Abstract
In January 2010, a panel of Canadian oncologists with particular expertise in colorectal cancer (crc) gathered to develop a consensus guideline on the use of therapies against the epidermal growth factor receptor (egfr) in the management of metastatic crc (mcrc). This paper uses a case-based approach to summarize the consensus recommendations developed during that meeting.
These are the consensus recommendations:
Testing for the KRAS status of the tumour should be performed as soon as an egfr inhibitor is being considered as an option for treatment.
Anti-egfr therapies are not recommended for the treatment of patients with tumours showing mutated KRAS status.
For a patient with wild-type KRAS and an Eastern Cooperative Oncology Group status of 0–2, whose mcrc has previously been treated with a fluoropyrimidine, irinotecan, and oxaliplatin, switching to an egfr inhibitor is a recommended strategy.
Cetuximab, cetuximab plus irinotecan, and panitumumab are all options for third-line therapy in patients with wild-type KRAS, provided that tolerability is acceptable.
Keywords: Anti-egfr, metastatic colorectal cancer, cetuximab, panitumumab, Canadian consensus
1. INTRODUCTION
In Canada, two epidermal growth factor receptor (egfr) inhibitors are currently indicated for the treatment of metastatic colorectal cancer (mcrc):
Cetuximab (Erbitux: Bristol–Myers Squibb, Princeton, NJ, U.S.A.)
Panitumumab (Vectibix: Amgen Canada, Mississauga, ON).
Table I shows the currently approved (2010) indications 1,2.
TABLE I.
Indications set out in Canadian product monographs for anti–epidermal growth factor receptor (egfr) therapies in metastatic colorectal cancer (mcrc)
| Cetuximab | Used in combination with irinotecan for the treatment of egfr-expressing mcrc in patients who are refractory to other irinotecan-based chemotherapy regimens. |
| Used as a single agent for the treatment of egfr-expressing mcrc in patients who are intolerant to irinotecan-based chemotherapy. | |
| Used as a single agent for the treatment of patients with egfr-expressing mcrc after failure of both irinotecan- and oxaliplatin-based regimens and after administration of a fluoropyrimidine, where the tumours have a wild-type (non-mutated) Kirsten rat sarcoma (kras) gene. | |
| Panitumumab | Used as monotherapy for the treatment of patients with egfr-expressing mcrc with non-mutated (wild-type) kras after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens. |
Cetuximab and panitumumab have been investigated in a number of clinical trials in mcrc, with active research ongoing. In January 2010, a panel of Canadian oncologists with representation from across the country and with particular expertise in crc gathered to develop a consensus guideline on the use of anti-egfr therapies in the management of mcrc.
2. OBJECTIVES
The objective of the consensus process was to
develop evidence-based recommendations for the selection of anti-egfr third-line monotherapy when both cetuximab and panitumumab are reimbursed.
define additional factors beyond efficacy that may influence choice of therapy, including toxicity and costs (comprising drug, chair time, and travel time costs).
develop a consensus opinion on the use of alternative dosing schedules for anti-egfr therapy (for example, cetuximab every 2 weeks, or panitumumab every 3 weeks).
provide evidence-based recommendations for the choice between anti-egfr monotherapy and combination therapy.
develop a consensus opinion on the choice between chemotherapy plus bevacizumab and chemotherapy plus anti-egfr in patients with potentially resectable liver metastases.
At the consensus meeting, the discussion of these topics took into account a series of actual cases selected to illustrate the potential scenarios in which anti-egfr therapies might be considered. Those cases provide the framework for the consensus recommendations.
Although the variable provincial funding and reimbursement statuses for cetuximab and panitumumab were discussed, the participants elected to make their consensus recommendations on best medical practice alone, in the hypothetical environment in which both anti-egfr therapies are fully reimbursed.
