Abstract
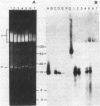
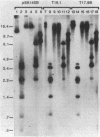
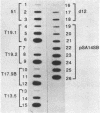
Paramecia of a given serotype express only one of several possible surface proteins called immobilization antigens (i-antigens). A 16-kilobase plasmid containing the gene for immobilization antigen A from Paramecium tetraurelia, stock 51, was injected into the macronucleus of deletion mutant d12, which lacks that gene. Approximately 40% of the injected cells acquired the ability to express serotype A at 34 degrees C. Expression appeared to be regulated normally. The transformed cells, like wild type, could be switched to serotype B by antiserum treatment and culture at 19 degrees C; on transfer to 34 degrees C, they switched back to serotype A expression. Many of the lines retained the ability to express serotype A until autogamy, when the old macronucleus is replaced by a new one derived from the micronucleus. DNA from transformants contained the injected plasmid sequences, which were replicated within the paramecia. No evidence for integration was obtained. The majority of replicated plasmid DNA comigrated with a linearized form of the input plasmid. Nonetheless, the pattern of restriction fragments generated by transformant DNA and that generated by input plasmid DNA are identical and consistent with a circular rather than a linear map. These conflicting observations can be reconciled by assuming that a mixture of different linear fragments is present in the transformants, each derived from the circular plasmid by breakage at a different point. Copy-number determinations suggest the presence of 45,000-135,000 copies of the injected plasmid per transformed cell. These results suggest that the injected DNA contains information sufficient for both controlled expression and autonomous replication in Paramecium.
Full text
PDF
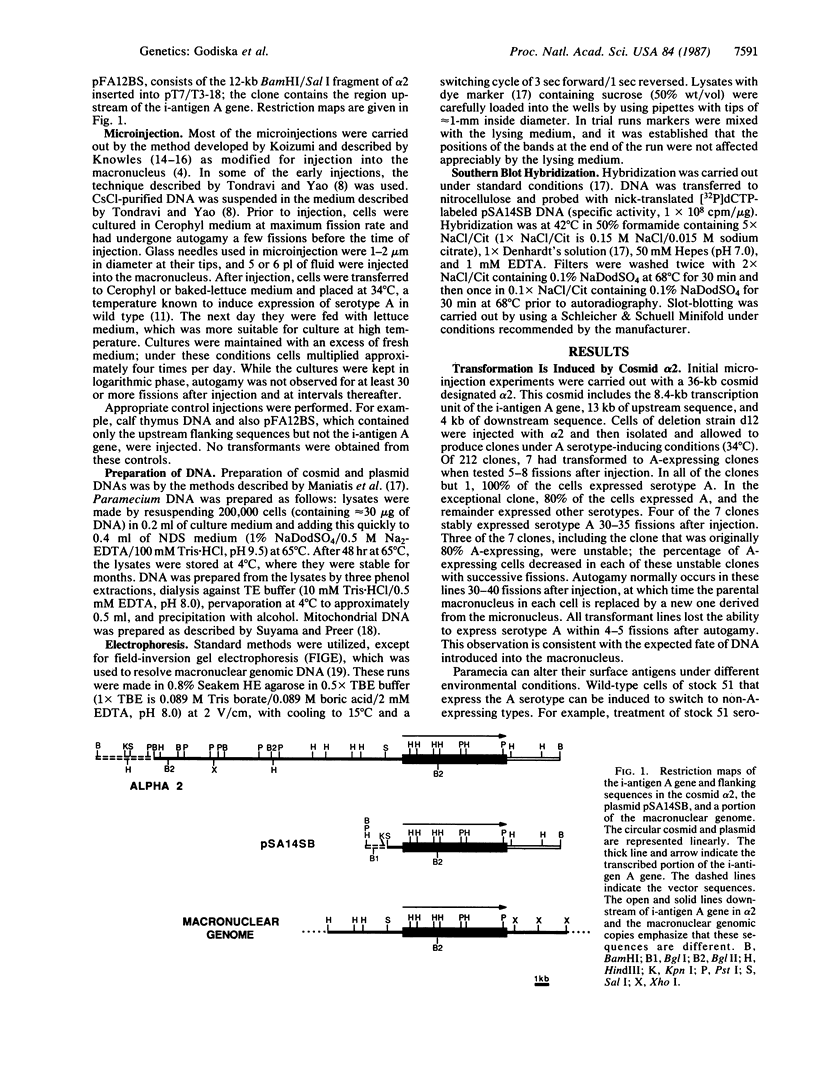



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascenzioni F., Lipps H. J. A linear shuttle vector for yeast and the hypotrichous ciliate Stylonychia. Gene. 1986;46(1):123–126. doi: 10.1016/0378-1119(86)90174-5. [DOI] [PubMed] [Google Scholar]
- Aufderheide K. J. Trichocyst phenotype transformation induced by macronuclear transplantation in Paramecium tetraurelia. Exp Cell Res. 1985 Jan;156(1):282–286. doi: 10.1016/0014-4827(85)90283-6. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Forney J. D., Epstein L. M., Preer L. B., Rudman B. M., Widmayer D. J., Klein W. H., Preer J. R., Jr Structure and expression of genes for surface proteins in Paramecium. Mol Cell Biol. 1983 Mar;3(3):466–474. doi: 10.1128/mcb.3.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Harumoto T. Induced change in a non-mendelian determinant by transplantation of macronucleoplasm in Paramecium tetraurelia. Mol Cell Biol. 1986 Oct;6(10):3498–3501. doi: 10.1128/mcb.6.10.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Knowles J. K. An improved microinjection technique in Paramecium aurelia. Transfer of mitochondria conferring erythromycin-resistance. Exp Cell Res. 1974 Sep;88(1):79–87. doi: 10.1016/0014-4827(74)90620-x. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Kobayashi S. The maintenance of kappa during macronuclear reorganization in Paramecium tetraurelia. J Cell Sci. 1978 Apr;30:187–191. doi: 10.1242/jcs.30.1.187. [DOI] [PubMed] [Google Scholar]
- Koizumi S. Microinjection and transfer of cytoplasm in Paramecium. Experiments on the transfer of kappa particles into cells at different stages. Exp Cell Res. 1974 Sep;88(1):74–78. doi: 10.1016/0014-4827(74)90619-3. [DOI] [PubMed] [Google Scholar]
- Mikami K., Koizumi S. Nuclear transplant studies of the determination of mating type in germ nuclei of Paramecium tetraurelia. Exp Cell Res. 1982 Feb;137(2):397–402. doi: 10.1016/0014-4827(82)90041-6. [DOI] [PubMed] [Google Scholar]
- Soldo A. T., Godoy G. A. The kinetic complexity of Paramecium macronuclear deoxyribonucleic acid. J Protozool. 1972 Nov;19(4):673–678. doi: 10.1111/j.1550-7408.1972.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H. The yeast ARS element, six years on: a progress report. Yeast. 1985 Sep;1(1):1–14. doi: 10.1002/yea.320010102. [DOI] [PubMed] [Google Scholar]
- Wünning I. U., Lipps H. J. A transformation system for the hypotrichous ciliate Stylonychia mytilus. EMBO J. 1983;2(10):1753–1757. doi: 10.1002/j.1460-2075.1983.tb01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Choi J., Yokoyama S., Austerberry C. F., Yao C. H. DNA elimination in Tetrahymena: a developmental process involving extensive breakage and rejoining of DNA at defined sites. Cell. 1984 Feb;36(2):433–440. doi: 10.1016/0092-8674(84)90236-8. [DOI] [PubMed] [Google Scholar]