Abstract
Aromatase inhibitors have not been adequately assessed in treatment of ovarian cancer. The aromatase inhibitor letrozole (2.5 mg daily) was administered in 2 cases of advanced endometrioid ovarian cancer with positive estrogen receptor.
Case 1
A 52-year-old woman with a grade 2–3, stage iiic endometrioid ovarian cancer was optimally debulked and received 6 cycles of intravenous paclitaxel and intraperitoneal cisplatin–paclitaxel. Post chemotherapy, one of several biopsies showed residual disease during the second-look laparoscopy. This patient was treated with letrozole and remained disease-free during 30 months of follow-up.
Case 2
A 47-year-old woman with a grade 3, stage iiic endometrioid ovarian cancer was optimally debulked and treated with intravenous carboplatin–paclitaxel. After a 15-month remission, her first recurrent disease was treated with carboplatin–docetaxel. The second remission lasted only 11 months, after which the patient was treated with splenectomy and subsequent liposomal doxorubicin. Letrozole was administered after the chemotherapy. The patient had a 30-month remission before the next recurrence of her disease.
Conclusions
Endometrioid ovarian carcinoma may benefit from aromatase inhibitors, especially when the tumour burden is low after primary chemotherapy or when the inhibitor is used as maintenance therapy between chemotherapies.
Keywords: Aromatase inhibitor, endometrioid ovarian cancer, letrozole, maintenance therapy, recurrent ovarian cancer
1. INTRODUCTION
Epithelial ovarian cancer can be considered an endocrine- related neoplasm 1. Despite surgical debulking and standard platinum–taxane initial chemotherapy, most patients ultimately develop recurrent or progressive disease within 12–36 months 2. Furthermore, patients often suffer significant morbidity secondary to the toxicity of the agents used to treat recurrent disease. A relatively nontoxic oral hormonal therapy may offer anti-neoplastic activity.
Aromatase is an enzyme complex that converts androgens to estrogens. Aromatase inhibitors have proven invaluable in the treatment of adjuvant and metastatic breast cancer, but their use has not been adequately assessed in ovarian cancer. Endometrioid ovarian carcinoma is the second most common type of epithelial ovarian cancer. Histologically, it resembles endometrial mucosa. Endometrioid tumour cells and the endometrium share similar growth characteristics. The 2 cases of endometrioid ovarian carcinoma reported here showed beneficial effects from administration of the aromatase inhibitor letrozole.
2. CASE DESCRIPTIONS
2.1. Case 1
A 52-year-old woman was diagnosed with grade 2–3, stage iiic endometrioid ovarian cancer positive for the estrogen (er) and progesterone receptors. Her cancer antigen 125 (CA125) level was 4791 U/mL before surgery and fell to 9 U/mL after optimal debulking. It then increased to 12 U/mL approximately 12 weeks later. She received 6 cycles of intravenous paclitaxel and intraperitoneal cisplatin–paclitaxel. After completion of chemotherapy, a biopsy during a second-look laparoscopy showed residual disease. Instead of chemotherapy, the patient was treated with letrozole 2.5 mg daily. She has remained disease-free as indicated by combined positron-emission and computed tomography (pet/ct) imaging, and her CA125 level has ranged from 4 U/mL to 7 U/mL during 30 months of follow-up. She has good quality of life and works full time.
2.2. Case 2
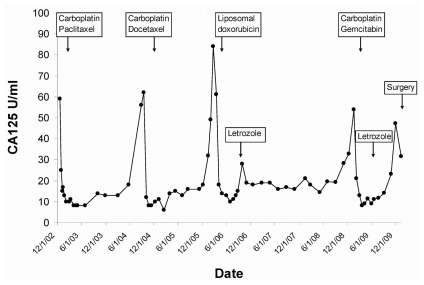
A 47-year-old woman was diagnosed with grade 3, stage iiic ovarian endometrioid adenocarcinoma. Her CA125 level was monitored during 7 years of treatments (Figure 1). She was optimally debulked and treated with 6 cycles of intravenous carboplatin– paclitaxel. Her first remission lasted 15 months. She then received 6 cycles of carboplatin–docetaxel. Her second remission lasted only 11 months, and a tumour returned in her spleen. She underwent splenectomy, followed by 4 cycles of liposomal doxorubicin, achieving a complete response (cr).