3. EGFR INHIBITORS AS THIRD-LINE THERAPY FOR PATIENTS WITH KRAS WILD-TYPE mCRC
3.1. Case Vignette 1: KRAS Wild-Type Tumour Status
Mrs. A, a 54-year-old woman with no family history of cancer and a personal history of well-controlled hypertension, quit smoking 20 years ago. She was later diagnosed with poorly differentiated colon cancer and underwent resection of a T3N0 (0 of 13 nodes) tumour. She was treated with adjuvant capecitabine for 24 weeks.
A year after that, follow-up revealed a single liver metastasis. The metastasis was resected, and Mrs. A was then treated with 12 cycles of capecitabine plus oxaliplatin (xelox).
Approximately 2 months after the completion of xelox, Mrs. A developed recurrent liver and lung metastases. She received leucovorin, fluorouracil, and irinotecan (folfiri), plus bevacizumab. She tolerated the irinotecan well and achieved a partial response to therapy. After 25 cycles, she showed progressive disease. Her current Eastern Cooperative Oncology Group (ecog) performance status is 1.
Tumour testing showed wild-type KRAS (KRAS-wt) status.
3.2. Case Vignette 2: KRAS-wt Tumour Status
Mrs. B, a 65-year-old woman, has a history of dyslipidemia, hypertension, and osteoarthritis. She was diagnosed with a mid-rectal cancer. Preoperatively, she received short-course radiotherapy; anterior resection of a pT3N2 (6 of 8 nodes) adenocarcinoma followed. She was also treated with adjuvant leucovorin, fluorouracil, and oxaliplatin (folfox) for 12 cycles. She developed grade 3 neuropathy.
Five months after conclusion of the folfox cycles, Mrs. B developed recurrent bilateral lung metastases. She had persistent grade 2 oxaliplatin neuropathy and was given folfiri plus bevacizumab. She developed hypertension related to bevacizumab and required irinotecan dose reductions for toxicity and myelosuppression.
After 16 cycles of folfiri plus bevacizumab, she experienced disease progression. Her ecog performance status is 2.
Tumour testing showed KRAS-wt status.
3.3. Goals of Therapy
In third-line therapy, the goal is to extend progression-free (pfs) and overall survival (os) while maximizing quality of life for the patient. Mrs. A and Mrs. B, who have both progressed on second-line therapy and have KRAS-wt tumours, are both candidates for anti-egfr therapy with either cetuximab (alone or in combination with irinotecan) or panitumumab monotherapy, per the approved indications.
3.3.1. Monotherapy: Cetuximab Compared with Panitumumab
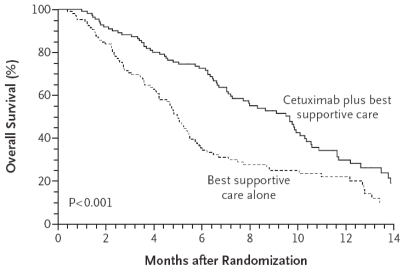
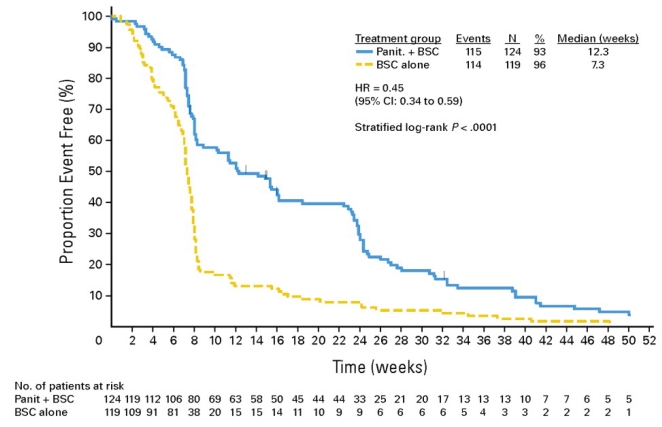
Studies of cetuximab and panitumumab have demonstrated that both agents have efficacy in improving pfs when used as third-line therapy in patients with KRAS-wt tumours 3–5. For cetuximab, analysis of the KRAS-wt subgroup in the co.17 study 3 showed that the median pfs was 3.7 months for cetuximab-treated patients and 1.9 months for those treated with best supportive care (bsc) alone [hazard ratio (hr): 0.40; p < 0.0001]. In addition, the investigators reported a significant 4.7-month improvement in median os (9.5 months vs. 4.8 months, p < 0.0001; Figure 1). For panitumumab, the KRAS subgroup analysis by Amado et al. 5 of the third-line efficacy trial (van Cutsem et al., 2007 4) showed that panitumumab-treated patients with KRAS-wt tumours had a mean pfs of 12.3 weeks as compared with 7.3 weeks with bsc alone (Figure 2), but because crossover was permitted, the trial was unable to demonstrate an os advantage.
