Abstract
Sophora flavescens (SF) is an herbal medicine widely used for the treatment of viral hepatitis, cancer, viral myocarditis, gastrointestinal hemorrhage, and skin diseases. It was recently reported that SF up-regulates CYP3A expression. The mechanism of SF-induced CYP3A expression is unknown. In the current study, we tested the hypothesis that SF-induced CYP3A expression is mediated by the activation of pregnane X receptor (PXR). We used two cell lines, DPX2 and HepaRG, to investigate the role of PXR in SF-induced CYP3A expression. The DPX2 cell line is derived from HepG2 cells with the stable transfection of human PXR and a luciferase reporter gene linked with a human PXR response element identified in the CYP3A4 gene promoter. In DPX2 cells, SF activated PXR in a concentration-dependent manner. We used a metabolomic approach to identify the chemical constituents in SF, which were further analyzed for their effect on PXR activation and CYP3A regulation. One chemical in SF, N-methylcytisine, was identified as an individual chemical that activated PXR. HepaRG is a highly differentiated hepatoma cell line that mimics human hepatocytes. In HepaRG cells, N-methylcytisine significantly induced CYP3A4 expression, and this induction was suppressed by the PXR antagonist sulforaphane. These results suggest that SF induces CYP3A expression via the activation of PXR.
Introduction
Sophora flavescens (SF) is widely used in traditional Chinese medicine for the treatment of viral hepatitis, cancer, viral myocarditis, gastrointestinal hemorrhage, and skin diseases (Dai et al., 1987; Chen et al., 2000; Sun et al., 2007; Jin et al., 2010). In recent decades, the market for SF has expanded worldwide (Sophora, http://www.itmonline.org/arts/sophora.htm). The potential SF-drug interactions are unknown. To assess SF-drug interactions, Ueng et al. (2009) investigated the effect of SF on the expression of hepatic cytochromes P450 (P450) in mice. Among the P450s tested, Cyp1a, 2a, 2b, and 3a were induced by SF (Ueng et al., 2009). Matrine and oxymatrine are two major pharmacologically active constituents in SF (Ling et al., 2007). Both matrine and oxymatrine have been found to induce CYP2B, but not CYP3A (Yuan et al., 2010). A cell-based reporter gene assay indicated that CYP2B induction by matrine and oxymatrine is mediated by a constitutive androstane receptor (CAR) (Yuan et al., 2010). CAR is a member of the nuclear receptor superfamily regulating the transcription of target genes involved in drug metabolism, such as CYP2B and CYP3A (Baes et al., 1994; Maglich et al., 2002). However, matrine and oxymatrine exhibited no significant effect on the regulation of CYP3A, which might be because 1) matrine and oxymatrine are weak CAR activators, because CAR can only be activated by the high concentration of matrine and oxymatrine (∼300 μM) (Yuan et al., 2010), 2) CAR exhibits a significantly higher selectivity for CYP2B induction than for CYP3A induction (Faucette et al., 2006), and 3) the basal level of CYP2B is much lower than that of CYP3A, so the induction of CYP2B reaches a statistically significant level more readily than that of CYP3A. The mechanism of SF-mediated CYP3A induction remains unclear.
Pregnane X receptor (PXR) is a ligand-activated transcriptional factor regulating drug metabolism. When activated, PXR induces a network of genes that encode phase I (including CYP3A) and phase II xenobiotic-metabolizing enzymes and transporters (Kliewer et al., 1998; Ma et al., 2008). Compared with CAR, PXR is a more important regulator of CYP3A (Kliewer et al., 1998; Goodwin et al., 2002; Faucette et al., 2006). We hypothesized that SF-induced CYP3A expression is mediated by the activation of PXR. Two cell lines, DPX2 and HepaRG, were used to investigate the role of PXR in SF-induced CYP3A expression. The DPX2 cell line is derived from HepG2 cells with the stable transfection of human PXR and a luciferase reporter gene linked with a human PXR response element identified in the CYP3A4 gene promoter (Raucy et al., 2002; Trubetskoy et al., 2005). The DPX2 cell line has been identified as an ideal tool to evaluate the PXR activators and CYP3A4 inducers. HepaRG is a highly differentiated cell line that mimics human hepatocytes with the expressions of multiple nuclear receptors and P450s, including PXR and CYP3A4 (Anthérieu et al., 2010). HepaRG is regarded as a valuable model to investigate the induction of drug-metabolizing P450s (Kanebratt and Andersson, 2008). We used a metabolomic approach to identify the chemical constituents in SF and further analyzed these relatively abundant chemicals for their effect on PXR activation and CYP3A regulation. We demonstrated that SF-induced CYP3A expression was mediated by the activation of PXR. One constituent in SF, N-methylcytisine, was identified as a novel PXR activator.