FIGURE 1.
Variation in cancer antigen 125 (CA125) levels during recurrence and treatment (case 2). At the time of diagnosis, CA125 was 832.7 U/mL (not shown). During 7 years of follow-up, CA125 was monitored every 1–3 months. At the time of the 1st, 2nd, 3rd, and 4th recurrences, CA125 was 56 U/mL, 32 U/mL, 32.9 U/mL, and 23.2 U/mL respectively. Recurrence was diagnosed based on clinical symptoms and imaging by combined positron-emission and computed tomography.
The tumour was found to be 99% positive for er and negative for her2/neu. The patient was then treated with letrozole 2.5 mg daily as a maintenance therapy, a regime that she tolerated well. She experienced a 30-month remission before her third disease recurrence, at which point she was treated with 6 cycles of carboplatin–gemcitabine. After a complete response (based on negative pet/ct and a CA125 level of 11 U/mL post treatment), she returned to letrozole. A repeat pet/ct 5 months later showed 2 tumours, and her CA125 level had risen to 23.2 U/mL. A tumour 2×4 cm2 in the right pelvis and another 2×2 cm2 on the right lobe of the liver were successfully removed during a recent laparotomy. Pathology showed that the specimen were 94% positive for er and negative for her2/neu.
3. DISCUSSION AND CONCLUSIONS
To our knowledge, the present case reports are the first to suggest a beneficial effect for letrozole as a maintenance therapy in advanced ovarian endometrioid adenocarcinoma.
A limited number of phase ii trials have investigated aromatase inhibitors for recurrent ovarian cancer with heterogeneous subtypes. Bowman et al. 3 treated 60 patients with letrozole at the time of CA125 relapse. Rates of cr, partial response (pr), and stable disease (sd) were reported as 0%, 9.4%, and 25% respectively in 54 patients who were evaluated based on CA125 response. In the full cohort of 60 patients, 11 had endometrioid carcinoma, which showed a higher response rate [4 of 11 (36.3%)] than did serous tumours [4 of 43 (9.3%)]. Treatment response was found to be associated with high er, and the authors concluded that letrozole can produce disease stabilization. Another aromatase inhibitor, anastrozole, was investigated in 53 women with asymptomatic recurrent or persistent Müllerian cancer, including 43 ovarian cancers 4. The median time to disease progression was 85 days. No statistically significant association was found between er status and response to anastrozole. Papadimitriou et al. 5 used letrozole to treat 27 patients for recurrent ovarian cancer. The overall rate of CA125 response was 33% (9 of 27). Those authors did not find a statistically significant association between response and er expression. Smyth et al. 6 investigated letrozole for recurrent er-positive ovarian cancer in 44 patients. Of 42 patients evaluated by CA125 criteria, 11 (26%) had sd after 6 months of treatment. A CA125 response was seen in 33.3% of patients (1 of 3) with endometrioid histology, as compared with 14.3% of patients (3 of 21) with serous tumours; however, no significant association was observed between response to letrozole and any of tumour stage, grade, or histology; number of previous chemotherapies; or platinum resistance. For platinum/taxane–resistant er-positive recurrent ovary cancer, Ramirez et al. 7 treated 32 patients with letrozole. No patient achieved a cr, 1 (3%) had a pr, and 7 (23%) achieved sd.
Based on the foregoing phase ii trials, aromatase inhibitors appear to be able to delay cancer progression. Patients in two of the trials were selected based on the er positivity of their tumours, and a heterogeneous population of ovarian cancers may respond differently to hormonal therapy. Identification of a potentially endocrine-sensitive subgroup for endocrine therapy may lead to tremendous benefit.
