Abstract
Purpose
To improve outcomes in localized osteosarcoma and to reduce the duration of preoperative chemotherapy, we conducted a phase ii trial assessing the efficacy of an intensive protracted regimen without methotrexate (api-ai regimen) in adolescent and adult patients with newly diagnosed disease.
Patients and Methods
Induction chemotherapy consisted of 2 cycles (4 courses) of doxorubicin 60 mg/m2 (days 1 and 15), cisplatin 100 mg/m2 (day 1), and ifosfamide 5 g/m2 (days 2 and 15). The primary endpoint was good histologic response [ghr (≤5% identifiable tumour cells)].
Results
From March 1993 to March 2000, 32 patients [median age: 21 years (range: 15–49 years)] were administered 126 induction courses. The median time between chemotherapy courses was 15 days (range: 12–32 days). All but 3 patients underwent conservative surgery. Toxicity was mainly hematologic, with febrile neutropenia occurring in 35% of patients and grades 3–4 thrombocytopenia in 35%. The ghr rate was 47%. The median follow-up was 64 months (range: 30–115 months). The 5-year event-free and overall survivals were 65% [95% confidence interval (ci): 48%–79%] and 69% (95% ci: 50%–83%) respectively. Two secondary hematologic malignancies occurred: 1 acute myelocytic leukemia (M5) in a poor responder with concomitant relapse, and 1 myelodysplastic syndrome in a patient achieving ghr.
Conclusions
Despite hematologic toxicity, the results observed with the api-ai regimen compare favourably with those observed during previous induction chemotherapy containing methotrexate in adult patients and the pediatric population treated at our institution. These promising results have to be validated by an ongoing national multicentre trial coordinated by the French Sarcoma Group.
Keywords: Induction chemotherapy, osteosarcoma
1. INTRODUCTION
Osteosarcoma is the most common primary malignant bone neoplasm; it occurs frequently in the first two decades of life and sporadically in adult patients. Because of a high rate of systemic spread, cure after surgical treatment alone is uncommon 1,2. The development of effective adjuvant or induction chemotherapy regimens has dramatically improved the prognosis of patients with localized disease at presentation, leading to a cure rate of 50%–70% and limb salvage in more than 90% of cases 3–19.
The most active drugs in osteosarcoma are high-dose methotrexate (mtx), cisplatin, doxorubicin, and ifosfamide. Compared with clinical response, histologic response is a more potent prognostic factor 14,16,18. Since the early 1970s and the first experience of high-dose-mtx–containing chemotherapy 3, all prospective trials have attempted to increase the rate of good histologic response (ghr) with induction chemotherapy schedules that are longer, or more intensified, or both. Despite the large preoperative use of these drugs in several combinations and at various dosages 4–19, it is still not clear which combination, in which doses, is the most effective, with the exception of high-dose mtx in children and teenagers.
Therapeutic options in adults with osteosarcoma are more limited because high-dose mtx is often highly toxic, with great individual variability in metabolism. Toxicity is mainly nonhematologic. Premature interruption of induction chemotherapy occurs in about one third of patients despite adequate folinic acid rescue 20,21. Consequently, the combination of doxorubicin and cisplatin has routinely been used in adults treated at our institution, in accord with the non-mtx arm of the European Osteosarcoma Intergroup phase iii trial 15. The results observed have been similar to those reported in that multicentre trial—that is, 30% of patients achieve a ghr, and 5-year overall survival (os) is 50% 21. But those results remain disappointing compared with the 50% of patients achieving a ghr and the 70% 5-year os observed in pediatric populations with the use high-dose mtx 3,11.
Ifosfamide has demonstrated relevant activity in osteosarcoma when used postoperatively in poorly responding patients and in the metastatic setting with or without etoposide 22,23. These promising results with ifosfamide prompted us to design a protracted intensive regimen of ifosfamide, cisplatin, and doxorubicin (api-ai) as induction chemotherapy in adults with localized osteosarcoma. Our aim was to improve outcomes and to reduce the duration of preoperative chemotherapy.