Materials and Methods
Herbs, Chemicals, and Reagents.
SF, Schisandra chinensis Baill (SCB) and Glycyrrhiza uralensis Fisch (GUF) were obtained from the Guangdong Kangmei Pharmaceutical Company (Puning, Canton, China). SCB and GUF served as positive controls of herbs that activate PXR (Mu et al., 2006). Chemical constituents of SF, sophocarpine, allomatrine, N-methylcytisine, and sophoranol, were purchased from Quality Phytochemicals LLC (Edison, NJ); oxymatrine, matrine, and tetrandrine were bought from INDOFINE Chemical Company (Hillsborough, NJ). The culturing medium and dosing medium for DPX2 cells were purchased from Puracyp Inc. (Carlsbad, CA). Williams' E medium with phenol red and fetal bovine serum for HepaRG cells was bought from Invitrogen (Carlsbad, CA). The luciferase assay system was provided by Promega (Madison, WI). TRIzol reagent was provided by Ambion (Austin, TX). TaqMan Universal PCR Master Mix was acquired from Applied Biosystems (Carlsbad, CA). Midazolam was purchased from Cerilliant Corporation (Round Rock, TX). All of the solvents for liquid chromatography and mass spectrometry were of the highest grade commercially available.
Preparations of Herbal Extracts.
Six grams of dried roots of SF, SCB, and GUF were individually immersed into 300 ml of H2O for 30 min and boiled for 1 h. Each aqueous extract was filtered and centrifuged at 10,000 relative centrifugal force. The supernatant was concentrated using a rotary evaporator to a final volume of 60 ml and served as stock solution. A 20 mM stock solution of each SF constituent was made in dimethyl sulfoxide. The stock solutions were diluted to different concentrations in the cell culture medium before the treatments.
Cell Cultures and Treatments.
Two cell lines, DPX2 and HepaRG, were used to determine the role of PXR in SF-induced CYP3A expression. The DPX2 cell line is derived from HepG2 cells with the stable transfection of human PXR and a luciferase reporter gene (Raucy et al., 2002; Trubetskoy et al., 2005). The DPX2 cell line was used for a large-scale screening of PXR activators. HepaRG cells mimic human hepatocytes with the expressions of multiple nuclear receptors and P450s, including PXR and CYP3A4 (Anthérieu et al., 2010). HepaRG cells were used for the functional analysis of PXR activation. The DPX2 cell line at passage 10 (lot number 4542) was provided by Puracyp, Inc. DPX2 cells were treated following standard operating procedures (105.04 and 116.03; Puracyp, Inc.). In brief, cells were cultured in a 96-well plate with a density of 2 × 105 cells/ml (100 μl in each well) in culturing medium. After a 24-h incubation, the culturing medium was removed and 150 μl of the dosing medium with test compound(s) was added. HepaRG cells were provided by Biopredic International (Rennes, France). The undifferentiated HepaRG cells were seeded at 0.2 million cells/well in a six-well plate, maintained in the growth medium (Biopredic International) for 2 weeks, and then cultured in the differentiation medium containing 2% dimethyl sulfoxide for 2 more weeks. HepaRG cells mimic human hepatocytes and express multiple xenobiotic receptors, including PXR, CAR, and aryl hydrocarbon receptor (Guillouzo et al., 2007). A highly selective PXR antagonist, sulforaphane (Zhou et al., 2007), was used in the treatment of HepaRG cells to specify the role of PXR. The SF aqueous extract and its major chemical constituents were incubated in both DPX2 and HepaRG cell lines, followed by analysis of luciferase activity and CYP3A4 expression and activity. The method for the luciferase assay was described in a previous study (Ma et al., 2007b). All experiments were performed in triplicate.
Cell Viability.
The viability of DPX2 and HepaRG cells was evaluated using the ATP Detection Assay System (PerkinElmer Life and Analytical Sciences, Waltham, MA) and a CytoToX-ONE Homogeneous Membrane Integrity assay for lactate dehydrogenase (Promega). Among the tested concentrations of SF and its constituents, no significant cytotoxicity was noted (data not shown).
Analysis of CYP3A4 Expression and Activity.
After 48 h of treatment with SF and its constituents in DPX2 and HepaRG cells, total RNA was extracted using TRIzol reagent. cDNA was prepared from 1 μg of total RNA. CYP3A4 mRNA was quantified using quantitative real-time polymerase chain reaction (qPCR) (Cheng et al., 2009). Values were quantified using the comparative cycle threshold method, and samples were normalized to glyceraldehyde-3-phosphate dehydrogenase. For CYP3A activity analysis, the culture medium containing SF aqueous extract and its constituents was withdrawn after 48 h of treatment and replaced by the medium containing 50 μM midazolam. Midazolam was used as a probe for CYP3A activity analysis (Ma et al., 2007a). After 2 h of incubation, 100 μl of the medium was taken out. An equal volume of cold acetonitrile was added, and the mixture was centrifuged at 10,000 relative centrifugal force. The top layer of the mixture was injected for ultraperformance liquid chromatography (UPLC) with time-of-flight mass spectrometry (TOFMS) analysis for midazolam metabolites.
