Abstract
Background
Soft-tissue sarcoma spreads predominantly to the lung. The frequency with which positron-emission tomography (pet) detects metastases not already obvious by chest computed tomography (ct) or clinical examination is currently unclear.
Methods
We retrospectively identified cases of soft-tissue sarcoma. Ewing sarcoma, rhabdomyosarcoma, and gastrointestinal stromal tumour were excluded, as were cases in which patients underwent imaging for follow-up, response assessment, or recurrence. Patients all had undergone diagnostic chest ct as part of their staging. Directed studies were requested to follow up on abnormal findings in the clinical history or physical examination. All charts and pre-treatment imaging were reviewed retrospectively.
Results
From 2004 to 2008, 75 patients met the criteria for the present review. Their median age was 51 years. In 21% of cases, the primary tumour had been removed (by excisional biopsy or unplanned excision) before staging. Of the previously unresected primary tumours, 97% were avid for fluorodeoxyglucose. Of all tumours, 81% were intermediate or high grade (Fédération Nationale des Centres de Lutte Contre le Cancer grades 2–3). The primary tumour was stage T2b in 69% of cases. The most common primary site was a lower extremity (55%). The most common pathologic diagnoses were leiomyosarcoma (21%), liposarcoma (19%), and synovial sarcoma (17%). At the end of staging, 17% of patients were considered to have metastatic disease.
Imaging by pet was negative for distant disease in 64 of the 75 cases. In 7 of the 64 cases, metastatic disease was evident on chest ct (negative predictive value: 88%). Imaging by pet was positive in 8 cases, with 5 of those already known to have metastases, 2 having pathologically proven false positives, and 1 being a new finding of a pulmonary metastasis (positive predictive value: 75%). The pet imaging was indeterminate in 3 patients (none of whom subsequently developed metastatic disease). Two incidental benign parotid tumours were found. Overall, only 1 patient was upstaged as a result of pet imaging (1.3%). In addition, pet did not alter the management of patients already know to have M1 disease (no new organ sites identified).
Conclusions
Although pet may be helpful in specific circumstances, routine use of fluorodeoxyglucose pet imaging for detection of metastatic disease as part of the initial staging of soft-tissue sarcoma added little to imaging by chest ct and was unlikely to alter management in our series.
Keywords: Soft-tissue sarcoma, positron-emission tomography, staging
1. INTRODUCTION
Soft-tissue sarcomas represent a histologically heterogeneous group of malignant tumours. These uncommon tumours account for 0.7% of adult malignancies and approximately 6.5% of childhood cancers. In 2009, 10,660 new soft-tissue sarcomas were expected to be diagnosed in the United States, with 3820 deaths expected from those tumours.
Soft-tissue sarcomas have a propensity for hematogenous metastasis, the risk of which varies with tumour size, grade, location, and histologic subtype 1. The most common site of metastasis, both at presentation and at recurrence, is the parenchyma of the lung. Because pulmonary lesions account for approximately 75% of metastases 2, the utility of adding abdominopelvic imaging has been debated 3. Exceptions to this pattern of spread are possible, such as in the case of myxoid or round-cell liposarcomas—tumours with a greater propensity for nodal and bony metastases 4.
Imaging by combined positron-emission tomography and computed tomography (pet/ct) has been investigated in soft-tissue sarcoma for biopsy guidance 5, response assessment 6, grading 7, and follow-up 8. The utility, beyond conventional staging, of fluorodeoxyglucose (fdg) pet/ct for the initial staging of soft-tissue sarcoma remains to be clarified.
At our institution, fdg pet/ct imaging has been performed in patients with large (American Joint Committee on Cancer T2) or high-grade [Fédération Nationale des Centres de Lutte Contre le Cancer (fnclcc) grades 2–3] sarcomas at the time of diagnosis. These studies were performed as part of a larger prospective study investigating the safety of fdg in imaging of cancer patients. Here, we review our initial experience.