2. PATIENTS AND METHODS
2.1. Patient Selection
Patients with newly diagnosed, biopsy-proven, and potentially resectable high-grade localized osteosarcoma were eligible for this unicentric study. Additional requirements at entry were
age 16–50 years,
performance status 0 or 1,
normal cardiac function,
adequate renal function (serum creatinine < 125 μmol/L),
adequate hepatic function (serum bilirubin < 25 μmol/L), and
bone marrow reserve (white cell count > 3×109/L, platelet count > 100×109/L).
The evaluation included conventional radiographs, magnetic resonance imaging (mri) of the primary site, computed tomography imaging of the lung, isotope bone scan, and abdominal ultrasound. Several laboratory tests, including alkaline phosphatase and lactate dehydrogenase were performed before any treatment was administered.
2.2. Induction Chemotherapy
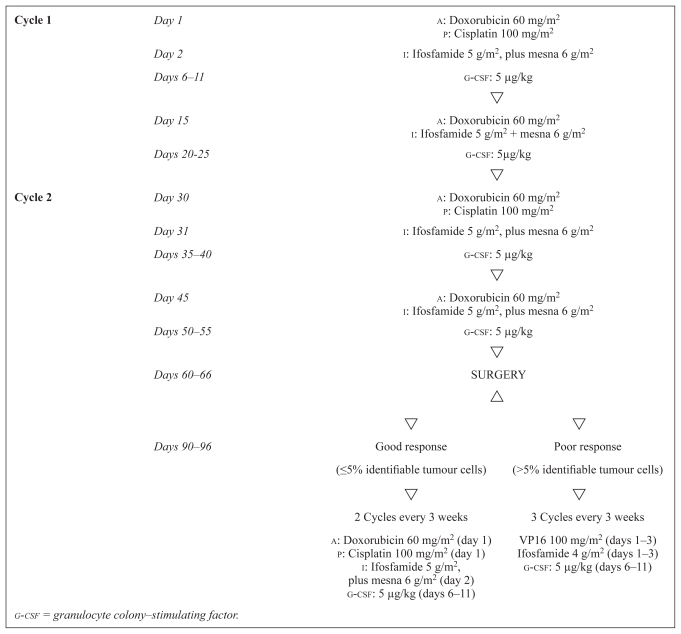
Figure 1 presents the api-ai induction chemotherapy schedule and the postoperative chemotherapy regimens according to histologic response. Patients received 2 cycles (4 courses) of induction chemotherapy consisting of doxorubicin 60 mg/m2 (days 1 and 15), cisplatin 100 mg/m2 (day 1), and ifosfamide 5 g/m2 (days 2 and 15), with an equivalent dose of mesna. The second cycle was planned for day 28. To maintain dose intensity, all patients received granulocyte colony–stimulating factor [g-csf (Granocyte: Chugai Pharmaceutical, Tokyo, Japan)] 5 μg/kg subcutaneously after each chemotherapy course (days 6–11 and days 20–25).
FIGURE 1.
Treatment schema for all patients enrolled in the study
2.3. Acute Toxicity Monitoring and Dose Modifications
Toxicity was evaluated according to World Health Organization criteria 24. Serum creatinine and electrolytes were assessed weekly during the induction api-ai regimen. Liver function tests and alkaline phosphatase were repeated before each chemotherapy course. Courses started as soon as peripheral blood counts had recovered, with an absolute neutrophil count above 1.5×109/L and platelets above 100×109/L. The total dose of cisplatin and ifosfamide was reduced by 20% in the case of grades 1–2 renal toxicity and completely stopped in the case of grades 3–4 renal toxicity. In addition, patients were required to leave the study if they experienced a life-threatening toxicity.
2.4. Surgery Type, Pathology Evaluation, and Definition of Histologic Response
Surgery was planned at 3 weeks after the end of pre-operative chemotherapy. Conservative surgery was performed only if preoperative imaging by mri assured the possibility of achieving wide surgical margins.
Multiple histologic sections were examined to classify response according to the Huvos classification 25: ghr (grade 4: no identifiable tumour cells; grade 3: 1%–5% identifiable tumour cells) or poor histologic response [phr (grade 2: 6%–50% identifiable tumour cells; grade 1: more than 50% identifiable tumour cells)].