UPLC-TOFMS Analysis.
UPLC-TOFMS was used to analyze SF constituents and to detect midazolam metabolite. In brief, a 100 mm × 2.1-mm (Acquity 1.7 μm) UPLC BEH C-18 column (Waters, Milford, MA) was used for chemical separation. The flow rate of the mobile phase was 0.3 ml/min with a gradient ranging from 2 to 98% aqueous acetonitrile containing 0.1% formic acid in a 10-min run. TOFMS was performed in a positive mode with electrospray ionization. The source temperature and desolvation temperature were set at 120 and 350°C, respectively. N2 was applied as the cone gas (10 l/h) and desolvation gas (700 l/h). Argon was applied as the collision gas. TOFMS was calibrated with sodium formate and monitored by the intermittent injection of lock mass leucine enkephalin in real time. The capillary voltage and the cone voltage were set at 3.5 kV and 35 V in positive ion mode. The structure of each chemical was elucidated by tandem mass spectrometry fragmentation with collision energy ramp ranging from 10 to 30 eV.
Data Analysis.
All values are expressed as mean ± S.D., and data were analyzed by a two-tailed Student's t test. p < 0.05 was regarded as significantly different between groups. For the metabolomic analysis of SF constituents, mass chromatograms and mass spectra were acquired by MassLynx software (Waters) in a centroid format from m/z 50 to 1000. Centroid and integrated mass chromatographic data were processed by MarkerLynx software (Waters) to generate a multivariate data matrix. The corresponding data matrices were then exported into SIMCA-P+ 12 (Umetrics, Kinnelon, NJ) for multivariate data analysis. Principal-component analysis (PCA) and orthogonal projection to latent structures-discriminant analysis (OPLS-DA) were conducted on Pareto-scaled data to analyze the chemical constituents of SF.
Results
PXR Activation by SF.
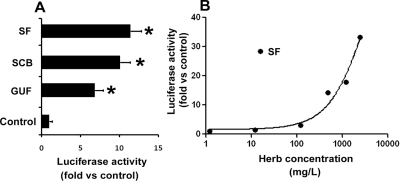
The effects of SF, SCB, and GUF extracts on PXR were evaluated in DPX2 cells. SCB and GUF aqueous extracts served as positive controls for herb-mediated PXR activation (Mu et al., 2006). Similar to SCB and GUF, SF strongly activated PXR, as the luminescence increased 11-fold compared with that for the vehicle control (Fig. 1A). In addition, dose-dependent PXR activation by SF was noted (Fig. 1B).
Fig. 1.
A cell-based PXR gene reporter assay of SF aqueous extract. DPX2 cells were incubated with SF aqueous extract for 24 h. Results are shown as the fold induction of luciferase activity over the vehicle control. A, the effect of SF aqueous extract (480 mg/l) on PXR activation in DPX2 cells. SCB and GUF aqueous extracts (480 mg/l) served as positive controls for herb-mediated PXR activation. The data are presented as means ± S.D. (n = 3; *, p < 0.05 versus control). B, concentration-dependent PXR activation in DPX2 cells by SF aqueous extract. The data are presented as means (n = 3 at each concentration).
Chemical Constituents of SF.