FIGURE 1.
Overall survival: cetuximab compared with best supportive care alone in patients with the wild-type KRAS gene 3. Copyright 2008, Massachusetts Medical Society. All rights reserved.
FIGURE 2.
Progression-free survival: panitumumab compared with best supportive care (bsc) alone in patients with a wild-type KRAS gene 5. Reprinted with permission. Copyright 2008, American Society of Clinical Oncology. All rights reserved.
3.3.2. Cetuximab Compared with Panitumumab: Factors Beyond Efficacy
Extrapolation from cross-trial comparisons suggest that cetuximab and panitumumab are comparable with respect to antitumour efficacy. The decision to recommend one therapy over the other may, therefore, in some jurisdictions, come down to provider preference, accessibility, and reimbursement criteria. However, assuming that the two egfr-inhibiting agents are available, certain differences between the agents and their regimens may lead to the selection of one agent over the other (Table II).
TABLE II.
Selecting an epidermal growth factor receptor (egfr) inhibitor for third-line treatment of metastatic colorectal cancer (mcrc): differences between cetuximab and panitumumab
| Variable | Cetuximab | Panitumumab |
|---|---|---|
| Antibody type | IgG1, chimeric | IgG2, human |
| Indicated in combination with irinotecan? | Yes (monotherapy or in combination with irinotecan) | No |
| Clinical trial data showing overall survival benefit? | Yes | No |
| Dose and schedule | Loading dose: 400 mg/m2 (maximum infusion rate: 10 mg/min) Subsequent doses: 250 mg/m2, weekly |
6 mg/kg body weight, once every 2 weeks |
| Chair time | Loading dose: 120 minutes Subsequent doses: 60 minutes |
60 minutes |
| Post-infusion observation | 60 minutes | None required |
| Premedication | Dexamethasone and diphenhydramine recommended | None required |
For example, if the patient has to travel a considerable distance for treatment, then a recommendation for panitumumab might be considered because of its recommended schedule of administration (once every 2 weeks, as compared with once weekly for cetuximab).
In addition, the toxicity profiles of the two anti-egfr therapies are not identical, and so that difference may be the deciding factor in selection of a therapy. Head-to-head trials are lacking, but manifestations of skin toxicity may differ somewhat between the two agents. Panitumumab is associated with a higher reported rate of paronychia. Cetuximab, a chimeric monoclonal antibody, is associated with a higher risk of infusion reaction. Post-infusion observation is therefore required, and mitigation with pre-treatment corticosteroids (dexamethasone, for instance) and antihistamines (diphenhydramine, for instance) may be considered 6.
It should be emphasized that cetuximab and panitumumab are considered cross-resistant. If progression occurs on the selected egfr inhibitor, there is no evidence to suggest that a switch to the other agent is a reasonable strategy.
Alternative Dosing Schedules
Limited, but widely accepted, phase ii data show that a schedule of cetuximab every 2 weeks may be possible, with similar efficacy outcomes 7,8 (Table III). However, the consensus opinion is that a routine recommendation for the administration of cetuximab every 2 weeks would be premature. Similarly, indications from phase i studies are that panitumumab administered once every 3 weeks may be feasible 9,10, but those preliminary findings also require further study before they can be implemented into current guidelines for administration.