2.5. Postoperative Chemotherapy and Follow-up
Chemotherapy started during the 4th week after surgery. The postoperative chemotherapy regimen was adapted to the histologic response (Figure 1). Patients with a ghr received 2 courses of the api regimen; those with a phr received a salvage regimen of 3 courses of chemotherapy combining ifosfamide 4 g/m2 (daily, days 1–3) in continuous infusion (with an equivalent dose of mesna) and etoposide 100 mg/m2 (daily, days 1–3). Prophylactic administration of g-csf was given from day 6 to day 12 after each course of chemotherapy. The postoperative chemotherapy was repeated every 3 weeks.
2.6. Statistical Design
The primary endpoint was a histologic ghr—that is, 5% or fewer identifiable tumour cells after induction chemotherapy. We estimated that our therapeutic combination would be of interest if the ghr rate reached or exceeded 50%. The treatment would be ineffective if the ghr rate was 25% or less. Patients were accrued using a Simon mini-max two-stage phase ii design 26: 16 patients had to be enrolled during the first stage of the study. If fewer than 5 ghrs were observed, no additional patients would be accrued. If 5 or more ghrs were observed, 17 additional patients would be enrolled. On the basis of 33 patients, chemotherapy would be considered ineffective if fewer than 13 ghrs were observed and effective if 13 or more ghrs were observed. The design as described tested the null hypothesis, H0 (that the true response rate was 25% or less), against the alternative hypothesis, H1 (that the response rate was 50% or greater). The significance level—that is, the likelihood of rejecting H0 when H1 is true—was 4.5%. The power—that is, the likelihood of rejecting H0 when H1 is true—was 90%.
Secondary endpoints were os, event-free survival (efs), and toxicity. Treatment results are expressed as percentages with 95% confidence intervals (95% ci) or as medians and ranges. The os rates were estimated using the Kaplan–Meier method, from the first day of chemotherapy to the date of death or to the date of the last follow-up visit for living patients. The efs rates were estimated from the first day of chemotherapy to the date of documented failure (time of relapse, time of second tumour, or time of death) or to the date of the last follow-up visit for those remaining in first complete response (cr) without second malignancy. The 95% cis for survival rates were estimated using the Rothman method 27. Median follow-up was estimated using the Schemper method. Statistical differences in os and efs were tested using the two-tailed log-rank test. Relative risks (rrs) and their 95% cis were estimated using a Cox model.
3. RESULTS
3.1. Patient Characteristics
Between March 1993 and March 2000, 34 adults with newly diagnosed localized operable osteosarcoma were enrolled into this phase ii trial. Two patients were excluded from analysis. One was ineligible because of metastasis at diagnosis; the second moved to another institution during preoperative treatment and was lost to follow-up. The analysis therefore includes 32 patients. Table I describes the characteristics of the patients.
TABLE I.
Patient characteristics
| Characteristic | Value |
|---|---|
| Sex [n (%)] | |
| Male | 19 (59) |
| Female | 13 (41) |
| Age (years) | |
| Median | 21 |
| Range | 15–49 |
| Tumour size [n (%)] | |
| <100 mm | 20 (62) |
| ≥100 mm | 12 (38) |
| Cartilage invasion [n (%)] | |
| Yes | 12 (38) |
| No | 20 (62) |
| Soft tissue invasion [n (%)] | |
| Yes | 28 (87) |
| No | 4 (13) |
| Alkaline phosphatasea [n (%)] | |
| <1.25 uln | 23 (79) |
| ≥1.25 uln | 6 (21) |
| Lactate dehydrogenaseb [n (%)] | |
| <1.25 uln | 25 (83) |
| ≥1.25 uln | 5 (17) |
| Primary tumour site [n (%)] | |
| Femur | 17 (53) |
| Tibia | 7 (22) |
| Humerus | 6 (19) |
| Girdle | 1 (3) |
| Second metacarpal | 1 (3) |
| Histologic subtype [n (%)] | |
| Common | 18 (56) |
| Osteoblastic | 9 (28) |
| Fibroblastic | 3 (10) |
| Chondroblastic | 2 (6) |
| Intended surgery [n (%)] | |
| Resection | 29 (91) |
| Amputation | 3 (9) |
| Postoperative chemotherapy [n (%)] | |
| Yes | 30 (94) |
| No | 2 (6) |
Data missing for 3 patients.
Data missing for 2 patients.
uln = upper limit of normal.
