Abstract
Introduction
Hepatitis B virus (hbv) reactivation is a recognized complication of chemotherapy. The U.S. Centers for Disease Control and Prevention recommend that all patients be screened for the hbv surface antigen (hbsag) before chemotherapy. We sought to determine the frequency of hbsag testing before chemotherapy at our hospital and to increase the frequency of testing to more than 90% of patients starting chemotherapy.
Methods
Using a retrospective electronic chart review, we identified the frequency of hbsag testing for patients initiated on intravenous chemotherapy at out institution between March 2006 and March 2007. The frequency of left ventricular function testing in the subgroup of patients receiving potentially cardiotoxic chemotherapy was identified as a comparator. An educational intervention was developed and delivered to the multidisciplinary oncology team. The frequency of hbsag testing was determined post intervention. Qualitative interviews were conducted with the members of the oncology team to identify risk perception and barriers to testing.
Results
Of 208 patients started on intravenous chemotherapy between March 2006 and March 2007, only 28 (14%) were tested for hbsag. All 138 patients scheduled for cardiotoxic chemotherapy (100%) underwent left ventricular function testing. In the post-intervention phase, of 74 patients started on intravenous chemotherapy, 24 (31%) underwent hbsag testing, with 1 patient testing positive.
Conclusions
The frequency of testing for hbsag before chemotherapy was very low at our institution. An educational intervention resulted in only a modest improvement. Potential barriers to routine screening include lack of awareness about existing guidelines, controversy about the evidence that supports hbsag testing guidelines, and a perception by physicians that hbv reactivation does not occur with solid tumours.
Keywords: Hepatitis B reactivation, quality assurance
1. INTRODUCTION
Infection with hepatitis B virus (hbv) is a global health problem, and the virus is prevalent in cities with large immigrant communities 1. Reactivation of hbv is a recognized complication associated with the use of immunosuppressive chemotherapy. Reactivation has been reported most frequently in patients with hematologic malignancies, but it has also been associated with chemotherapy use in patients with solid tumours 2–5. In patients with lymphoma who are positive for the hbv surface antigen (hbsag) and who are undergoing chemotherapy, reactivation of hbv can be as high as 73% 6. The potential clinical consequences of hbv reactivation include liver damage from reactivation hepatitis, interruption of cancer treatment, and a mortality rate of up to 5% 7. Prophylaxis with anti-hbv nucleoside/nucleotide analogs such as lamivudine reduces the incidence and severity of reactivation hepatitis in hbv carriers receiving chemotherapy 8–12.
Chronic hbv carriers are often asymptomatic and unrecognized. Screening patients before the initiation of immunosuppressive treatment is therefore important so that prophylaxis can be commenced to prevent hbv reactivation. Guidelines published by the U.S. Centers for Disease Control and Prevention recommend that all patients about to receive chemotherapy for malignant disease be tested for hbsag before cancer treatment is initiated 13,14. In Canada, consensus guidelines from the Canadian Association for the Study of the Liver and the Association of Medical Microbiology and Infectious Disease Canada also recommend testing for hbsag before chemotherapy. Nonetheless, anecdotal reports suggest that institutional practices regarding hbsag testing before chemotherapy are diverse. At our institution, there is no official hbsag screening policy for patients receiving chemotherapy. We therefore designed a quality assurance study to assess the baseline incidence of hbsag testing before chemotherapy start and to try to increase to 90% the frequency with which patients scheduled for intravenous chemotherapy are tested.
2. MATERIALS AND METHODS
2.1. Overview
The objectives of this single-centre quality assurance study were
to determine the frequency of hbsag testing before chemotherapy start,
to compare the rate of hbsag testing with testing for left ventricular function (lvf), and
to attempt to increase the frequency of hbsag testing to 90% among all patients receiving intravenous chemotherapy.
This quality assurance project used the Plan, Do, Study, Act cycle 15. Qualitative interviews were also conducted at the conclusion of the study to uncover barriers to hbsag testing. The setting for this study was an outpatient oncology clinic at St. Michael’s Hospital, an inner-city academic hospital in Toronto, Ontario. The clinic is staffed primarily by nurses, a pharmacist, and 5 physicians (3 medical oncologists, 2 hematologists). Ethics approval was obtained from the hospital’s research ethics board.