2. PATIENTS AND METHODS
2.1. Patients
A review of the database of the pet/ct unit of the Department of Nuclear Medicine at the McGill University Health Center covering May 2004 to April 2008 revealed 219 individual patients with known soft-tissue or osseous sarcomas who presented for a fdg pet/ct study. After cases of Ewing sarcoma, rhabdomyosarcoma, and gastrointestinal stromal tumours were excluded, together with cases in which patients were imaged for follow-up, response assessment, or recurrence, 75 patients with a pet/ct study for initial staging of their soft-tissue sarcoma remained for evaluation. The hospital charts of those patients were then reviewed for pertinent clinical information.
2.2. Conventional Staging Studies
In addition to imaging—typically by magnetic resonance (mr)—of the primary tumour site, every patient underwent a dedicated ct study of the chest. Patients underwent additional studies at the treating physician’s discretion, typically to investigate symptoms or clinical findings suspicious for metastatic disease. The clinical reports used for patient care are also used here as the basis for considering a study positive, negative, or indeterminate. An indeterminate study was typically a chest ct on which millimetric parenchymal or pleural nodules were seen.
2.3. FDG PET/CT Imaging
After written informed consent was obtained from patients, fdg pet studies were acquired on a hybrid pet/ct scanner (Discovery ST: General Electric Medical Systems, Waukesha, WI, U.S.A.), which combines a dedicated, full-ring pet scanner with a 16-slice spiral ct scanner. Between 370 MBq and 500 MBq of fdg was injected intravenously. At 60 minutes after fdg injection, ct and pet images were consecutively acquired from the base of the skull to the upper thighs, with additional images acquired according to the sarcoma location. In the pet portion of the study, two-dimensional acquisition was performed, and images were acquired at 4–5 minutes per bed position (depending on the patient’s body weight) in 5–6 bed positions (depending on the patient’s height). Attenuation-corrected and non-attenuation-corrected pet images, ct images, and fused images were reconstructed in the transaxial, coronal, and sagittal planes using an ordered-subset expectation-maximization iterative algorithm.
For accuracy and uniformity of measurements of the standardized uptake value (suv) in patients in whom the primary tumour remained in place, 2 physicians retrospectively conducted a systematic review and came to consensus on the maximum suv measurements (suvMax). The suvMax values of each primary lesion were measured using a rounded region-of- interest tool and a systematic slice-by-slice search for the most intense voxel within a given lesion. The primary tumour was considered pet-positive if its suvMax was 2.5 or greater. As in the conventional staging studies, the clinical reports used for patient care were also used as the basis for considering the study positive, negative, or indeterminate for the presence of metastatic disease. An “indeterminate study” was a study with a fdg-avid lesion considered to be atypical for a metastasis or with a non-avid abnormality on the ct component of the study.
3. RESULTS
Table I presents patient demographics and tumour characteristics for the 75 patients who underwent total-body fdg pet/ct imaging as part of the initial staging of a soft-tissue sarcoma at the McGill University Health Centre from May 2004 to April 2008. In 21% of the patients, the primary tumour had been removed (by excisional biopsy or unplanned excision) before staging. Of the previously unresected primary tumours, 97% were fdg-avid (median suvMax: 7.4; range: 1.8–35.8). Of all tumours, 81% were intermediate or high grade (fnclcc grades 2–3), including all 11 T1a tumours. The primary tumour was stage T2b in 69% of cases. The most common primary site was a lower extremity (55%). The most common pathologic diagnoses were leiomyosarcoma (21%), liposarcoma (19%), and synovial sarcoma (17%).
TABLE I.
Patient demographics and tumour characteristics
| Variable | Value |
|---|---|
| Age (years) | |
| Median | 51 |
| Range | 16–90 |
| Sex (%) | |
| Female | 55 |
| Male | 45 |
| Tumour stage (%) | |
| T1a | 15 |
| T1b | 13 |
| T2a | 3 |
| T2b | 69 |
| Tumour grade (%) | |
| TNM low (fnclcc grade 1) | 9 |
| TNM high (fnclcc grades 2–3) | 81 |
| Tumour location (%) | |
| Lower extremity | 55 |
| Upper extremity | 23 |
| Other | 22 |
| Tumour histology (%) | |
| Leiomyosarcoma | 21 |
| Liposarcoma | 19 |
| Synovial sarcoma | 17 |
| Undifferentiated | 13 |
| Other | 40 |
fnclcc = Fédération Nationale des Centres de Lutte Contre le Cancer.