3.2. Neoadjuvant Chemotherapy
A total of 126 courses of api-ai chemotherapy were administered, with 24 patients (75%) receiving the 2 planned api-ai cycles (4 courses), 3 patients (9%) receiving 1 additional ai course because of a delayed surgery date, and 5 patients (16%) receiving only 1.5 cycles (3 courses) because of hematologic toxicity. All courses were given according to protocol. The median time between chemotherapy courses was 15 days (range: 12–32 days). The median time from the start of chemotherapy to surgery was 10 weeks (range: 8–15 weeks).
3.3. Acute Toxicity of Induction Chemotherapy
Overall, 56% of patients experienced at least 1 episode of grade 3 or 4 neutropenia, 28% an episode of grade 3 or 4 thrombocytopenia, and 6% an episode of grade 3 or 4 anemia. A febrile neutropenia episode occurred in 34% of the patients. Table II describes the toxicity of the api-ai regimen per course. Hematologic toxicities (grades 3–4) were significantly more frequent with api courses than with ai courses (p = 0.03), and two thirds of the grade 4 hematologic toxicities were observed after the second api course.
TABLE II.
Grade 3 or 4 hematologic toxicity during the induction regimen
| Variable |
api |
ai |
Total |
Patients |
||||
|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Courses of treatment (n)a | 57 | — | 46 | — | 103 | — | 32 | — |
| Neutropenic fever | 12 | 21 | 2 | 4 | 14 | 14 | 11 | 34 |
| Neutropenia | 34 | 60 | 22 | 48 | 56 | 54 | 18 | 69 |
| Thrombocytopenia | 20 | 35 | 1 | 2 | 21 | 20 | 9 | 34 |
| Anemia | 8 | 14 | 7 | 15 | 15 | 15 | 2 | 8 |
Toxicity data missing for 23 courses (8 api, 15 ai).
api = doxorubicin–cisplatin–iphosphamide; ai = doxorubicin– iphosphamide.
Nausea and vomiting were the major nonhematologic side effects, with grades 3–4 nausea and vomiting occurring in 27% of patients, mostly with api courses. No acute renal, cardiac, neurologic, or hepatologic toxicities were observed. No toxic deaths were recorded during this induction chemotherapy.
3.4. Surgery
A limb-sparing surgery was possible in 29 patients (91%); 3 patients underwent amputation because of large tumour volume or neurovascular bundle involvement at diagnosis. The surgical margins were optimal (“wide margins” in all patients). No major surgical complications were encountered.
3.5. Histologic Response to Preoperative Chemotherapy
Histologic ghrs to chemotherapy were observed in 6 of the first 16 recruited patients; thus, 16 additional patients were recruited. Overall, among the 32 patients evaluated, a histologic ghr was observed in 15 patients (ghr rate: 47%; 95% ci: 29%–65%), including 6 (19%) who exhibited a histologic cr (grade 4).
3.6. Adjuvant Chemotherapy
All but 2 patients with phr (1 refusal and 1 patient with a chondroblastic osteosarcoma who was deemed to be chemotherapy-resistant) received the planned postoperative chemotherapy.
The 15 patients with a ghr received a mean of 2.2 api courses (range: 2–3 courses). As in the induction chemotherapy, toxicities were mainly hematologic: 13 courses (39%) were associated with grades 3–4 neutropenia, including 7 courses (21%) complicated by febrile neutropenia. Grades 3–4 thrombocytopenia was associated with 12 courses (36%). Grades 3–4 nausea and vomiting occurred in 6% of courses.
The 15 patients with a phr received a mean of 2.8 courses of ifosfamide–etoposide. Toxicity with that regimen was also mainly hematologic, with a grade 3 or 4 neutropenia occurring after 10 courses (24%), including 6 courses (14%) with febrile neutropenia and 8 courses (19%) followed by grade 3 or 4 thrombocytopenia. No evidence of grades 3–4 nonhematologic toxicities was observed in these patients.