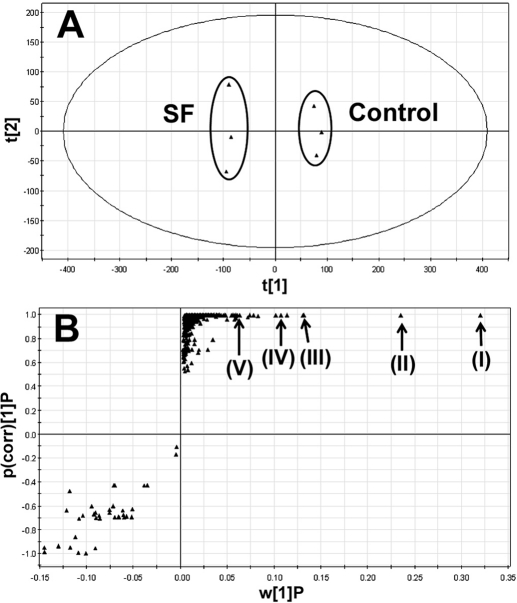
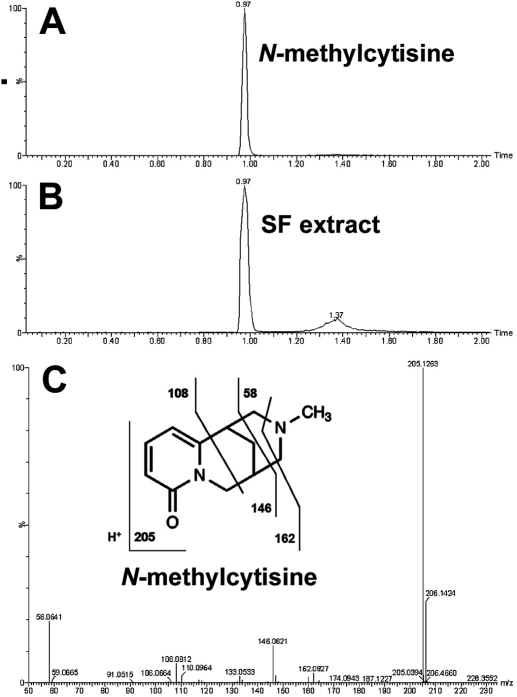
SF aqueous extract is expected to contain chemical(s) that activate PXR. The chemical constituents of SF aqueous extract were profiled using a liquid chromatography-mass spectrometry-based metabolomic approach. The results of chemometric analysis on the ions produced by UPLC-TOFMS are shown in Fig. 2. The score plot of unsupervised PCA (Fig. 2A) revealed two clusters corresponding to the control and SF aqueous extract. The corresponding S-plot (Fig. 2B) generated from OPLS-DA displayed the ion contribution to the group separation of control and SF aqueous extract. Top ranking ions in SF aqueous extract were marked in the S-plots (Fig. 2B), which were identified as oxymatrine (I), oxysophocarpine (II), matrine (III), sophocarpine (IV), and N-methylcytisine (V). The structures of these chemicals were confirmed by comparing their retention times and mass fragments with those of commercially available standards. The confirmation of N-methylcytisine (C12H16N2O) in SF extract is shown in Fig. 3. The N-methylcytisine had exactly the same retention time (0.97 min) and mass fragments (58, 108, 146, and 162) as the authentic standard. Individual chemicals were further evaluated for their effect on PXR activation and CYP3A expression.
Fig. 2.
Global profiling of the chemical constituents of SF aqueous extract using a liquid chromatography-mass spectrometry-based metabolomic approach. UPLC-TOFMS was used to analyze SF constituents. Centroid and integrated mass chromatographic data were processed by MarkerLynx software to generate a multivariate data matrix. PCA and OPLS-DA were conducted on Pareto-scaled data to analyze the chemical constituents of SF. A, separation of SF aqueous extract group and vehicle group in a PCA score plot. The t[1] and t[2] values represent the scores of each sample in principal component 1 and 2, respectively. B, loading S-plot generated by OPLS-DA. The y-axis represents the correlation of each ion to the model, and the x-axis represents the relative abundance of ions. The ions from SF aqueous extract are presented in the upper-right window, and several top-ranking ions were identified as oxymatrine (I), oxysophocarpine (II), matrine (III), sophocarpine (IV), and N-methylcytisine (V).
Fig. 3.
Structural elucidation of N-methylcytisine in SF extract. The analysis of N-methylcytisine and SF extract was performed under the same conditions using UPLC-TOFMS. A, a chromatogram of authentic N-methylcytisine, retention time at 0.97 min. B, a chromatogram of N-methylcytisine detected in SF extract, retention time at 0.97 min. C, tandem mass spectrometry fragmentation of N-methylcytisine. Tandem mass spectrometry fragmentation was conducted with collision energy ramping from 10 to 30 eV. Major daughter ions from fragmentation are interpreted in the inlaid structural diagram.
Screening PXR Activator(s) from SF Constituents.
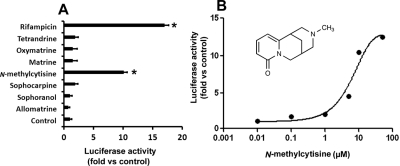
The top ranking ions in the metabolomic analysis of SF aqueous extract were screened for their effects on PXR. N-Methylcytisine significantly activated PXR, as the luciferase activity increased ∼10-fold in DPX2 cells (Fig. 4A). The other SF constituents tested, such as oxymatrine, matrine, sophocarpine, and allomatrine, had no significant effect on PXR. As shown in Fig. 4B, N-methylcytisine activated PXR in a concentration-dependent manner in DPX2 cells with a median effective concentration at ∼8.9 μM.
Fig. 4.
