Abstract
Objectives
The present study investigated factors affecting outcome at relapse after previous surgery and adjuvant chemoradiation (crt) in high-risk esophageal cancer patients.
Patients and Methods
From 1989 to 1999, we followed high-risk resected esophageal cancer patients who had completed postoperative crt therapy. Patients who relapsed with a disease-free interval of less than 3 months were treated with palliative crt when appropriate. Patients with a disease-free interval of 3 months or more were treated with best supportive care. Post-recurrence survival was estimated using the Kaplan–Meier technique, and statistical comparisons were made using log-rank chi-square tests and Cox regression.
Results
Of the 69 patients treated with adjuvant crt after esophagectomy, 46 experienced recurrence. Median time to relapse was 28 months (range: 0.1–40 months). Among the 46 relapsed patients, median age was 61 years (range: 37–82 years), and 42 were men. At the initial staging, 44 of 46 were node-positive; 31 of 46 had adenocarcinoma. In 33 of 46, post-esophagectomy resection margins were clear. Median follow-up after recurrence was 30.5 months (range: 1.3–100 months). Median overall survival after recurrence was 5.8 months, and the 12-month, 24-month, and 36-month survival rates were 20%, 10%, and 5% respectively. Of the prognostic factors analyzed, only resection margin status and interval to recurrence were statistically significant for patient outcome in univariate and multivariate analysis.
Patients who had positive resection margins and who relapsed 12 or fewer months after surgery and adjuvant crt had a median post-recurrence overall survival of 0.85 months as compared with 6.0 months in other patients (more than 12 months to relapse, or negative resection margins, or both; log-rank p = 0.003).
Conclusions
Resection margin status and interval to disease relapse are significant independent prognostic factors for patient outcome after adjuvant crt therapy.
Keywords: Esophagus, cancer, relapse, resection margin, interval
1. INTRODUCTION
Management of patients who experience disease relapse after completion of surgery and adjuvant chemoradiation (crt) is controversial. Post-esophagectomy resection margin status and interval to recurrence have been reported as prognostic factors for patient outcome 1–5. Postoperative crt has been reported to improve patient outcome in high-risk esophageal cancer patients 6,7. We previously reported a cohort of patients who experienced disease relapse after adjuvant crt 8.
To manage patients who experience disease recurrence after adjuvant treatment, some oncologists advocate intensive therapeutic intervention because of promising experience with treatment for recurrence disease 9; others recommend palliative support 10 because of concerns for poor patient outcome after disease recurrence. In addition, it is not clear whether patient outcomes improve after adjuvant crt when the patients at risk have resection margin involvement, and whether interval to recurrence can affect patient survival after relapse. This clinical information may be useful in providing appropriate guidance for oncologists who must manage esophageal cancer patients after disease relapse.
The present study was conducted to determine the factors that affect outcome at relapse after previous surgery and adjuvant crt in high-risk esophageal cancer patients.
2. PATIENTS AND METHODS
We analyzed data for patients with a diagnosis of high-risk resected esophageal cancer who attended the London Regional Cancer Program from 1989 to 1999. “High-risk” pathology findings were defined as T3 or T4 disease 11 or regional nodal (N1) involvement, or both.
Adjuvant therapy consisted of chemotherapy followed by concurrent crt 4–6 weeks after esophagectomy. Chemotherapy consisted of 4 cycles of either ecf [epirubicin 50 mg/m2 on day 1 and every 21 days, 5-fluorouracil (5fu) 200 mg/m2 continuous infusion for 21 days, and cisplatinum 60 mg/m2 on day 1 and every 21 days], with epirubicin omitted during the concurrent phase with radiotherapy (rt), or 4 cycles of cf (cisplatinum 100 mg/m2 on day 1 and every 21 days, and 5fu 1000 mg/m2 continuous infusion days 1–4 and every 21 days). Total rt dose ranged from 45 Gy to 60 Gy using 1.8–2.0 Gy fractions at the discretion of the treating radiation oncologist. In general, 45–50 Gy was used for microscopic disease; higher doses (up to 60 Gy) were reserved for patients with margin involvement or residual disease.