TABLE III.
Alternative dosing trials with cetuximaba
| Parameter | Cetuximab dosing |
|||
|---|---|---|---|---|
| 250 mg/m2 weeklyb |
500 mg/m2 every 2 weeksc |
|||
| bond11 | mabel6 | Danish study 8 | Danish study 8 | |
| Patients (n) | 329 | 1147 | 65 | 74 |
| Response rate (%) | 22.9 | 20 | 18.5 | 25.7 |
| Median ttp (months) | 4.1 | nr | 5.4d | 5.4d |
| Median os (months) | 8.6 | 9.2 | 10.4 | 8.9 |
The main irinotecan regimens were 180 mg/m2 every 2 weeks and 350 mg/m2 every 3 weeks.
Preceded by an initial dose of 400 mg/m2 (according to the approved dosing regimen).
The first course is infused over 120 minutes, followed 1 hour later by irinotecan at a dose of 180 mg/m2 as a 30-minute infusion. Subsequent courses of cetuximab are infused over 60 minutes, immediately followed by irinotecan, resulting in a total treatment time of only 90 minutes.
In the Danish study, the values were reported as median progression-free survival.
bond = Bowel Oncology with Cetuximab Antibody; mabel = Monoclonal Antibody Erbitux in European Pre-License; ttp = time to progression; nr = not reported; os = overall survival.
Monotherapy or Combination Therapy
Once the decision to treat with an anti-egfr therapy is made, the next decision is whether to use combination therapy with irinotecan. Although phase ii and iii trials of panitumumab in combination with second-line folfiri have been reported, panitumumab is not currently indicated for combination therapy in the third-line setting.
There is evidence to suggest that the combination of cetuximab and irinotecan is superior to cetuximab monotherapy. In the randomized bond (Bowel Oncology with Cetuximab Antibody) study of pre-treated patients with irinotecan-refractory mcrc, higher response rates (22.9% vs. 10.8%) and a longer pfs (4.1 months vs. 1.5 months) were observed for the combination of cetuximab and irinotecan than for cetuximab alone 11. Reversal of irinotecan resistance has been reported in heavily pre-treated patients with mcrc who are subsequently treated with the cetuximab–irinotecan combination 12. It should be noted, however, that the population in the bond study was unselected and included both KRAS-wt and mutated-type KRAS (KRAS-mt) tumours. Moreover, patients were allowed to cross over, and hence the potential survival benefit of third-line combination therapy remains unknown.
The efficacy data seem to favour combination therapy, but deciding whether a patient should be prescribed a cetuximab–irinotecan combination is largely a question of irinotecan tolerability. If the patient’s history indicates acceptable tolerance of irinotecan, and if current risk factors for that patient do not preclude the use of irinotecan, then the cetuximab–irinotecan combination is a reasonable choice. However, if the patient has previously demonstrated significant dose-limiting toxicity or would be expected to be adversely affected by irinotecan, treatment with monotherapy would be the preferred option—in which case, either cetuximab or panitumumab would be a reasonable choice depending on the additional factors reviewed in Table II.
3.4. Conclusions for Case Vignettes 1 and 2
Mrs. A appeared to tolerate irinotecan quite well during her earlier 25 cycles of folfiri. Cetuximab plus irinotecan would be the most reasonable choice for her situation.
In Mrs. B’s case, her history of limited tolerance for folfiri chemotherapy and her current performance status of 2 both indicate that monotherapy would be a better choice.
4. EGFR-INHIBITOR THERAPY IN PATIENTS WITH POTENTIALLY RESECTABLE LIVER METASTASES
4.1. Case Vignette: KRAS-wt with Potentially Resectable Liver Metastases
Mr. C, a 70-year-old man, was recently diagnosed with colon cancer after he presented with anemia. He has no other significant illnesses. He was found to have 3 large bi-lobar liver metastases that have been determined to be potentially resectable if downstaged. His tumour status is KRAS-wt.