Identification of PXR activator(s) in SF aqueous extract. DPX2 cells were incubated for 24 h with individual chemicals identified from SF aqueous extract. Results are shown as the fold induction of luciferase activity over the vehicle control. A, effect of individual chemicals (10 μM) on PXR activation. These chemicals were identified in SF aqueous extract. Rifampicin served as a positive control for PXR activation. The data are presented as mean ± S.D. (n = 3; *, p < 0.05 versus control). B, concentration-dependent PXR activation in DPX2 cells by N-methylcytisine. The data are presented as means (n = 3 at each concentration).
SF and N-Methylcytisine-Mediated CYP3A4 Induction.
After the treatment with different concentrations of SF aqueous extract and N-methylcytisine in DPX2 and HepaRG cells, CYP3A4 mRNA expression was quantified by qPCR. SF aqueous extract induced CYP3A4 mRNA expression in a concentration-dependent manner in DPX2 cells (Fig. 5A). Significant induction of CYP3A4 by N-methylcytisine was also noted in DPX2 cells (Fig. 5A). Similar to the results in DPX2 cells, both SF aqueous extract and N-methylcytisine up-regulated CYP3A4 mRNA expression in HepaRG cells (Fig. 5B). Sulforaphane, a PXR antagonist (Zhou et al., 2007), significantly abolished N-methylcytisine-mediated CYP3A4 up-regulation in HepaRG cells (Fig. 5C), suggesting that CYP3A4 induction by N-methylcytisine is PXR-dependent. Consistent with the CYP3A4 expression level, CYP3A activity was significantly increased in DPX2 cells after the pretreatment of SF aqueous extract and N-methylcytisine (Fig. 6). Pretreatment with oxymatrine, matrine, sophocarpine, and allomatrine had no significant effect on CYP3A activity (Fig. 6).
Fig. 5.
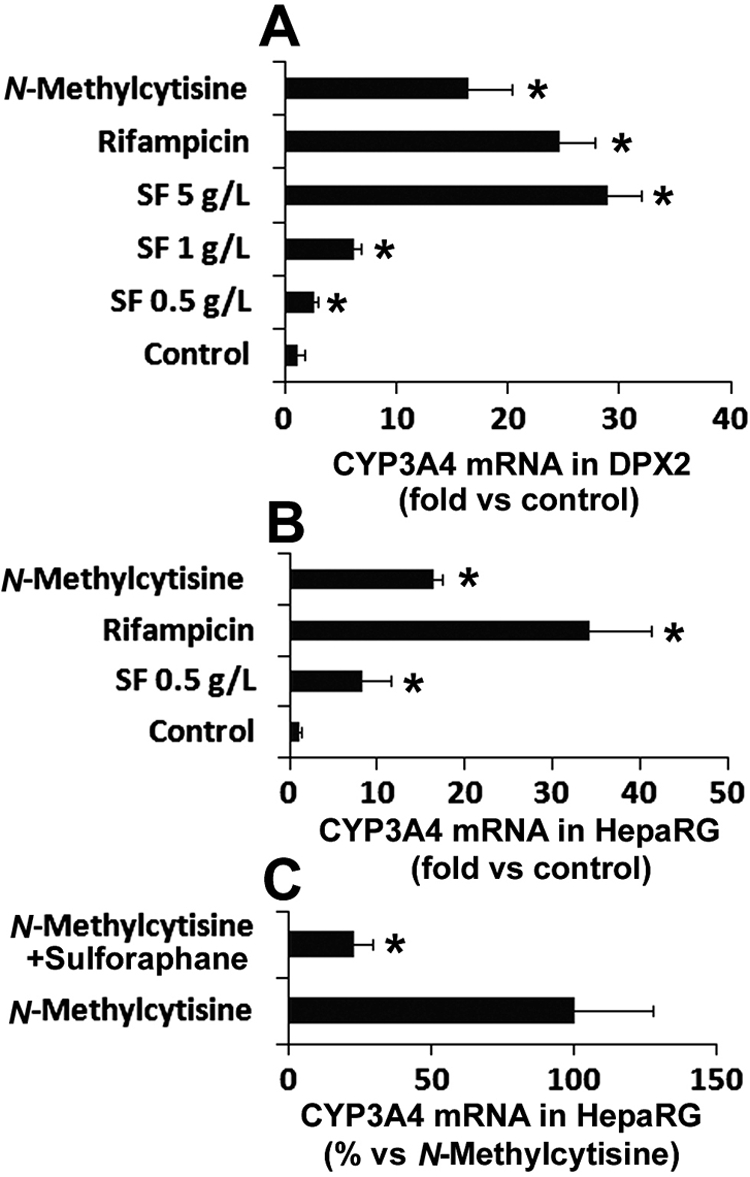
Induction of CYP3A4 by SF aqueous extract and N-methylcytisine. DPX2 cells and HepaRG cells were incubated with SF aqueous extract and N-methylcytisine (10 μM) for 48 h. CYP3A4 mRNA expression was analyzed by qPCR. Values were quantified using the comparative cycle threshold method, and samples were normalized to glyceraldehyde-3-phosphate dehydrogenase. Rifampicin (10 μM) served as a positive control of CYP3A4 inducer. A, effect of SF aqueous extract and N-methylcytisine on CYP3A4 expression in DPX2 cells. The data are shown as the fold induction versus control (n = 3; *, p < 0.05 versus control). B, effect of SF aqueous extract and N-methylcytisine on CYP3A4 expression in HepaRG cells. The data are shown as the fold induction versus control (n = 3; *, p < 0.05 versus control). C, effect of PXR antagonist sulforaphane (20 μM) on N-methylcytisine-mediated CYP3A4 induction in HepaRG cells. CYP3A4 expression in the N-methylcytisine-treated group was set as 100% (n = 3; *, p < 0.05 versus N-methylcytisine-treated group).