2.2. Patients and Data
The records of all patients with a cancer diagnosis (hematologic cancers and solid tumours) who were started on intravenous chemotherapy between March 2006 and August 2007 were retrospectively reviewed for this study. Patients receiving second-or third-line chemotherapy were excluded.
Patients were identified using existing clinical databases in the outpatient oncology clinic. Electronic charts were reviewed to determine the proportion of the study group who received hbsag testing before chemotherapy start (any time in the year preceding the chemotherapy).
A subgroup of patients receiving potentially cardiotoxic chemotherapy (such as an anthracycline or trastuzumab) was also identified. The proportion of those patients who underwent lvf testing either by echocardiography or by multi-gated acquisition imaging before chemotherapy start was identified as a comparator.
2.3. Intervention
In developing interventions to increase hbsag screening, several factors were taken into consideration:
Reactivation of hbv and screening for hbsag before chemotherapy start were relatively new concepts for some members of the oncology team. An educational component was therefore required to introduce those concepts to staff.
An algorithm for a hbsag screening protocol had to be developed for staff.
Because hbsag screening before chemotherapy is a new concept, reminders about screening had to be developed as reinforcement.
Because a checklist routinely signed off by the pharmacists was successfully developed as a reminder for liver and kidney function tests, the same strategy was adopted for hbsag screening.
Four strategies to increase hbsag screening before chemotherapy start were implemented:
All members of the oncology team—physicians, pharmacists, and nurses—attended a 20-minute educational seminar presented by a physician who described the risks and consequences of hbv reactivation and the benefits of hbsag screening.
Algorithms outlining an approach to hbsag testing before chemotherapy start and how to proceed in the absence of test results were developed for the physicians, pharmacists, and nurses. These algorithms were presented to the members of the oncology team as part of the educational seminar.
Posters reminding members of the oncology team to test for hbsag were developed and displayed in treatment areas.
Pharmacists at our institution routinely review and sign off the records of complete blood counts, liver enzymes, and creatinine levels before approving chemotherapy. Testing for hbsag was added to the checklist reviewed by our pharmacists before a first chemotherapy administration. If hbsag testing had not been done, pharmacists were asked to convey that information to the physician who had ordered the chemotherapy and to request hbsag testing.
The educational intervention was delivered in a 2-month run-in phase to give members of the oncology team time to adopt and to become accustomed to the hbsag screening program.
2.4. Outcome Assessment
For 3 consecutive months starting 2 months after the completion of the educational intervention, data on the proportion of patients who had received hbsag testing before chemotherapy start were collected but not fed back to the clinical team. During the same period, data were also recorded on the proportion of patients receiving potentially cardiotoxic chemotherapy who had received lvf testing before chemotherapy start.
The proportions of patients who received hbsag testing before chemotherapy start were then compared for the periods before and after the educational intervention.
2.5. Qualitative Interviews
Based on the results from the study phase, qualitative interviews were conducted to identify perceived obstacles and challenges to hbsag testing before chemotherapy start. Interviews were conducted with the principal members of the oncology team, including the physicians and pharmacists. For this purposive sample, all team members were contacted by e-mail to arrange interviews. In total, 4 physicians (3 medical oncologists, 1 hematologist) and 2 pharmacists (1 inpatient pharmacist, 1 outpatient pharmacist) participated in a one-on-one interview with a member of the research team. All participants were interviewed separately.
Each core oncology team member was asked these standard questions:
Do you think that hbsag testing before chemotherapy start is important? Why or why not?
What are some of the challenges with regard to hbsag testing?
How would you feel about an automatic (standard) order for hbsag testing before chemotherapy start for all cancer patients?
Can you think of other strategies to raise the frequency of testing?
Do you have any previous experience with reactivation hepatitis? If yes, what was the outcome?
The interviews were audiotaped and transcribed. Two reviewers listened to the interviews separately and extracted themes. The two lists of themes were then compared, and common themes were identified.
2.6. Statistical Analyses
Quantitative data were analyzed using Statistical Analysis System (version 9.1: SAS Institute, Cary, NC, U.S.A.). Dichotomous variables were compared using the chi-square or Fisher exact test, as appropriate. A two-sided p value of 5% or less was considered significant.