4.2. Goals of Therapy
At the time of writing (February 2010), anti-egfr therapies are not indicated or funded for use in first- or second-line therapy in Canada. Data from phase iii trials of anti-egfr therapy compared with chemotherapy alone show a significantly higher response rate in patients randomized to anti-egfr therapy plus chemotherapy 13. Currently, no phase iii data address the setting of conversion chemotherapy for potentially resectable liver-limited metastases, but promisingly high response rates and rates of hepatic resection have been demonstrated in phase ii liver-limited trials of cetuximab combined with standard chemotherapy regimens.
The celim (Cetuximab in Neoadjuvant Treatment of Non-Resectable Colorectal Liver Metastases) study 14 included 111 patients with non-resectable liver metastases who were randomized to receive folfox6 plus cetuximab or folfiri plus cetuximab, planned for 8 cycles. Most of the tumours (71%) were KRAS-wt. The reported response rate (partial and complete combined) was 68% in the folfox plus cetuximab group and 57% in the folfiri plus cetuximab group. The R0 resection rates were 34% in the folfox plus cetuximab group and 30% in the folfiri plus cetuximab group (first-, second-, or third-line use). Median time to resection in the trial was 5.1 months; some patients underwent resection after the second or third line of therapy.
Although those findings are encouraging, the consensus opinion is that, in this population, it is still early to endorse the routine use of first-line chemotherapy in combination with cetuximab or panitumumab as a recommended strategy. Bevacizumab, a monoclonal antibody that inhibits vascular endothelial growth factor, remains the standard targeted therapy in the first-line setting 15,16. Prospective randomized studies are currently underway to compare the benefit of chemotherapy and anti-egfr therapy in the first-line setting with that of the current approach of chemo-therapy plus bevacizumab. Those studies include the ongoing National Cancer Institute of Canada crc.5 study (Cancer and Leukemia Group B 80405), which is comparing bevacizumab or cetuximab added to folfox or folfiri in approximately 3000 patients in the first line. The results of crc.5 and other similar trials will be a welcome addition to the evidence base in mcrc, helping clinicians to decide on the targeted agent that is the most beneficial in patients with potentially resectable metastases.
4.3. Conclusions for Case Vignette 3
Based on emerging data for cetuximab and Mr. C’s known KRAS-wt status, conversion therapy with folfox/folfiri and cetuximab can reasonably be considered. However, at present, neither cetuximab nor panitumumab is indicated for use in this setting in Canada.
4.4. Additional Discussion Points
4.4.1. Dual Antibody Therapy
In an effort to optimize first-line response, the approach of first-line doublet chemotherapy in combination with dual antibody inhibition using bevacizumab and an antiegfr antibody has been investigated in two published randomized phase iii trials. In both studies, the addition of cetuximab or panitumumab to first-line chemotherapy, particularly a fluoropyrimidine and oxaliplatin regimen, was associated with inferior survival 17,18. Given the negative and consistent findings of these highly publicized studies, anti-egfr therapy should not be used in combination with chemotherapy and bevacizumab.
4.4.2. KRAS Testing
KRAS is a robust predictive biomarker for anti-egfr use. Cetuximab and panitumumab are not recommended for use in patients with KRAS-mt tumours, and so determination of KRAS status is a critical requirement. However, the decision about when to order KRAS testing is a matter of some debate. The consensus is that tumour testing for KRAS should be timely—that is, as soon as egfr-inhibitor therapy is being considered as an option in the treatment of metastatic disease.