Patients were treated with high-energy megavoltage photons (>6 MV) in the supine position. Follow-up evaluations were performed every 3 months and included chest radiography and screening blood cell counts and chemistries according to the model of Herskovic et al. 12. At the time of relapse, investigations— endoscopy; barium swallow esophagram; brain, chest, and abdomen computerized tomography; and bone scan—were carried out as clinically indicated.
Margins of the surgical specimens were reviewed with a pathologist specializing in thoracic tumours. Patient disease status was determined from clinic progress notes or updated information provided by the family physician. Relapse was defined as disease recurrence at local, regional, or distant sites as the first event in the follow-up. Local relapse was defined as recurrence at or immediately adjacent to the anastomotic site. Regional relapse was defined as recurrence at the mediastinum or peri-esophageal region (excluding local relapse), or both. Distant relapse was defined as recurrence at a distant site (for example, brain, liver, lung).
If relapse occurred after an interval of more than 3 months, the relapsed patient was salvaged with chemotherapy with or without rt. Chemotherapy consisted of 4 cycles of ecf as described earlier, with epirubicin omitted during the phase concurrent with rt. A radiation therapy dose ranging between 20 Gy and 60 Gy was delivered at the discretion of the treating radiation oncologist. For patients with a relapse interval of 3 months or less, management consisted of best supportive care, including pain medications with or without palliative rt.
Post-recurrence cause-specific survival (css) was defined as the interval between the date of first disease recurrence and the date of death or last follow-up, with death attributable to cancer being defined as an event. Post-recurrence overall survival (os) was defined as the interval between the date of first disease recurrence and the date of death or last follow-up, with death attributable to any cause being defined as an event. Survival estimates were obtained using Kaplan–Meier methodology 13. Log-rank chi-square tests are presented graphically and were used in exploratory analyses. Univariate and stepwise multivariate Cox proportional hazards regressions 14 were used to evaluate the association of os with various prognostic factors, including age, sex, pathologic stage, histology, resection margin status, relapse, and interval to recurrence. For the multivariate model, entry and removal were set at the 0.05 level. Values of p less than 0.05 were considered statistically significant.
3. RESULTS
We previously reported a cohort of 69 patients with high-risk esophageal cancer after esophagectomy and crt 8. At the time of analysis, 12 (13%) of the patients were living, 54 (83%) had died, and 3 (4%) were lost follow-up. Of the 69 patients, 46 (67%) had experienced disease relapse. Median time to recurrence after adjuvant treatment was 28 months (range: 0.1–40 months).
Table I shows the patient demographics of the relapse group. Surgery was either transhiatal (86%) or transthoracic (14%), with 33 patients (72%) having negative resection margins. In 13 patients, a resection margin was positive, with 9 of those (70%) having a positive circumferential resection margin (crm) and 4 (30%) having a positive proximal resection margin. Follow-up for the relapse cohort ranged from 1.3 months to 100 months (median: 30.5 months). All 46 patients with relapse died of their disease. The median post-recurrence os was 5.8 months, and the 12-month, 24-month, and 36-month survival rates were 20%, 10%, and 5% respectively.
TABLE I.
Demographics of the patient cohort
| Variable | Value |
|---|---|
| Age (years) | |
| Median | 61 |
| Range | 37–82 |
| Sex [n (%)] | |
| Male | 42 (91) |
| Female | 4 (8) |
| Pathologic stage [n (%)] | |
| T1 | 1 (2) |
| T2 | 9 (20) |
| T3 | 34 (74) |
| T4 | 2 (4) |
| N0 | 2 (4) |
| N1 | 44 (96) |
| Histology [n (%)] | |
| Adenocarcinoma | 31 (67) |
| Squamous | 15 (33) |
| Resection margin status [n (%)] | |
| Negative | 33 (72) |
| Positive | 13 (28) |
| Time to recurrence (months) | |
| Median | 28 |
| Range | 0.1–40 |
Table II shows the pattern and the sites of relapse. Distant relapses constituted 45% of all relapses, and bone, liver, lung, and brain were the common sites.
TABLE II.