3. RESULTS
3.1. Before the Educational Intervention
Between March 2006 and March 2007, 208 patients received first-line intravenous chemotherapy at our institution. Of the 208 patients, 163 (78%) had solid tumours, and 45 (22%) had a hematologic malignancy. Table I outlines the baseline characteristics of the patients. During the same time period, 138 patients received cardiotoxic chemotherapy.
TABLE I.
Baseline patient characteristics
| Characteristic | Group |
|
|---|---|---|
| Pre-intervention | Post-intervention | |
| Patients (n) | 208 | 77 |
| Average age (years) | 58 | 54 |
| Sex [n (%)] | ||
| Female | 141 (68) | 53 (69) |
| Male | 67 (32) | 24 (31) |
| Cancer type [n (%a)] | ||
| Solid tumours | 163 (78) | 60 (78) |
| Breast | 94 (45) | 31 (40) |
| Lung | 14 (7) | 3 (4) |
| Colorectal | 20 (10) | 20 (26) |
| Other gastrointestinal | 14 (7) | 5 (6) |
| Genitourinary | 7 (3) | 0 (0) |
| Kaposi sarcoma | 7 (3) | 1 (1) |
| Others | 7 (3) | 0 (0) |
| Hematologic cancers | 45 (22) | 17 (22) |
| Non-Hodgkin lymphoma | 36 (17) | 11 (14) |
| Hodgkin lymphoma | 6 (3) | 4 (5) |
| Multiple myeloma | 3 (1) | 1 (1) |
| Others | 0 (0) | 1 (1) |
Because of rounding, percentages may not add to exactly 100%.
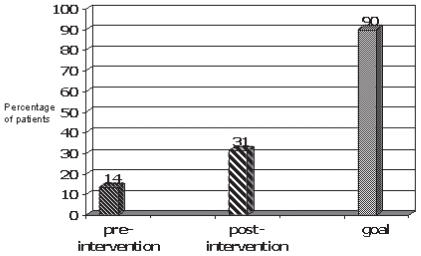
Of the 208 patients, 28 (14%) were tested for hbsag before receiving their chemotherapy. This proportion was well below our target level of 90% (Figure 1). By comparison, 100% of patients receiving cardiotoxic chemotherapy during the same period underwent a lvf assessment (n = 138, p < 0.001, Figure 2). None of the 28 patients tested were positive for hbsag. Fewer than 1% of the patients who received lvf testing had an abnormal result. As compared with patients receiving treatment for a solid tumour, patients receiving treatment for a hematologic malignancy were tested for hbsag in significantly larger proportion before chemotherapy start (38% vs. 7%, p < 0.0001).
FIGURE 1.
Comparison of testing rates for hepatitis B surface antigen (hbsag) in patients receiving chemotherapy: pre-intervention phase (n = 208), post-intervention phase (n = 77), and 90% testing goal.
FIGURE 2.
Comparison of testing rates for hepatitis B surface antigen (hbsag) and for left ventricular (lv) function in patients receiving chemotherapy: hbsag testing rate in the pre-intervention phase (n = 208), and lv function testing rate in the subgroup of patients receiving potentially cardiotoxic chemotherapy in the same period (n = 138).
3.2. After the Educational Intervention
During the 3-month period after the run-in phase for the educational intervention, 77 patients were started on intravenous chemotherapy. Of those 77 patients, 24 (31%) were tested for hbsag before chemotherapy start. That rate represented a 130% increase in testing frequency as compared with the period before the educational intervention (Figure 1), but it was still far below our testing target of 90% of patients before chemotherapy start. Of the 24 patients in this cohort that were tested, 1 was positive for hbsag. That patient had a hematologic malignancy.
3.3. Qualitative Interviews
All 5 members of the oncology team who were contacted participated in the qualitative interviews. Table II lists the themes identified for each question.
TABLE II.