Fig. 6.
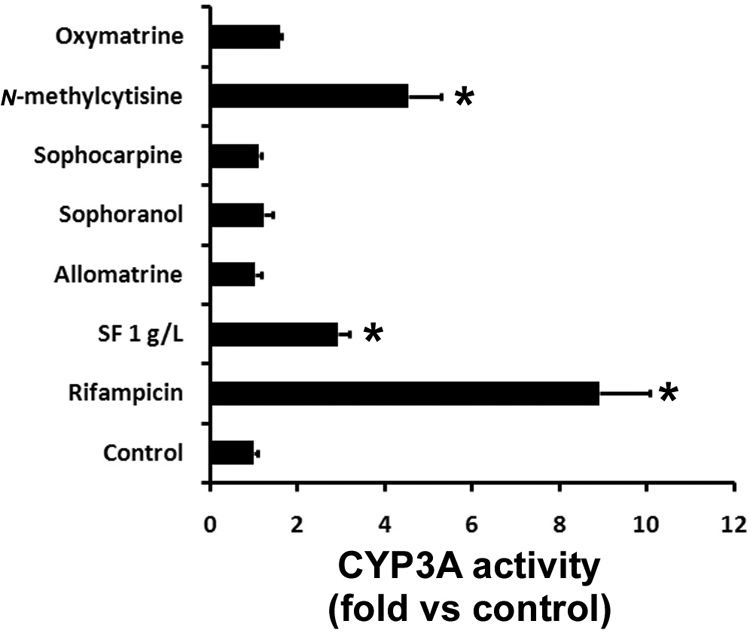
The effect of the pretreatment of SF aqueous extract and its constituents on CYP3A activity in DPX2 cells. DPX2 cells were exposed to SF aqueous extract and its constituents (10 μM each) for 48 h. After the treatment, the culture medium containing SF aqueous extract or its constituents was withdrawn and changed to the medium containing 50 μM midazolam. Midazolam was used as a probe for CYP3A activity analysis. 1′-Hydroxymidazolam was analyzed by UPLC-TOFMS. CYP3A activity in the control group was set as 1. Rifampicin (10 μM) served as a positive control. All data are presented as means ± S.D. (n = 3; *, p < 0.05 versus control).
Discussion
Herbal medicines or supplements are used worldwide (Kraft, 2009). The safety and efficacy of herb-drug combinations are largely unknown. Research on herb-drug interactions is urgently needed to guide herbal usage (Chan et al., 2010). Metabolism-mediated herb-drug interactions are very common, which might decrease the efficacy and/or increase the toxicity of the combined drug. The nuclear receptor PXR is a xenobiotic sensor, which regulates a large number of enzymes and transporters that contribute to drug metabolism and disposition (Kliewer et al., 1998; Ma et al., 2008). St. John's wort is an herb that has drawn a lot of attention because of herb-drug interactions. PXR was identified as the key mediator in St. John's wort-drug interactions (Moore et al., 2000; Mannel, 2004). In recent years, PXR has been considered as a molecular target to predict herb-drug interactions. SCB, GUF, Ginkgo biloba, Tian Xian, and Coleus forskohlii were reported as herbs that activate PXR (Ding and Staudinger, 2005; Mu et al., 2006; Lichti-Kaiser and Staudinger, 2008; Li et al., 2009). In the current study, we determined that SF activates PXR.