3.7. Outcome
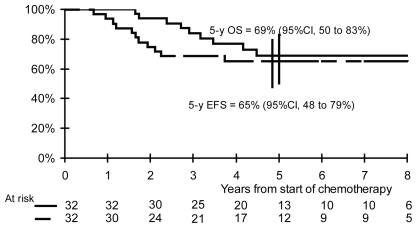
After a median follow-up of 64 months (range: 30–115 months), 21 patients remain continuously free of disease, 10 patients have relapsed, and 2 patients have developed secondary hematologic malignancies (1 with concomitant relapse). For the overall population, the 5-year efs and os rates are 65% (95% ci: 48%–79%) and 69% (95% ci: 50%–83%) respectively (Figure 2).
FIGURE 2.
Event-free (efs) and overall survival (os) for all patients. ci = confidence interval.
The site of first relapse was local in 1 patient and distant in 9 patients (lung in 7, bone in 2). Among the 10 patients who relapsed, 8 died of tumour progression, and 2 are living in complete remission.
A secondary hematologic malignancy developed in 2 patients: 1 acute myelocytic leukemia (M5) with translocation t(9;11) occurred in a phr patient 14 months after the start of treatment, and 1 myelodysplastic syndrome (mds) with chromosome 7 abnormality occurred in a ghr patient 28 months after the start of treatment. The first patient showed a concomitant relapse and died of primary tumour progression; the second failed to respond to chemotherapy and died of mds.
3.8. Outcome According to Histologic Response to Preoperative Chemotherapy
Among the 15 patients with a ghr, 3 experienced failure: 2 patients relapsed, and 1 of those died of disease progression; 1 patient died from mds, without evidence of recurrent osteosarcoma. Among the 17 patients with a pr, 8 relapsed, and 7 of those died of disease progression.
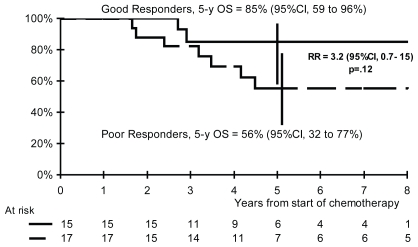
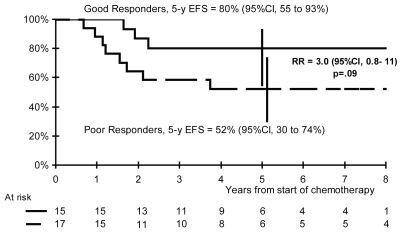
The 5-year efs rates are 80% in ghr patients and 52% in phr patients (rr: 3.0; 95% ci: 0.8–11; p = 0.09; Figure 3), and the 5-year os rates are 85% and 56% respectively (rr: 3.2; 95% ci: 0.7–15; p = 0.12; Figure 4).
FIGURE 3.
Overall survival (os) according to histologic response. ci = confidence interval; rr = risk ratio.
FIGURE 4.
Event-free survival (efs) according to histologic response. ci = confidence interval; rr = risk ratio.
4. DISCUSSION AND CONCLUSIONS
Previous studies have shown that high-dose mtx is highly toxic in adults. Many reports suggest that higher 24-hour mtx residual concentrations are associated with older patients, who should therefore be monitored for toxicity 18,28. In the first French Sarcoma Group trial (osad 93), 3 of the first 8 patients randomized to receive high-dose mtx alternately with cisplatin and ifosfamide developed severe, life-threatening (neurologic and renal) nonhematologic toxicities 20. That study was prematurely interrupted because of the high-dose-mtx–induced toxicity. It later proceeded with a combination of cisplatin–ifosfamide as the induction chemotherapy, with favourable updated results recently being reported 29. Similarly, use of high-dose mtx in adult patients at a single institution 30 showed relevant toxicities, resulting in chemotherapy being given with considerable delay and not according to the planned protocol.
The worse survival observed for patients older than 30 years in some studies is more related to in-adequate compliance with chemotherapy protocols than to the tumour itself (negative predictive prognostic factor) 5,8,14,31. However, when patients 30 years of age and older underwent complete chemotherapy according to the protocol, their life expectancy was the same as that for younger patients 10,32. Many authors 17,19,28 have called attention to the importance of initial intensive chemotherapy and to the close relationship between mtx pharmacokinetics and prognosis in osteosarcoma. In agreement with others, we consider drug tolerance to be an important prognostic factor and delayed low-dose chemotherapy to possibly lead to the development of chemoresistant tumour cell populations, with poor clinical outcome in adult patients.