Summary of responses from the qualitative interviews
| Is testing for hepatitis B surface antigen (hbsag) important? | |
| Oncologist 1 | Yes, because it can prevent reactivation hepatitis, which can be fatal, but only for patients who have risk factors for hepatitis B. |
| Oncologist 2 | Important only for hematologic tumours, not for solid tumours. |
| Oncologist 3 | Yes, because it can prevent something that is potentially fatal. |
| Hematologist | Yes, because it can prevent something that is potentially fatal. |
| Pharmacist | |
| Outpatient | Yes, because it can prevent reactivation hepatitis. |
| Inpatient | Yes, because it can prevent reactivation hepatitis. |
| Challenges inhbsag testing? | |
| Oncologist 1 | Results not returned in time, or test ordered but not done. |
| Oncologist 2 | Cost-effectiveness (no hepatitis B reactivation in solid tumours seen in 15 years of practice). |
| Oncologist 3 | Remembering to test before starting a patient on chemotherapy. |
| Hematologist | None identified (always tests for hbsag before chemotherapy in hematologic cancers). |
| Pharmacist | |
| Outpatient | Test not ordered by the oncologists. |
| Inpatient | Remembering to check whether testing was done, especially if patient is sick. |
| Implement an automatic or standard order forhbsag testing? | |
| Oncologist 1 | No, because if a patient lacks risk factors for hepatitis B, then testing is not needed. |
| Oncologist 2 | Not at this point, because testing may not be cost-effective. |
| Oncologist 3 | Yes, it would be useful. |
| Hematologist | Not necessary |
| Pharmacist | |
| Outpatient | Yes, because no further reminders for checking are needed. |
| Inpatient | Yes, because testing would not be missed. |
| Other strategies to increase the frequency ofhbsag testing? | |
| Oncologist 1 | More checks needed at the nursing and pharmacy levels in the process of testing. |
| Oncologist 2 | More convincing data are needed to show that hepatitis B testing is cost-effective. |
| Oncologist 3 | Automated testing before chemotherapy start. |
| Hematologist | Checklist to remind whether hepatitis B testing was done. |
| Pharmacist | |
| Outpatient | No additional strategies identified. |
| Inpatient | Implement a chemotherapy flow sheet inquiring about hbsag on the inpatient unit. |
| Previous experience with hepatitis B reactivation hepatitis? | |
| Oncologist 1 | No |
| Oncologist 2 | No |
| Oncologist 3 | No |
| Hematologist | Patient with lymphoma developed fulminant hepatic failure from hepatitis B reactivation. |
| Pharmacist | |
| Outpatient | No |
| Inpatient | No |
Most of the staff interviewed (4 of 5) believe that hbsag screening before chemotherapy start is important for all patients with cancer. Most interviewees believe that hbv reactivation involves serious and potentially fatal consequences.
According to the medical oncologists, challenges related to hbsag testing include administrative errors in ordering the test (that is, the test is ordered by the physician, but not done by the laboratory), concerns that testing for hbsag may not be cost-effective, and difficulty in remembering to order the test. The sole hematologist interviewed routinely tests for hbsag and therefore identified no barriers to testing. The major challenge identified by the pharmacists was a failure on the part of the physicians to order the hbsag test—even when the physicians were reminded.
In regard to implementing an automated hbsag order, only 1 of the 4 physicians interviewed thought that an automated order is a good idea; both pharmacists interviewed liked that option. The only respondent who had experience with hbv reactivation was the hematologist, one of whose patients developed fulminant fatal hepatic failure secondary to hbv reactivation.
4. DISCUSSION
Despite recommendations to test for hbsag before chemotherapy start, the proportion of patients being tested for hbsag in that situation at our institution was low. After educational interventions were implemented, the frequency of hbsag testing increased to 30% of all targeted patients, but still fell far short of our 90% target for testing in patients starting firstline intravenous chemotherapy at our institution.
By contrast, lvf was routinely tested at our institution before first administration of cardiotoxic chemotherapy. The high rate of cardiac function testing indicates that the programs currently implemented at our institution to predict and prevent side effects of chemotherapy can have a high compliance rate. At our institution, a “ready for chemotherapy” list is reviewed and signed off by pharmacists before a first chemotherapy administration. That list includes complete blood count, liver enzymes, creatinine, and cardiac function testing. As part of our intervention, hbsag was added to the “ready for chemotherapy” list. However, despite the success of the list for lvf testing, it did not produce high rates of testing for hbsag. That finding is interesting, because the paperwork and lead time required for hbsag testing is much less than that for cardiac function assessment. At our institution, it typically takes 1–2 weeks to obtain non-urgent cardiac assessment. By contrast, hbsag is available 24–48 hours after blood is drawn.