Patterns and sites of relapse
| Variable | Value |
|---|---|
| Patients (n) | 46 |
| Relapse pattern [n (%)] | 46 |
| Local | 9 (20) |
| Regional | 16 (35) |
| Distal | 21 (45) |
| Relapse site [n (%)] | 58 |
| Anastomosis | 9 (16) |
| Neck/mediastinum | 9 (16) |
| Bone | 9 (16) |
| Abdomen | 6 (10) |
| Liver | 7 (12) |
| Lung | 7 (12) |
| Brain | 5 (9) |
| Skin | 2 (3) |
| Stomach | 2 (3) |
| Adrenal | 2 (3) |
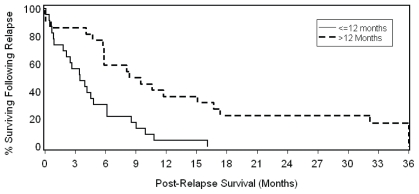
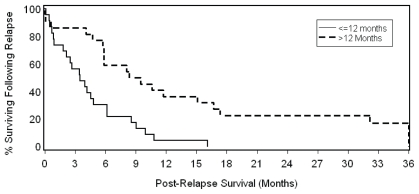
Of the possible prognostic factors (age, sex, pathologic stage, histology, resection margin status, relapse pattern, and interval to recurrence—Table III), only resection margin status and interval to recurrence were significantly associated with os. In the multivariate model, the significant association with survival for resection margin status [p = 0.038; hazard ratio (hr): 0.46; 95% confidence interval (ci): 0.23 to 0.96] and interval to recurrence (p = 0.024; hr: 2.27; 95% ci: 1.12 to 4.63) remained even after adjustment for the use of systemic therapy. Patients experiencing late relapse (>12 months) had a median post-recurrence os of 8.4 months as compared with 3.5 months for those experiencing early relapse [≤12 months (Cox univariate p = 0.008; Figure 1)]. Relapse patients with negative resection margins had a median post-recurrence os of 5.8 months as compared with 2.6 months for those with a positive resection margin (Cox univariate p = 0.002; Figure 2). When the same characteristics were considered in a stepwise multivariate model, interval to relapse (≤12 months vs. >12 months) and margin status were independently associated with os (p = 0.027 for relapse interval and p = 0.003 for margin status).
TABLE III.
Factors potentially prognostic for overall survival after relapse
| Prognostic factor | p Value | hr (95% ci) |
|---|---|---|
| Univariate | ||
| Agea | 0.39 | 1.14 (0.84–1.56) |
| Sex (female) | 0.63 | 1.29 (0.46–3.68) |
| Pathologic stage (stage iii) | 0.16 | 1.75 (0.79–3.81) |
| Histology (adenocarcinoma) | 0.25 | 1.48 (0.77–2.87) |
| Resection margin (positive) | 0.008b | 2.62 (1.28–5.32) |
| Relapse pattern | 0.24 | |
| Local/regional | 0.10 | 1.71 (0.89–3.29) |
| Distant | 0.43 | 1.43 (0.58–3.54) |
| Time to recurrence (≤12 months) | 0.002b | 0.97 (0.95–0.99) |
| Multivariate | ||
| Resection margin (positive) | 0.027b | 0.45 (0.22–0.91) |
| Time to recurrence (≤12 months) | 0.003b | 2.81 (1.42–5.01) |
Hazard ratio for age can be interpreted in terms of decade increments.
Statistically significant.
hr = hazard ratio; ci = confidence interval.
FIGURE 1.
Effect of interval to relapse on post-recurrence overall survival in high-risk resected esophageal cancer patients (log-rank p = 0.007).
FIGURE 2.
Effect of resection margin status on post-recurrence overall survival in high-risk resected esophageal cancer patients (log-rank p = 0.006).
The median post-recurrence css was 8.1 months for patients with an interval greater than 12 months as compared with 4.1 months for those with an interval of 12 months or less (log-rank p = 0.01). The median post-recurrence css intervals for negative and positive resection margins were 6.1 months and 2.7 months respectively (log-rank p = 0.01).