In 64 of 75 cases, pet imaging was negative for distant disease. Of those 64 cases, 7 showed metastatic disease on chest ct. The negative predictive value of pet was 88%, and the specificity, 97%. Imaging by pet was positive in 8 patients, with 5 of those already known to have metastases, 2 having pathologically proven false positives, and 1 being a new finding of a pulmonary metastasis. Both false-positive studies resulted in additional surgical interventions: in one case, the patient underwent an open biopsy of tibial fibrous dysplasia (Figure 1), and in the other, axillary dissection of reactive lymph nodes (Figure 2). Imaging by pet was indeterminate in 3 patients. In one patient, a non-fdg-avid vertebral lesion was confirmed by subsequent mr imaging to be a hemangioma; fdg-avid mediastinal lymph nodes were presumed to represent granulomatous disease in another patient with a hand primary; and multiple fdg-avid lymph nodes in the third patient were considered to reflect a known indolent lymphoma. During follow-up (13–21 months), no patient with an indeterminate study developed metastatic disease. Two incidental benign parotid tumours were found.
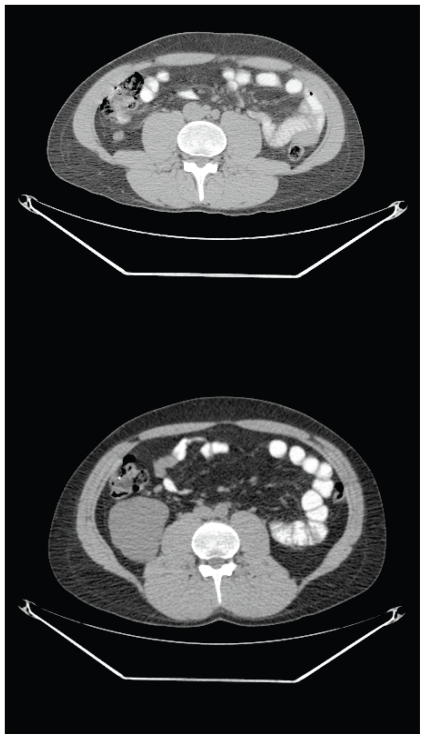
FIGURE 1.
False positive. A 48-year-old patient with a large myxoid liposarcoma of the thigh required biopsy to demonstrate that an avid (standard uptake value 7.4) lesion of the distal tibia was fibrous dysplasia.
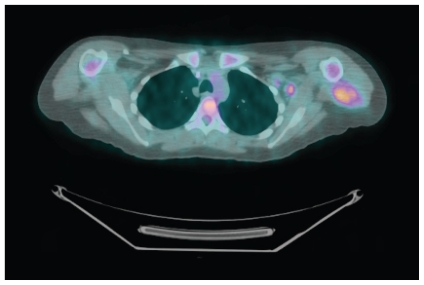
FIGURE 2.
False positive. A 29-year-old patient with what was thought to be an epithelial sarcoma on biopsy had an axillary lymph node with a standard uptake value of 4.4. On final pathology, this was found to be an angiomatoid fibrous histiocytoma with a reactive lymph node.
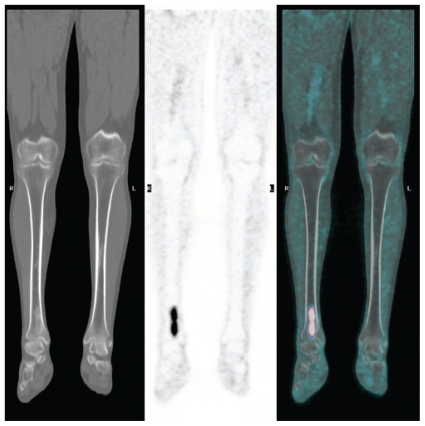
At the end of staging, 17% of patients (n = 13) were considered to have metastatic disease. Of those 13 patients, 11 had pulmonary metastases. Of the 2 patients with only extrathoracic metastases, one had an adrenal lesion (seen in the abdominal portion of the chest ct), and the other, bone metastases [suspected on Tc99m–methylene diphosphonate (Tc99m-mdp) bone scintigraphy conducted before the pet]. In retrospect, 1 patient with a large round-cell liposarcoma of the ankle (Figure 3) had a 1.4-cm non-avid suprarenal lymph node on the ct portion of the pet/ct. The lesion enlarged substantially on a follow-up study, and on resection, it was confirmed to be a metastatic lesion. The original pet study is therefore counted in this series as a false negative.