One study identified the relative risks of epithelial ovarian cancer after estrogen replacement therapy at 2.81 [95% confidence interval (ci): 1.15 to 6.89] for endometrioid carcinoma and 2.03 (95% ci: 1.04 to 3.97) for serous carcinoma 8. In another study, subjects on estrogen replacement therapy had a relative risk of 3.1 (95% ci: 1.0 to 9.8) for endometrioid carcinoma, 1.6 (95% ci: 1.0 to 2.6) for serous carcinoma, and 0.7 (95% ci: 0.3 to 1.4) for mucinous carcinoma 9. Those studies suggest a strong association between exogenous estrogen and endometrioid ovarian carcinoma.
Interestingly, Kuhnel et al. 10 reported that the highest aromatase activity was found in endometrioid carcinomas. In 40 ovarian cancer tissues, aromatase activity was 40% in endometrioid, 23% in serous, and 25% in mucinous ovarian cancer. This in situ endogenous production of estrogen in tumours may be an important growth-promoting factor, especially for endometrioid carcinoma.
Although the estrogen receptor inhibitor tamoxifen and aromatase inhibitors are both anti-estrogen therapies, tamoxifen has a proliferative effect on the endometrium, which can lead to endometrial hyperplasia, polyps, cysts, and endometrial cancer. This effect may be a result of the agonistic pathway on estrogen receptors of the endometrial epithelial and stromal cells 11,12. Switching from tamoxifen to aromatase inhibitors significantly reverses the endometrial thickness seen with tamoxifen 13,14. Those data certainly suggest that aromatase inhibitors are better candidates than tamoxifen as hormonal therapy for ovarian endometrioid carcinoma.
We hypothesize that patients with certain tumour characteristics are more likely to respond to an endocrine therapy such as an aromatase inhibitor. Gene mutations, the presence or absence of hormone receptors, the likelihood of response to chemotherapy, disease-free interval, and tumour burden all may be important factors. For ovarian cancer, the histologic tissue type has not been used as a patient selection criterion in clinical trials. Because endometrioid ovarian carcinoma histologically resembles the endometrium, which is highly sensitive to estrogen regulation, aromatase inhibitors may have a more substantial anti-neoplastic effect on this type of ovarian cancer. Unlike clinical trials of cytotoxic chemotherapies (which are used for all ovarian cancers in heterogeneous settings), future trials of targeted therapy should consider using specific subtypes of ovarian cancer as inclusion criteria.
In addition to patient selection, we think that the timing of aromatase inhibitor treatment is important. The data from the phase ii trials 3–7 suggest that aromatase inhibitors rarely induce a cr for recurrent ovarian cancer; more often, the result is sd or a pr. In contrast to the phase ii clinical trials, which treat patients at the time of recurrence, the aromatase inhibitors in our cases were administered at the time of chemotherapy completion, as a maintenance therapy. The excellent toxicity profile of aromatase inhibitors favours this role. In case 1, letrozole immediately followed first-line chemotherapy. In case 2, letrozole was given after completion of the third- and fourthline chemotherapies with the aim of prolonging the platinum-free interval for recurrent disease. During the initial letrozole treatment, the patient had a remission that was twice as long as her first remission, and 3 times the length of her second remission. Unfortunately, the remission when letrozole was resumed after carboplatin–gemcitabine was only 5 months.