First- and second-line evidence
Although cetuximab and panitumumab are not currently indicated for first- or second-line therapy, several trials have demonstrated efficacy for egfr inhibitors in those settings. The first-line crystal (Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer) 13 and opus (Oxaliplatin and Cetuximab in First-Line Treatment of mcrc) 19 studies showed that the use of cetuximab with, respectively, folfiri and folfox4 led to significantly extended pfs. In the prime (Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy) 20 study, similar results were seen with panitumumab plus folfox as compared with folfox alone. In the second line, after folfox, the epic (European Prospective Investigation into Cancer and Nutrition) study 21 showed that, for pfs, irinotecan plus cetuximab is superior to irinotecan alone.
5. CONSENSUS RECOMMENDATIONS FOR THE USE OF ANTI-EGFR THERAPIES IN mCRC
-
Testing for the KRAS status of the tumour should be performed as soon as an egfr inhibitor is being considered as an option for treatment.
Anti-egfr therapies are not recommended for the treatment of patients with tumours showing mutated-type KRAS status.
For a patient with wild-type KRAS and an Eastern Cooperative Oncology Group status of 0–2, whose mcrc has previously been treated with a fluoropyrimidine, irinotecan, and oxaliplatin, switching to an egfr inhibitor is a recommended strategy.
-
Cetuximab, cetuximab plus irinotecan, and panitumumab are all options for third-line therapy in patients with wild-type KRAS, provided that tolerability is acceptable.
The choice of cetuximab or panitumumab monotherapy should be made patient by patient, depending on the individual’s suitability for the treatment regimen.
Although a greater body of evidence exists for cetuximab, cetuximab and panitumumab are deemed to have comparable efficacy in the third-line setting.
Combination therapy (cetuximab plus irinotecan) is associated with a higher likelihood of response and may be preferred over cetuximab monotherapy if irinotecan tolerability is not a concern.
If cetuximab plus irinotecan is selected and if the patient has irinotecan tolerability issues, then switching to cetuximab monotherapy is a reasonable strategy.
Although cetuximab and panitumumab are both accepted options as monotherapy, panitumumab is associated with greater convenience of administration and scheduling.
6. SUMMARY
The availability of the anti-egfr therapies cetuximab and panitumumab is an important advance in the management of patients with mcrc. Cetuximab and panitumumab have both demonstrated a pfs benefit in the third-line setting, and for cetuximab, a significant os benefit as compared with bsc alone. Tumours with KRAS-mt status do not benefit from egfr antibody inhibition; cetuximab and panitumumab are indicated only for KRAS-wt tumours.
To date, no studies have compared the two anti-egfr therapies head-to-head in the third line. In the absence of data, selection of a particular treatment regimen (cetuximab, cetuximab plus irinotecan, or panitumumab) in eligible patients should be made based on tolerability and on patient preference and suitability for the regimen.
Although cetuximab and panitumumab are currently approved only in the third-line setting in Canada, numerous trials are reporting a benefit for cetuximab and panitumumab in combination with chemotherapy in the first- and second-line settings. Data are also emerging for use of cetuximab in conversion chemotherapy for patients with non-resect-able liver-limited metastases. At the time of writing, the data definitely justify routine consideration of egfr therapy in the conversion setting pending further confirmatory prospective studies.
The present consensus report is meant to serve as a practical tool for Canadian clinicians. The recommendations contained herein were made based on evidence available in February 2010. The authors recognize that the role of egfr inhibition in mcrc is evolving, and ongoing research is sure to provide further insight into the utility of these agents for various settings (first line, second line) and as part of various combination therapies in the treatment of mcrc. These recommendations will therefore probably have to be updated on a regular basis.
7. CONFLICT OF INTEREST DISCLOSURES
All authors disclosed potential financial conflicts of interest:
Shahid Ahmed has worked as a consultant for Bristol–Myers Squibb.
Bruce Colwell has received grant and research support and speaker honoraria from Abraxis Bio-Science, Amgen, AstraZeneca, Bristol–Myers Squibb, Genentech/Roche, and Sanofi Aventis.
Christine Cripps declares no financial conflicts.