FIGURE 3.
False negative. A 35-year-old patient with a large round-cell liposarcoma of the ankle required nephrectomy for a large infrarenal mass (lower panel), which in retrospect was a lesion non-avid for fluorodeoxyglucose on staging imaging by positron-emission tomography (upper panel).
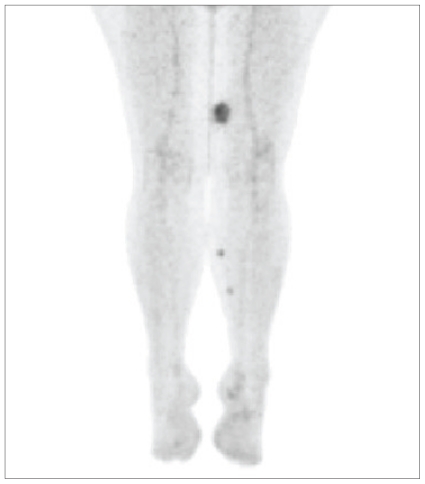
Overall, only 1 patient (1.3%) was upstaged by pet imaging (Table II; Figure 4 shows a true positive pet of unsuspected extrathoracic disease). In addition, pet did not alter the management of patients already known to have M1 disease. The sensitivity of pet/ct for the detection of metastatic disease was 43%, and the positive predictive value, 75%.
TABLE II.
Results from positron-emission tomography imaging
| Result | [n (%)] |
|---|---|
| True negative | 57 (76) |
| False negative | 7 (9) |
| True positive | |
| Known | 5 (6) |
| New | 1 (1) |
| False positive | 2 (2) |
| Indeterminate | 3 (4) |
FIGURE 4.
True positive (patient contemporary to the current series). In this 58-year-old patient with a peripheral nerve sheath tumour of the left thigh, positron-emission imaging demonstrated distal soft tissue metastases not initially seen on physical examination.
4. DISCUSSION
In our series of 75 patients, the use of pet appropriately altered management in only 1 patient. Few other reports document the use of metabolic imaging in the initial staging of soft-tissue tumours. These series tend to include more heterogeneous diagnoses and bone tumours.
In a retrospective study, Tateishi et al. 9 reviewed images from 117 patients who had undergone staging for a suspected bone or soft-tissue tumour. In addition to conventional imaging, which included Tc99m-mdp bone scintigraphy, chest radiography, and total body ct, a fdg pet scan was performed in each case. Compared with conventional imaging, the metabolic imaging found distant metastases in an additional 14% of cases. The anatomic sites of those metastases were not reported. Notably, 41% of the patients had metastases, and the series included osseous tumours, soft-tissue osteosarcomas, and Ewing sarcomas, which were excluded from our review.
In another retrospective study, Iagaru et al. 10 reported on 44 patients with osseous and soft-tissue sarcomas imaged with combination pet/ct. The ct and metabolic portions of the scan were reviewed separately. Imaging by pet was found to be less sensitive than imaging by ct for the detection of metastases (78.6% vs. 82.3%), but more specific (92.8% vs. 76%). In addition to bone tumours and Ewing sarcomas, that series included rhabdomyosarcomas. From the manuscript, it was impossible to tell if any patient would have been upstaged by the addition of metabolic imaging.
In a small series of 16 patients imaged for initial staging of a bone or soft-tissue sarcoma, Piperkova et al. 11 reported that no additional metastatic lesions were detected by metabolic imaging as compared with ct imaging.
