar]
- Wagner RK. Phonological processing abilities and reading: implications for disabled readers. Journal of Learning Disabilities. 1986;19:623–630. doi: 10.1177/002221948601901009. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Development of Reading-Related Phonological Processing Abilities - New Evidence of Bidirectional Causality from A Latent Variable Longitudinal-Study. Developmental Psychology. 1994;30:73–87. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, Hecht SA, Barker TA, Burgess SR, Donahue J, Garon T. Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: a 5-year longitudinal study. Developmental Psychology. 1997;33:468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- Wainwright M, Wright MJ, Geffen GM, Geffen LB, Luciano M, Martin NG. Genetic and environmental sources of covariance between reading tests used in neuropsychological assessment and IQ subtests. Behavior Genetics. 2004;34:365–376. doi: 10.1023/B:BEGE.0000023642.34853.cb. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children, Revised. The Psychological Corporation; New York, NY: 1974. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children, Third Edition. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Whitehouse AJ, Spector TD, Cherkas LF. No clear genetic influences on the association between dyslexia and anxiety in a population-based sample of female twins. Dyslexia. 2009;15:282–290. doi: 10.1002/dys.378. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. Understanding comorbidity between reading disability and ADHD. Annual Meeting of the International Dyslexia Association. 2008 [Google Scholar]
- Willcutt EG, Betjemann RS, Wadsworth SJ, Samuelsson S, Corley R, DeFries JC, Byrne B, Pennington BF, Olson RK. Preschool twin study of the relation between attention-deficit/hyperactivity disorder and prereading skills. Reading and Writing. 2007;20:103–125. [Google Scholar]
- Willcutt EG, Chhabildas NA, Pennington BF. Validity of the DSM-IV subtypes of ADHD. The ADHD Report. 2001;9:2–5. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF. Comorbidity of reading disability and attention-deficit/hyperactivity disorder: differences by gender and subtype. Journal of Learning Disabilities. 2000;33:179–191. doi: 10.1177/002221940003300206. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, Olson RK. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, DeFries JC. Etiology of inattention and hyperactivity/impulsivity in a community sample of twins with learning difficulties. Journal of Abnormal Child Psychology. 2000a;28:149–159. doi: 10.1023/a:1005170730653. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, DeFries JC. Twin study of the etiology of comorbidity between reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000b;96:293–301. doi: 10.1002/1096-8628(20000612)96:3<293::aid-ajmg12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: in search of the common deficit. Developmental Neuropsychology. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: a twin study of reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Sonuga-Barke EJS, Nigg JT, Sergeant JA. Recent developments in neuropsychological models of childhood disorders. Advances in Biological Psychiatry. 2008;24:195–226. [Google Scholar]
- Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. Journal of Abnormal Child Psychology. 1998;26:141–152. doi: 10.1023/a:1022673906401. [DOI] [PubMed] [Google Scholar]
- Zhou K, Dempfle A, Arcos-Burgos M, Bakker SC, Banaschewski T, Biederman J, Buitelaar J, Castellanos FX, Doyle A, Ebstein RP, Ekholm J, Forabosco P, Franke B, Freitag C, Friedel S, Gill M, Hebebrand J, Hinney A, Jacob C, Lesch KP, Loo SK, Lopera F, McCracken JT, McGough JJ, Meyer J, Mick E, Miranda A, Muenke M, Mulas F, Nelson SF, Nguyen TT, Oades RD, Ogdie MN, Palacio JD, Pineda D, Reif A, Renner TJ, Roeyers H, Romanos M, Rothenberger A, Schafer H, Sergeant J, Sinke RJ, Smalley SL, Sonuga-Barke E, Steinhausen HC, van der Meulen E, Walitza S, Warnke A, Lewis CM, Faraone SV, Asherson P. Meta-analysis of genome-wide linkage scans of attention deficit hyperactivity disorder. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:1392–1398. doi: 10.1002/ajmg.b.30878. [DOI] [PMC free article] [PubMed] [Google Scholar]