The European Osteosarcoma Intergroup 15 reported the results of the first prospective clinical trial comparing a high-dose-mtx–containing chemotherapy regimen with a 2-drug regimen, doxorubicin plus cisplatin (ap). That study showed that ap over a short period gives results equivalent to those with a high-dose-mtx–based regimen administered over 44 weeks, with less morbidity and better tolerance. However, the cure rate was still unsatisfactory (5-year os: 50%), and a conventional ap regimen cannot be recommended as standard induction chemotherapy in localized osteosarcoma. A more intensive ap regimen (ap every 2 weeks, 3 cycles before surgery) increases the histologic response rate (46%), but without a concomitant (or any) improvement in os 33.
Because salvage chemotherapy regimens containing high-dose ifosfamide demonstrate relevant activity in metastatic patients 23, ifosfamide has progressively been incorporated into front-line chemotherapy regimens. However, the use of ifosfamide in combination with ap in a neoadjuvant setting precludes any association with high-dose mtx.
As expected, the toxicities associated with the api-ai schedule were primarily hematologic, with 69% of patients experiencing a grade 3 or 4 neutropenia that led to fever in 34% despite prophylactic use of g-csf. However, the relative dose intensity of the regimen was optimal, given that all patients received the full intended dose of each drug, the median delay between each course of chemotherapy was 15 days, and the median time from beginning of chemotherapy to surgery was 10 weeks (instead of the 9 weeks of the planned protocol). In contrast with previous mtx-based chemotherapy regimens, no renal, cardiac, or other dose-limiting nonhematologic toxicities were observed.
Most patients in our study had good prognostic factors 28, such as osteosarcoma of an extremity (78%) limited to less than one third of the long bone (63%), and a histologic subtype associated with ghr (up to 65%). However, 60% of the patients were men, which confers a higher risk of phr 28. Despite the good prognostic factors for the patients included in this trial, the 69% 5-year os rate in this cohort of patients compares favourably with the rates in other prospective unicentric or multicentric trials reported to date 4–19, highlighting the possibility of cure for adult patients as well as younger ones. Furthermore, the 5-year efs and os rates in our patient cohort also compare well with those from three studies that included high-dose mtx in neoadjuvant chemotherapy 34–36 in substantially younger patients 34,35, and we report lower toxicity rates.
The positive impact of this induction chemotherapy regimen on efs and os rates could be explained by
a shift toward a better histologic response rate (as compared with previous trials at our institution)— even in grades1–2 histologic responses;
an intensive, rapidly recycled chemotherapy design that reduces the preoperative period of treatment and avoids the emergence of resistant cell clones in a preoperative context;
incorporation of ifosfamide in the neoadjuvant setting; and
optimal salvage therapy with a fractionated high-dose-ifosfamide–containing regimen for phr patients 23.
The 47% histologic ghr rate observed with this api-ai regimen in adult patients is promising and compares favourably with other active induction regimens, including mtx-containing chemotherapy.
The failure of adjuvant therapy to improve outcome in patients with a phr to induction therapy likely reflects inherent resistance to conventional therapies. Modifications of the postsurgical treatment have limited efficacy. Randomized trials comparing various therapeutic options and investigational strategies such as noncytotoxic agents or intensive chemotherapy with hematologic supports have to be implemented in these patients with a dismal prognosis. In contrast, results in patients with a histologic ghr are optimal, and the acute and delayed toxicities of adjuvant chemotherapy could be detrimental for them. Predictive prognostic factors of relapse in this favourable group of patients need to be carefully analyzed to avoid non-useful additional systemic therapies.
The relatively high incidence of second malignancy in patients treated for an osteosarcoma is a matter of concern, as documented by several authors in previous reports 5,14,17,37–40. The design of the present dose-dense induction chemotherapy (including alkylating agents, cisplatin, and doxorubicin), its use of etoposide in phr patients, and the impact, if any, of hematologic growth factors 40 may contribute to the development of secondary hemopathy 38. However, more than 150 patients have been recruited into other trials based on the same api-ai regimen in locally advanced soft-tissue sarcoma 41 and the Ewing family of tumours 42, and no second malignancy has been reported to date in those populations.