Our study has a few limitations that merit mention. First, only electronic charts were reviewed. As a result, hbv status was known only for patients who were tested at our institution. It is possible that some small number of individuals underwent a hbsag test at a different institution and that the treating oncologist or hematologist knew about the results and thus did not order the test. However, given the very low level of testing at our institution, it is unlikely that this bias had a major effect on our results. Additional limitations include the single-centre nature of our study and the relatively small sample sizes. Further studies are necessary to confirm whether the results obtained at our institution reflect widespread practice.
Several factors likely contributed to the low frequency of hbv screening.
Low compliance to screening by the oncologists plays a role. In our qualitative interviews, one of the barriers identified was the perception that hbv reactivation in patients with solid tumours is a rare event and that testing is of low importance in that setting. But those beliefs are not supported by published literature. Most studies on hbv reactivation have focused on patients with a hematologic malignancy, but the few studies that actually assessed the risks in patients with solid tumours suggest a concerning rate of reactivation. For instance, among patients receiving intravenous chemotherapy for breast cancer, 31%–55.6% of hbsag-positive patients developed hbv reactivation 2,3. Reactivation of hbv has also been reported in other solid tumours, including hepatocellular carcinoma and nasopharyngeal carcinoma (reported reactivation rates of 36% and 28.6% respectively) 4,5. Case reports of hbv reactivation in bladder, pancreatic, and non-small-cell lung cancer have also been reported 16–18 (no cohort data are available for those populations). Still, rates of adverse outcomes related to reactivation remain poorly characterized. One study reported a fatal hepatic failure rate of 11%. Another study showed no associated mortality; patients with hbv reactivation resolved without treatment 3,19. These inconsistencies in the literature may contribute to confusion about best practice.
Difficulty in changing physician behaviour has also been documented. Significant discrepancies between best available scientific evidence and actual clinical practice have been widely reported 20,21, as has a disconnect between knowledge and consistent practice. For instance, hand-washing has been demonstrated to be an integral part of infection control, and educational initiatives to drive home this point are widespread. However, studies have shown that adherence by physicians to this measure has remained unsatisfactory despite interventions and guidelines 21. The discrepancy between knowledge, education, and actual practice highlights the difficulty of changing physician behaviour.
A systematic requirement to test for hbsag before chemotherapy start is also lacking. The low rate of hbsag testing observed in the present study could potentially be circumvented by automatic testing when chemotherapy is ordered for the first time. However, an automated order was not popular among our interviewees because of the perception that it may not be cost-effective and that patients should be tested only if they have recognized risk factors for hbv. However, testing based on risk factors may not be effective in capturing all patients at risk. Studies of Americans born in hbv-endemic areas show that only two thirds of the subjects are ever screened for hbv 22. A second study reported that fewer than 50% of survey respondents received recommendations from their physicians on hbsag testing 23.
Our study showed that the baseline hbsag testing rate at our institution was only 13%, with most tests being ordered by a single physician. Based on the demographics of the population served by our inner-city hospital, we would expect that substantially more than 13% of presenting patients would have risk factors for hbv. Overall, our study suggests that, although physicians express an intention to screen at-risk individuals for hbsag, that intention is not always realized.
5. CONCLUSIONS
Our study of hbsag testing before start of intravenous chemotherapy found that the rate of testing at our institution was very low. A modest improvement in testing occurred after multiple educational and other interventions. Potential barriers to testing included a lack of knowledge about existing guidelines that recommend testing before chemotherapy start, a perception that hbv reactivation does not occur in patients with solid tumours, and confusion caused by inconsistencies in the evidentiary basis of existing guidelines.
To increase the frequency of hbsag testing before chemotherapy start, we feel that additional steps are needed. Future steps at our institution include planning for additional education that highlights hbv reactivation in both hematologic and solid tumours, and a consideration of automatic orders for hbsag testing. More studies are also needed to clarify the clinical effects of routine hbsag screening before chemotherapy start.