PXR is the dominant activator of CYP3A transcription (Kliewer et al., 1998, 2002; Goodwin et al., 2002). Activation of human PXR results in the transcriptional activation of CYP3A involving the formation of a heterodimer with retinoid X receptor, which binds to PXR response elements in the 5′-flanking region of the CYP3A4 gene (Goodwin et al., 1999). By determining that SF activates PXR, we uncovered the mechanism of SF-mediated CYP3A induction. CYP3A is an important metabolic enzyme that is responsible for metabolizing more than 50% of current prescription drugs (Guengerich, 1999). The most common clinical reason for the activation of the human PXR is the occurrence of drug-drug interactions mediated by up-regulated CYP3A isozymes. However, there is no clinical report on SF-drug interactions, although SF can induce CYP3A and some other P450s (Ueng et al., 2009). It is possible that the clinical reports do not exist because no clinical trials were done on the SF-drug interactions. On the other hand, it might be due to the inhibitory effect of SF on P450s. During the screening of herbal medicines that inhibit CYP3A activity, SF showed the highest potency, which suggested that there are CYP3A inhibitors in SF constituents (Lee et al., 2007). When CYP3A inducers and inhibitors are cotreated with drugs as CYP3A substrates, the effect of CYP3A inducers in drug-drug interactions is not significant, because the CYP3A inhibitors mask the effect of CYP3A inducers (Hafner et al., 2010). However, when the inhibitor is withdrawn, the effect of CYP3A inducers will be present because of the high expression of CYP3A. In the current study, we cultured the cells with the medium containing SF aqueous extract or its constituents for 48 h and then withdrew the culture medium before CYP3A activity analysis. As expected, a significant increase in CYP3A activity was observed, which suggested that SF-drug interactions might occur in an early period after SF withdrawal.
Studies on herb-drug interaction are always challenging because an herb is a chemical mixture, and its constituents vary when the herb is collected from different locations and in different seasons or is extracted by different methods. Profiling the chemicals in an herb is a critical step in understanding potential herb-drug interactions. In the current study, we adopted the metabolomic approach to profile the chemical constituents in SF. Metabolomics is the systematic study of small molecule metabolite profiles that are left behind as unique chemical fingerprints by biological processes. The metabolomic approach has been used and is identified as a powerful tool to disclose the chemical components of traditional herbs (Rochfort, 2005; Xie et al., 2008). By combining the speed and resolving power of UPLC, the accurate mass determination of TOFMS, and multivariate data analysis such as PCA and OPLS-DA, we can collect the overall chemical information of an herb, which can subsequently be used to guide research on herbal medicine. This method has a big advantage in the large-scale analysis of herbs and thus accelerates research on herbs. In the current study, we profiled the chemical constituents of SF, and the top-ranking chemicals were analyzed for their effect on PXR. N-Methylcytisine was identified as a novel PXR activator. N-Methylcytisine has also been isolated from other herbs, such as Sophora secundiflora and Caulophyllum thalictroides (Izaddoost et al., 1976; Ding et al., 2006). We expect that these herbs can also activate PXR at different levels based on their abundance of N-methylcytisine. These data indicated that the metabolomic approach is an ideal tool to profile the chemical constituents in an herb, and it has great promise in pharmacological and toxicological research on herbs.
In summary, SF-induced CYP3A expression is mediated by the activation of PXR. N-Methylcytisine is a chemical in SF that activates PXR. Potential SF-drug interactions should be monitored in clinical practice.
Acknowledgments.
We thank Puracyp, Inc., for providing DPX2 cells and Biopredic International for providing HepaRG cells. We thank Dr. Martha Montello for editing the article.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant COBRE 5P20-RR021940].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.035253.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- SF
- Sophora flavescens
- P450
- cytochrome P450
- CAR
- constitutive androstane receptor
- PXR
- pregnane X receptor
- SCB
- Schisandrae chinensis Baill
- GUF
- Glycyrrhizae uralensis Fisch
- qPCR
- quantitative real-time polymerase chain reaction
- UPLC
- ultraperformance liquid chromatography
- TOFMS
- time-of-flight mass spectrometry
- PCA
- principal-component analysis
- OPLS-DA
- orthogonal projection to latent structures-discriminant analysis.