We feel that our results are not surprising given the prevalence of distant metastases in our patient population and the expected patterns of spread of soft-tissue sarcoma. If the pre-test probability of having metastatic disease is less than 20%, and if 75% of metastatic lesions will be intrathoracic (where ct is more sensitive 12), a yield of less than 2% is not unexpected, especially after accounting for symptomatic lesions, lesions visible on conventional imaging, and false negatives. In selected clinical scenarios, pet imaging may be justified despite the low yield because of a large impact on clinical care—for example, before a proposed amputation. In other cases, increasing the yield of the staging pet by limiting its use to patients who either are at greater risk of systemic disease or are expected to have a higher proportion of extrathoracic metastases would appear to be appropriate. The former approach is relatively straightforward, because simple clinical factors such as tumour size and grade are strongly predictive of metastatic risk. The latter approach is not as obvious. For example, although myxoid liposarcoma is known to have a higher propensity for bony metastases, screening for them may be better handled by mr imaging 4.
In our series, 2 false-positive and 3 indeterminate pet findings resulted in additional investigations and surgical procedures. Beyond the costs incurred, such findings can lead to delayed management of the primary tumour and additional patient morbidity.
Our findings are somewhat tempered by the small size of the series. For the immediate future, we plan to continue using pet in the staging of patients with soft-tissue sarcoma, although we plan to revisit our indications when more patients have been imaged.
5. CONCLUSIONS
In our patient population, routine use of fdg pet imaging for the detection of metastatic disease in the initial staging of soft-tissue sarcoma added little to conventional chest ct imaging.
6. ACKNOWLEDGMENT
Data from this series were presented at the annual meeting of the American Society of Clinical Oncology, Orlando, Florida, May 29–June 2, 2009.
7. REFERENCES
- 1.Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402–8. doi: 10.1002/cncr.20778. [DOI] [PubMed] [Google Scholar]
- 2.Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3:353–66. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 3.King DM, Hackbarth DA, Kilian CM, Carrera GF. Soft-tissue sarcoma metastases identified on abdomen and pelvis ct imaging. Clin Orthop Relat Res. 2009;467:2838–44. doi: 10.1007/s11999-009-0989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conill C, Setoain X, Colomo L, et al. Diagnostic efficacy of bone scintigraphy, magnetic resonance imaging, and positron emission tomography in bone metastases of myxoid liposarcoma. J Magn Reson Imaging. 2008;27:625–8. doi: 10.1002/jmri.21298. [DOI] [PubMed] [Google Scholar]
- 5.Hicks RJ, Toner GC, Choong PF. Clinical applications of molecular imaging in sarcoma evaluation. Cancer Imaging. 2005;5:66–72. doi: 10.1102/1470-7330.2005.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz MR, Czernin J, Allen–Auerbach MS, et al. fdg-pet/ct imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–63. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folpe AL, Lyles RH, Sprouse JT, Conrad EU, 3rd, Eary JF. 18F Fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–87. [PubMed] [Google Scholar]
- 8.Bastiaannet E, Groen H, Jager PL, et al. The value of fdg-pet in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev. 2004;30:83–101. doi: 10.1016/j.ctrv.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Kim EE. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose pet/ct and conventional imaging. Radiology. 2007;245:839–47. doi: 10.1148/radiol.2453061538. [DOI] [PubMed] [Google Scholar]
- 10.Iagaru A, Quon A, McDougall IR, Gambhir SS. F-18 fdg pet/ct evaluation of osseous and soft tissue sarcomas. Clin Nucl Med. 2006;31:754–60. doi: 10.1097/01.rlu.0000246846.01492.31. [DOI] [PubMed] [Google Scholar]
- 11.Piperkova E, Mikhaeil M, Mousavi A, et al. Impact of pet and ct in pet/ct studies for staging and evaluating treatment response in bone and soft tissue sarcomas. Clin Nucl Med. 2009;34:146–50. doi: 10.1097/RLU.0b013e3181966f9d. [DOI] [PubMed] [Google Scholar]
- 12.De Wever W, Meylaerts L, De Ceuninck L, Stroobants S, Verschakelen JA. Additional value of integrated pet-ct in the detection and characterization of lung metastases: correlation with ct alone and pet alone. Eur Radiol. 2007;17:467–73. doi: 10.1007/s00330-006-0362-7. [DOI] [PubMed] [Google Scholar]