Despite the delayed complications, this short, intensive api-ai chemotherapy regimen produces promising results in adults with localized osteosarcoma and compares favourably (for both histologic response and survival) with those obtained using complex and longer-duration regimens (such as those based on the widely used modified T10 regimen or the less intensive doxorubicin–cisplatin combination). Because of the well-known discrepancies between the results of unicentric non-controlled studies and multicentric trials in osteosarcoma, we urgently await results on the activity and tolerance of this regimen from the ongoing national phase ii trial in a larger population of adults with localized osteosarcoma. The design of a future phase iii study will be planned after analysis of those final results, bearing in mind that patients with osteosarcoma have to be treated in centres with experience in the regimen, which seems a prerequisite for optimizing the cure rate of this curable disease. Future studies will include a pediatric population, and other phase ii and iii studies may compare our protocol with standard neoadjuvant therapies such as the ap combination, with or without high-dose mtx, with interim futility and toxicity analyses after phase ii that will consider a dual endpoint of ghr rate and toxicity rates.
6. REFERENCES
- 1.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 2.Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–6. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–77. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–30. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (coss-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–37. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Picci P, Ruggieri P, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Istituto Rizzoli experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990;65:2539–53. doi: 10.1002/1097-0142(19900601)65:11<2539::aid-cncr2820651125>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Saeter G, Alvegård TA, Elomaa I, Stenwig AE, Holmström T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9:1766–75. doi: 10.1200/JCO.1991.9.10.1766. [DOI] [PubMed] [Google Scholar]
- 8.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan–Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin RS, Chawla SP, Carrasco CH, et al. Preoperative chemotherapy for osteosarcoma with intravenous Adriamycin and intra-arterial cis-platinum. Ann Oncol. 1992;3(suppl 2):S3–6. doi: 10.1093/annonc/3.suppl_2.s3. [DOI] [PubMed] [Google Scholar]
- 10.Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579–91. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 11.Kalifa C, Razafindrakoto H, Vassal G, et al. Chemotherapy in osteogenic sarcoma: the experience of the Pediatric Department of the Gustave Roussy Institute. Cancer Treat Res. 1993;62:347–9. doi: 10.1007/978-1-4615-3518-8_42. [DOI] [PubMed] [Google Scholar]
- 12.Bacci G, Picci P, Ferrari S, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–38. doi: 10.1002/1097-0142(19931201)72:11<3227::aid-cncr2820721116>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari S, Bacci G, Picci P, et al. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol. 1997;8:765–71. doi: 10.1023/a:1008221713505. [DOI] [PubMed] [Google Scholar]
- 14.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 15.Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–17. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan–Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German–Austrian–Swiss osteosarcoma study group’s protocol coss-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–9. doi: 10.1023/a:1008391103132. [DOI] [PubMed] [Google Scholar]
- 18.Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–27. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 19.Bacci G, Briccoli A, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli’s 4th protocol. Eur J Cancer. 2001;37:2030–9. doi: 10.1016/s0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 20.Blay J, Thyss A, Demaille M, et al. osad93: a multicentric pilot study of high dose ifosfamide and cddp in patients > 16 years old with nonmetastatic osteosarcoma [abstract 110] Med Pediatr Oncol. 1996;27:314. [Google Scholar]
- 21.LeCesne AL, Delord JP, Ledeley MC, et al. Localized osteosarcoma (os) in adults: the experience of the Institut Gustave Roussy (igr) [abstract 1963] Proc Am Soc Clin Oncol. 1998;16 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=31&abstractID=14068; cited October 3, 2010] [Google Scholar]