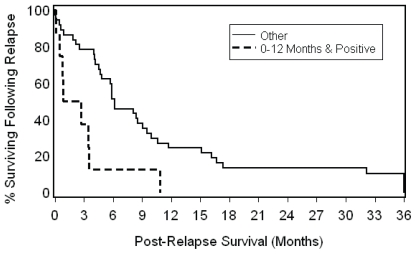
Patients who had positive resection margins and who relapsed 12 or fewer months after surgery and adjuvant crt had a median post-recurrence os of 0.85 months as compared with 6.0 months in other patients (more than 12 months to relapse, or negative resection margins, or both; log-rank p = 0.003; Figure 3). The median post-recurrence csss for patients with positive resection margins and with relapse at 12 or fewer months, as compared with all other patients, were 1.7 months and 8.1 months respectively (log-rank p = 0.006).
FIGURE 3.
Combined effect of interval to relapse and resection margin status on post-recurrence overall survival in high-risk resected esophageal cancer patients (log-rank p = 0.003).
4. DISCUSSION
Alexiou et al. 4 reported 621 cases in a series of patients who underwent esophagectomy with curative intention for squamous cell carcinoma or adenocarcinoma. Multivariate analysis showed that completeness of resection was a significant (p = 0.028) predictor of survival. In their study, the presence of tumour within 1 mm of the crm after potentially curative resection was an important independent prognostic variable. Dexter et al. 2 reported that patients with crm involvement had a median survival of 21 months as compared with 39 months for patients without crm involvement (p = 0.015). Roch et al. 15 concurred, with a similar observation that patients with crm involvement have a poor 3-year survival of 26.8% as compared with 61.8% for those without such involvement. In addition, different grades of proliferative activity (determined using the Ki-67 labelling index) between the central and peripheral tumour portion were seen in 38.3% of those with crm involvement and in 9.1% of those without (p = 0.007). It was suggested by Roch and coworkers 15 that tumour cells involving the crm might be of a different subclone type, with more invasive properties and higher proliferative activity. They also suggested that crm involvement is more an indicator of advanced and aggressive disease than of incomplete excision.
In our patient cohort with resection margin involvement, 70% had crm involvement. That disease recurred despite adjuvant crt accords with the suggestion from Roch et al. 15 that resection margin involvement such as crm is more an indicator of advanced disease than of incompletion excision. Margin involvement such as crm was suggested for inclusion as a subcategory of T3 in a revised TNM staging. A complete pathology report is important for quality control in patient management and for predicting treatment outcome.
Shimada et al. 5 reported that treatment response is significantly associated with time to recurrence. As compared with patients experiencing disease recurrence more than 1 year after radical esophagectomy, those experiencing recurrence at 1 year or less had a poorer response to nonsurgical treatment (p = 0.001). Time to recurrence was significant in both univariate (p = 0.01) and multivariate analyses of survival (p = 0.015). Our results are consistent with those of Shimada and coworkers, in that time to recurrence was an independent predictor of patient outcome after treatment in our cohort—not only after surgery, but also after adjuvant crt. In our series, recurrence within 12 months was more likely to develop in distant organs than in regional lymph nodes. That observation accords with those of Shimada et al. 5 that distant organ recurrence happens earlier (within 6–12 months) than nodal recurrence does. Those authors also observed that time to recurrence and number of recurrences may be associated with the growth rate of recurrent tumours.
Our data showed that patients with resection margin involvement and a short interval to recurrence (≤12 months) had a poor outcome. That observation is consistent with the analysis showing that both variables seem to be independent factors, contributing their detrimental effects in an additive manner. It is possible that patients with resection margin involvement are a subgroup of patients with subclones of tumour cells that represent advanced disease and that patients experiencing early recurrences possess fast-growing tumour cells that do not respond to crt. Recognition of those factors may be important in patient management. Appropriate supportive care may be considered for such patients to spare them the significant toxicity, time, and financial expense associated with intensive therapeutic regimens.