6. REFERENCES
- 1.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39(suppl 1):S64–9. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 2.Yeo W, Ho WM, Hui P, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004;88:209–15. doi: 10.1007/s10549-004-0725-1. [DOI] [PubMed] [Google Scholar]
- 3.Dai MS, Wu PF, Shyu RF, Lu JJ, Chao TY. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int. 2004;24:540–6. doi: 10.1111/j.1478-3231.2004.0964.x. [DOI] [PubMed] [Google Scholar]
- 4.Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–6. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 5.Yeo W, Hui EP, Chan AT, et al. Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol. 2005;28:379–84. doi: 10.1097/01.coc.0000159554.97885.88. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (hbv) reactivation in hbvcarriers with lymphoma. Hepatology. 2003;37:1320–8. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 7.Liang R, Lau GKK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patients who are also chronic hepatitis B carriers: a review of the problem. J Clin Oncol. 1999;17:394–8. doi: 10.1200/JCO.1999.17.1.394. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri F, Ermacora A, Sperotto A, et al. Lamivudine allows completion of chemotherapy in lymphoma patients with hepatitis B reactivation. Br J Haematol. 2000;108:394–6. doi: 10.1046/j.1365-2141.2000.01847.x. [DOI] [PubMed] [Google Scholar]
- 9.Persico M, De Marino F, Russo GD, et al. Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus– infected patients treated for non-Hodgkin lymphoma. Blood. 2002;99:724–5. doi: 10.1182/blood.v99.2.724. [DOI] [PubMed] [Google Scholar]
- 10.Li YH, He YF, Jiang WQ, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–5. doi: 10.1002/cncr.21701. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Hahn JS, Park SY, et al. Long-term outcome after prophylactic lamivudine treatment on hepatitis B virus reactivation in non-Hodgkin’s lymphoma. Yonsei Med J. 2007;48:78–89. doi: 10.3349/ymj.2007.48.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz LH, Fraser A, Gafter-Gvili, Leibovici L, Tur–Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepat. 2008;15:89–102. doi: 10.1111/j.1365-2893.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 14.Sherman M, Shafran S, Burak K, et al. Management of chronic hepatitis B: consensus guidelines. Can J Gastroenterol. 2007;21(suppl C):5C–24C. [PMC free article] [PubMed] [Google Scholar]
- 15.Guinane CS, Sikes JI, Wilson RK. Using the pdsa cycle to standardize a quality assurance program in a quality improvement driven environment. Jt Comm J Qual Improv. 1994;20:696–705. doi: 10.1016/s1070-3241(16)30118-3. [DOI] [PubMed] [Google Scholar]
- 16.Nigashiyama H, Harabayashi T, Shinohara N, Chuma M, Hige S, Nonomura K. Reactivation of hepatitis in a bladder cancer patient receiving chemotherapy. Int Urol Nephrol. 2007;39:461–3. doi: 10.1007/s11255-006-9006-8. [DOI] [PubMed] [Google Scholar]
- 17.Öksüzoglu B, Kilickap S, Yalcin S. Reactivation of hepatitis B virus infection in pancreatic cancer: a case report. Jpn J Clin Oncol. 2002;32:543–5. doi: 10.1093/jjco/hyf113. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Zee B, Chan AT, et al. A phase ii study of gemcitabine plus oral etoposide in the treatment of patients with advanced nonsmall cell lung carcinoma. Cancer. 2000;89:543–50. [PubMed] [Google Scholar]
- 19.Yeo W, Chan PK, Hui P, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–61. doi: 10.1002/jmv.10430. [DOI] [PubMed] [Google Scholar]
- 20.Sladek RM, Bond MJ, Huynh LT, Chew DP, Phillips PA. Thinking styles and doctors’ knowledge and behaviors relating to acute coronary syndromes guidelines. Implement Sci. 2008;3:23. doi: 10.1186/1748-5908-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sladek RM, Bond MJ, Phillips PA. Why don’t doctors wash their hands? A correlational study of thinking styles and hand hygiene. Am J Infect Control. 2008;36:399–406. doi: 10.1016/j.ajic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Taylor VM, Yasui Y, Burke N, et al. Hepatitis B testing among Vietnamese American men. Cancer Detect Prev. 2004;28:170–7. doi: 10.1016/j.cdp.2004.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CA, Lin SY, So SK, Chang ET. Hepatitis B and liver cancer knowledge and preventive practices among Asian Americans in the San Francisco Bay Area, California. Asian Pac J Cancer Prev. 2007;8:127–34. [PubMed] [Google Scholar]