Scot Dowden has received speaker honoraria from Sanofi Aventis, Roche, Amgen, and Bristol– Myers Squibb.
Sharlene Gill has worked as a consultant for and received speaker honoraria from Amgen and Bristol–Myers Squibb.
Hagen Kenneke has received grant and research support and speaker honoraria from Bristol–Myers Squibb, Amgen, Roche, and Sanofi Aventis.
Jean Maroun declares no financial conflicts.
Benoît Samson has received grant and research support from Sanofi Aventis and Pfizer, and speaker honoraria from Amgen, Roche, AstraZeneca, Eli Lilly, and Bristol–Myers Squibb.
Michael Thirlwell has received grant and research support from Pfizer and Roche. He has also served as an advisory board member for AstraZeneca, Bristol–Myers Squibb, Pfizer, Roche, and Sanofi Aventis, and he has received speaker honoraria from Bristol–Myers Squibb and Sanofi Aventis.
Ralph Wong has worked as a consultant for Bristol–Myers Squibb and Amgen, and received speaker honoraria from Bristol–Myers Squibb.
8. REFERENCES
- 1.Bristol–Myers Squibb Canada. Erbitux (Cetuximab) [product monograph] Montreal, QC: Bristol–Myers Squibb Canada; Feb 1, 2010. [Google Scholar]
- 2.Amgen Canada. Vectibix (Panitumumab) [product mono-graph] Mississauga, ON: Amgen Canada; Feb 26, 2010. [Available online at: www.amgen.ca/Vectibix_PM.pdf; cited October 8, 2010] [Google Scholar]
- 3.Karapetis CS, Khambata–Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 4.van Cutsem E, Peeters M, Siena S, et al. Open-label phase iii trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 5.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Glynne–Jones R, Thaler J, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: mabel Study. J Clin Oncol. 2008;26:5335–43. doi: 10.1200/JCO.2008.16.3758. [DOI] [PubMed] [Google Scholar]
- 7.Tabernero J, Pfeiffer P, Cervantes A. Administration of cetuximab every 2 weeks in the treatment of metastatic colorectal cancer: an effective, more convenient alternative to weekly administration? Oncologist. 2008;13:113–19. doi: 10.1634/theoncologist.2007-0201. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer P, Nielsen D, Bjerregaard J, Qvortrup C, Yilmaz M, Jensen B. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol. 2008;19:1141–5. doi: 10.1093/annonc/mdn020. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson JJ, Gregory C, Burris H, et al. An open-label clinical trial evaluating safety and pharmacokinetics of two dosing schedules of panitumumab in patients with solid tumors. Clin Colorectal Cancer. 2009;8:29–37. doi: 10.3816/CCC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 10.Weiner LM, Belldegrun AS, Crawford J, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:502–8. doi: 10.1158/1078-0432.CCR-07-1509. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Humblet Y, Siena S, et al. Cetuximab mono-therapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 12.Lévi FA, Karaboué A, Bralet M, et al. Cetuximab reversal of resistance to chronomodulated chemotherapy in heavily-pretreated patients with metastatic colorectal cancer (mcc) without amplification of epidermal growth factor receptor (egfr) gene [abstract 13104] Proc Am Soc Clin Oncol. 2006;24 [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=31328; cited October 8, 2010] [Google Scholar]
- 13.van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemo-therapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 14.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the celim randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase ii trial. J Clin Oncol. 2005;23:3697–705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 17.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 18.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase iiib trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–80. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 19.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 20.Douillard J, Siena S, Cassidy J, et al. Randomized phase 3 study of panitumumab with folfox4 compared to folfox4 alone as 1st-line treatment (tx) for metastatic colorectal cancer (mcrc): the prime trial [abstract 10LBA] Eur J Cancer. 2009;7:6. [Google Scholar]
- 21.Sobrero AF, Maurel J, Fehrenbacher L, et al. epic: phase iii trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–19. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]