Independent predictors of early recurrence and death in esophageal cancer treated with tri-modality therapy, including immunohistochemical analysis of markers of resistance to platinum-based chemotherapy such as glutathione S-transferase, P-glycoprotein, and the 5fu marker thymidylate synthetase, have been reported 16. Serum concentrations of C-reactive protein (scrp) and albumin were found to be associated with poor survival in patients with esophageal cancer undergoing rt 17. Shimada et al. 5 also reported that serum anti-p53 antibodies (s-p53Ab, associated with decreased chemosensitivity to cisplatinum and 5fu) and scrp were associated with treatment response after recurrence in esophageal cancer patients. In multivariate analyses, S-p53Ab and scrp were independent prognostic factors. Future studies can investigate the presence of molecular markers such as glutathione S-transferase, S-p53Ab, and scrp in patients with a positive resection margin and 12 months or less to recurrence.
5. CONCLUSIONS
Our results demonstrate that a positive surgical resection margin and a short interval to relapse (≤12 months) are independent variables having negative effect on patient outcome. Identification of these prognostic factors should aid physicians in delivering appropriate care, particularly to patients with poor outcomes. On the other hand, patients experiencing recurrent disease confined to limited areas and not possessing these poor prognostic features may benefit from more aggressive treatment.
6. ACKNOWLEDGMENTS
This work was presented in part at the 4th Annual Ontario Thoracic Cancer Conference, Niagara-on-the- Lake, Ontario, May 2009.
The authors thank Mary-Ellen Coughlin for her skilful preparation of this manuscript, and Drs. P. Truong, R. Ash, G. Videtic, I. Craig, M. Lefcoe, A. Tomiak, and W. Kocha for team support.
7. REFERENCES
- 1.Saga PM, Johnston D, McMahon MJ, Dixon MF, Quirke P. Significance of circumferential resection margin involvement after oesphagectomy for cancer. Br J Surg. 1993;80:1386–8. doi: 10.1002/bjs.1800801109. [DOI] [PubMed] [Google Scholar]
- 2.Dexter SPL, Sue–Ling H, McMahon MJ, Quirke P, Mapstone N, Martin IG. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48:667–70. doi: 10.1136/gut.48.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan OA, Fitzgerald JJ, Soomro I, Beggs FD, Morgan WE, Duffy JP. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer. Br J Cancer. 2003;88:1549–52. doi: 10.1038/sj.bjc.6600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexiou C, Khan O, Black E, et al. Survival after esophageal resection for carcinoma: the importance of the histologic cell type. Ann Thorac Surg. 2006;82:1037–7. doi: 10.1016/j.athoracsur.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Shimada H, Kitabayasshi H, Nabeya Y, et al. Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery. 2003;133:24–31. doi: 10.1067/msy.2003.31. [DOI] [PubMed] [Google Scholar]
- 6.Bedard E, Inculet RI, Malthaner R, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423–30. [PubMed] [Google Scholar]
- 7.Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590–6. doi: 10.1016/s0022-5223(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu E, Dar R, Rodrigues G, et al. Is extended volume external beam radiation therapy covering the anastomotic site beneficial in post-esophagectomy high risk patients? Radiother Oncol. 2004;73:141–8. doi: 10.1016/j.radonc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Ota M, Narumiya K, et al. Multimodal treatment for lymph node recurrence of esophageal carcinoma after curative resection. Annu Surg Oncol. 2008;15:2451–7. doi: 10.1245/s10434-008-0016-x. [DOI] [PubMed] [Google Scholar]
- 10.Javle M, Ailawadhi S, Yang GY, Nwogu CE, Schiff MD, Nava HR. Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol. 2006;4:365–73. 379. [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 12.Herskovic A, Montz LK, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of esophagus. N Engl J Med. 1992;326:1593–8. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 14.Hosmer DW, Jr, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York, NY: Wiley; 1999. [Google Scholar]
- 15.Roch MS, Lee JI, Choi PJ. Significance of circumferential resection margin involvement following esophagectomy for esophageal cancer. Korean J Pathol. 2004;38:23–8. [Google Scholar]
- 16.Harpole DH, Moore MB, Herndon JE, et al. The prognostic valve of molecular marker analysis in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2001;7:562–9. [PubMed] [Google Scholar]
- 17.Wang CY, Hsieh MJ, Chiu YC, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92:270–5. doi: 10.1016/j.radonc.2009.01.002. [DOI] [PubMed] [Google Scholar]