References
- Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, et al. (2010) Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos 38:516–525 [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14:1544–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE. (2010) Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 13:50–65 [PubMed] [Google Scholar]
- Chen C, Guo SM, Liu B. (2000) A randomized controlled trial of kurorinone versus interferon-α2a treatment in patients with chronic hepatitis B. J Viral Hepat 7:225–229 [DOI] [PubMed] [Google Scholar]
- Cheng J, Ma X, Krausz KW, Idle JR, Gonzalez FJ. (2009) Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab Dispos 37:1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Chan MY, Lee SS, Ogle CW. (1987) Effects of Sophora flavescens Ait. on haemodynamics and ventricular fibrillation threshold in anaesthetized dogs. Am J Chin Med 15:53–57 [DOI] [PubMed] [Google Scholar]
- Ding PL, Huang H, Zhou P, Chen DF. (2006) Quinolizidine alkaloids with anti-HBV activity from Sophora tonkinensis. Planta Med 72:854–856 [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. (2005) Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther 312:849–856 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. (2006) Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther 317:1200–1209 [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. (1999) The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 56:1329–1339 [DOI] [PubMed] [Google Scholar]
- Goodwin B, Redinbo MR, Kliewer SA. (2002) Regulation of cyp3a gene transcription by the pregnane X receptor. Annu Rev Pharmacol Toxicol 42:1–23 [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17 [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. (2007) The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact 168:66–73 [DOI] [PubMed] [Google Scholar]
- Hafner V, Jäger M, Matthée AK, Ding R, Burhenne J, Haefeli WE, Mikus G. (2010) Effect of simultaneous induction and inhibition of CYP3A by St John's wort and ritonavir on CYP3A activity. Clin Pharmacol Ther 87:191–196 [DOI] [PubMed] [Google Scholar]
- Izaddoost M, Harris BG, Gracy RW. (1976) Structure and toxicity of alkaloids and amino acids of Sophora secundiflora. J Pharm Sci 65:352–354 [DOI] [PubMed] [Google Scholar]
- Jin JH, Kim JS, Kang SS, Son KH, Chang HW, Kim HP. (2010) Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J Ethnopharmacol 127:589–595 [DOI] [PubMed] [Google Scholar]
- Kanebratt KP, Andersson TB. (2008) Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab Dispos 36:1444–1452 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. (2002) The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23:687–702 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82 [DOI] [PubMed] [Google Scholar]
- Kraft K. (2009) Complementary/alternative medicine in the context of prevention of disease and maintenance of health. Prev Med 49:88–92 [DOI] [PubMed] [Google Scholar]
- Lee SS, Zhang B, He ML, Chang VS, Kung HF. (2007) Screening of active ingredients of herbal medicine for interaction with CYP450 3A4. Phytother Res 21:1096–1099 [DOI] [PubMed] [Google Scholar]
- Li L, Stanton JD, Tolson AH, Luo Y, Wang H. (2009) Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res 26:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Staudinger JL. (2008) The traditional Chinese herbal remedy tian xian activates pregnane X receptor and induces CYP3A gene expression in hepatocytes. Drug Metab Dispos 36:1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JY, Zhang GY, Cui ZJ, Zhang CK. (2007) Supercritical fluid extraction of quinolizidine alkaloids from Sophora flavescens Ait. and purification by high-speed counter-current chromatography. J Chromatogr A 1145:123–127 [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Gonzalez FJ. (2008) The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol 4:895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. (2007a) The pregnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 35:194–200 [DOI] [PubMed] [Google Scholar]
- Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. (2007b) Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 322:391–398 [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. (2002) Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol 62:638–646 [DOI] [PubMed] [Google Scholar]
- Mannel M. (2004) Drug interactions with St John's wort: mechanisms and clinical implications. Drug Saf 27:773–797 [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. (2000) St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA 97:7500–7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, Huang L, Yaramus M, Baum A, Venkataramanan R, et al. (2006) Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther 316:1369–1377 [DOI] [PubMed] [Google Scholar]
- Raucy J, Warfe L, Yueh MF, Allen SW. (2002) A cell-based reporter gene assay for determining induction of CYP3A4 in a high-volume system. J Pharmacol Exp Ther 303:412–423 [DOI] [PubMed] [Google Scholar]
- Rochfort S. (2005) Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J Nat Prod 68:1813–1820 [DOI] [PubMed] [Google Scholar]
- Sun M, Han J, Duan J, Cui Y, Wang T, Zhang W, Liu W, Hong J, Yao M, Xiong S, et al. (2007) Novel antitumor activities of Kushen flavonoids in vitro and in vivo. Phytother Res 21:269–277 [DOI] [PubMed] [Google Scholar]
- Trubetskoy O, Marks B, Zielinski T, Yueh MF, Raucy J. (2005) A simultaneous assessment of CYP3A4 metabolism and induction in the DPX-2 cell line. AAPS J 7:E6–E13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueng YF, Chen CC, Tsai CC, Soucek P. (2009) Differential inductive profiles of hepatic cytochrome P450s by the extracts of Sophora flavescens in male and female C57BL/6JNarl mice. J Ethnopharmacol 126:437–446 [DOI] [PubMed] [Google Scholar]
- Xie G, Plumb R, Su M, Xu Z, Zhao A, Qiu M, Long X, Liu Z, Jia W. (2008) Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci 31:1015–1026 [DOI] [PubMed] [Google Scholar]
- Yuan F, Chen J, Wu WJ, Chen SZ, Wang XD, Su Z, Huang M. (2010) Effects of matrine and oxymatrine on catalytic activity of cytochrome P450s in rats. Basic Clin Pharmacol Toxicol doi: 10.1111/j.1742-7843.2010.00596.x [DOI] [PubMed] [Google Scholar]
- Zhou C, Poulton EJ, Grün F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. (2007) The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol 71:220–229 [DOI] [PubMed] [Google Scholar]