- 22.Pratt CB, Horowitz ME, Meyer WH, et al. Phase ii trial of ifosfamide in children with malignant solid tumors. Cancer Treat Rep. 1987;71:131–5. [PubMed] [Google Scholar]
- 23.Gentet JC, Brunat–Mentigny M, Demaille MC, et al. Ifosfamide and etoposide in childhood osteosarcoma. A phase ii study of the French Society of Paediatric Oncology. Eur J Cancer. 1997;33:232–7. doi: 10.1016/s0959-8049(96)00439-x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (who) WHO Handbook for Reporting Results for Cancer Treatment. Geneva, Switzerland: who; 1979. p. 48. [Available online at: whqlibdoc.who.int/publications/9241700483.pdf; cited October 3, 2010] [Google Scholar]
- 25.Huvos AG. Bone Tumors: Diagnosis, Treatment and Prognosis. Philadelphia, PA: WB Saunders; 1991. [Google Scholar]
- 26.Simon R. Optimal two-stage designs for phase ii clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ. Estimation of confidence limits for the cumulative probability of survival in life table analysis. J Chronic Dis. 1978;31:557–60. doi: 10.1016/0021-9681(78)90043-7. [DOI] [PubMed] [Google Scholar]
- 28.Bielack SS, Kempf–Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 29.Biron P, Rolland F, Thyss A, et al. osad 93: a multicentric prospective phase ii study of preoperative high dose ifosfamide and cddp in adult patients with non metastatic osteosarcoma [abstract 9019] Proc Am Soc Clin Oncol. 2004;22 [Available online at: meeting.ascopubs.org/cgi/content/abstract/22/14_suppl/9019; cited October 3, 2010] [Google Scholar]
- 30.Le Cesne A, Le Deley M, Brugières L, et al. Localized osteosarcoma of adult patients: comparison with pediatric population in the same institution over a 16-year period [abstract 315] Eur J Cancer. 2001;37(suppl 7):S87. [Google Scholar]
- 31.French Bone Tumor Study Group. Age and dose of chemotherapy as major prognostic factors in a trial adjuvant therapy of osteosarcoma combining two alternating drug combinations and early prophylactic lung irradiation. Cancer. 1988;61:1304–11. doi: 10.1002/1097-0142(19880401)61:7<1304::aid-cncr2820610705>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Lindner NJ, Ramm O, Hillmann A, et al. Limb salvage and outcome of osteosarcoma. The University of Muenster experience. Clin Orthop Relat Res. 1999;(358):83–9. doi: 10.1097/00003086-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Lewis J, Nooij M. Chemotherapy at standard or increased dose intensity in patients with operable osteosarcoma of the extremity: a randomised controlled trial conducted by the European Osteo Sarcoma Intergroup (isrctn 86294690) [abstract 3281] Proc Am Soc Clin Oncol. 2003;22:816. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=23&abstractID=102563; cited October 3, 2010] [Google Scholar]
- 34.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 35.Le Deley MC, Guinebretiere JM, Gentet JC, et al. sfop OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007;43:752–61. doi: 10.1016/j.ejca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 37.Pappo A, Schneider NR, Sanders JM, Buchanan GR. Secondary myelodysplastic syndrome complicating therapy for osteogenic sarcoma. Cancer. 1991;68:1373–5. doi: 10.1002/1097-0142(19910915)68:6<1373::aid-cncr2820680631>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case–control study by the Société Francaise d’Oncologie Pédiatrique. J Clin Oncol. 2003;21:1074–81. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 39.Jeha S, Jaffe N, Robertson R. Secondary acute non-lymphoblastic leukemia in two children following treatment with a cisAPI- diamminedichloroplatinum-ii–based regimen for osteosarcoma. Med Pediatr Oncol. 1992;20:71–4. doi: 10.1002/mpo.2950200116. [DOI] [PubMed] [Google Scholar]
- 40.Briasoulis E, Tzouvara E, Tsiara S, Vartholomatos G, Tsekeris P, Bourantas K. Biphenotypic acute leukemia following intensive adjuvant chemotherapy for breast cancer: case report and review of the literature. Breast J. 2003;9:241–5. doi: 10.1046/j.1524-4741.2003.09323.x. [DOI] [PubMed] [Google Scholar]
- 41.Gomez Abuin G, Lassalle M, Bonvalot S, et al. Intensive induction chemotherapy (api-ai regimen) followed by conservative surgery in adult patients with locally advanced soft tissue sarcoma (sts): survival is predicted by the histological response [abstract 9036] Proc Am Soc Clin Oncol. 2004;22:827s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=26&abstractID=1599; cited October 3, 2010] [Google Scholar]
- 42.Assi H, Fizazi K, Missenard G, et al. api-ai: an intensive dosedense induction regimen followed by surgery and radiotherapy in adults patients with localized Ewing sarcoma family of tumors (les). Final results [abstract 756P] Ann Oncol. 2004;15(suppl 3):iii200. [Google Scholar]