Brodie et al. 15 used tumour xenograft models in mice to investigate the mechanisms of resistance to letrozole. Those authors found that er was initially upregulated in letrozole-responsive tumours, but that the er subsequently declined to below control levels in tumours no longer responsive to letrozole. In letrozole-resistant tumours, her2 and other signalling proteins were increased. In our case 2, we found no significant change in er or her2 before and after 3 years of letrozole treatment. The lack of effectiveness for letrozole re-treatment may be the result of a high tumour burden (tumours 2×4 cm2 and 2×2 cm2 were found surgically). It is possible that the negative pet/ct imaging after chemotherapy was attributable to inactive tumour cells transiently inhibited by carboplatin–gemcitabine, leading only to a pr instead of a cr, given that the next recurrent tumours arose in the same locations as the tumours that were present before carboplatin–gemcitabine. The intended maintenance therapy with letrozole may actually have been treating recurrent disease during a pr after chemotherapy, delaying the progression of tumours for 5 months, a mechanism suggested by the phase ii trials data 4,6.
We suggest that women with endometrioid ovarian carcinoma may benefit from aromatase inhibitors as an endocrine therapy, especially when their tumour burden is low after primary chemotherapy or when the aromatase inhibitor is used as a maintenance therapy between chemotherapies. The optimal use of aromatase inhibitors in ovarian cancer warrants further investigation.
4. REFERENCES
- 1.Makar AP. Hormone therapy in epithelial ovarian cancer. Endocr Relat Cancer. 2000;7:85–93. doi: 10.1677/erc.0.0070085. [DOI] [PubMed] [Google Scholar]
- 2.Hamerlynck JV, Maskens TH, Mangioni C, et al. Phase ii trial of medroxyprogesterone acetate in advanced ovarian cancer: an eortc Gynecological Cancer Cooperative Group Study. Gynecol Oncol. 1985;2:313–16. doi: 10.1016/0090-8258(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 3.Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase ii trial of letrozole in ovarian cancer: identification of an endocrine sensitive subgroup. Clin Cancer Res. 2002;8:2233–9. [PubMed] [Google Scholar]
- 4.del Carmen MG, Fuller AF, Matulonis U, et al. Phase ii trial of anastrozole in women with asymptomatic Müllerian cancer. Gynecol Oncol. 2003;91:596–602. doi: 10.1016/j.ygyno.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase ii study. Oncology. 2004;66:112–17. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 6.Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen-receptor positive patients. Clin Cancer Res. 2007;13:3617–22. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez PT, Schmeler KM, Milam MR, et al. Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecol Oncol. 2008;110:56–9. doi: 10.1016/j.ygyno.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. 1996;63:254–7. doi: 10.1006/gyno.1996.0315. [DOI] [PubMed] [Google Scholar]
- 9.Weiss NS, Lyon JL, Krishnamurthy S, Dietert SE, Liff JM, Daling JR. Noncontraceptive estrogen use and the occurrence of ovarian cancer. J Natl Cancer Inst. 1982;68:95–8. [PubMed] [Google Scholar]
- 10.Kuhnel R, Delemarre JF, Rao BR, Stolk JG. Correlation of aromatase activity and steroid receptors in human ovarian carcinoma. Anticancer Res. 1986;6:889–92. [PubMed] [Google Scholar]
- 11.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004;94:256–66. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Salazar EL, Paredes A, Calzada L. Endometrial thickness of postmenopausal breast cancer patients treated with tamoxifen. Gynecol Endo. 2005;21:312–16. doi: 10.1080/09513590500430450. [DOI] [PubMed] [Google Scholar]
- 13.Garuti G, Cellani F, Centinaio G, Montanari G, Nalli G, Luerti M. Prospective endometrial assessment of breast cancer patients treated with third generation aromatase inhibitor. Gynecol Oncol. 2006;103:599–603. doi: 10.1016/j.ygyno.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bertelli G. Hall E, Ireland E, et al. Long-term endometrial effects in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (ies)—a randomized controlled trial of exemestane versus continued tamoxifen after 2–3 years tamoxifen. Ann Oncol. 2010;21:498–505. doi: 10.1093/annonc/mdp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodie A, Sabnis G, Macedo L. Xenograft models for aromatase inhibitor studies. J Steroid Biochem Mol Biol. 2007;106:119–24. doi: 10.1016/j.jsbmb.2007.05.010. [DOI] [PubMed] [Google Scholar]