Abstract
UDP-glucuronosyltransferases (UGTs) are enzymes involved in the metabolism of steroid hormones, carcinogens, cancer chemotherapy agents, and addictive agents from cigarettes. Because the UGT2B family of genes has been linked to hormonal regulation in human cell lines in vitro, we hypothesized that there may be sex-related differences in the expression and activity of these genes in human tissues. To evaluate whether there are sex differences in UGT2B expression and activity, we examined 103 normal human liver specimens for UGT2B expression by real-time polymerase chain reaction and in vitro glucuronidation activities in human liver microsomes (HLM). Men exhibited an approximately 4-fold higher level of expression of UGT2B17 than women (p = 0.007). Consistent with the increased expression of UGT2B17 in men, HLM from men also had a higher level of glucuronidation activity than HLM from women against three UGT2B17 substrates: 3-fold higher for 17-dihydroexemestane (p = 0.002); 3-fold higher for 3-hydroxycotinine (p < 0.001); and 1.5-fold higher for suberoylanilide hydroxamic acid (p = 0.014). When we stratified by UGT2B17 gene deletion genotype, similar patterns were observed for all three substrates, with HLM from men with the UGT2B17 (+/+) or (+/0) genotypes exhibiting significantly higher levels of glucuronidation activity against all three substrates compared with HLM from women. These data suggest that men have a higher amount of UGT2B17 glucuronidation activity then women. This sex difference in UGT2B17 gene expression and corresponding protein activity could potentially result in different levels of carcinogen detoxification or drug elimination in men versus women.
Introduction
UDP-glucuronosyltransferases (UGTs) are phase II enzymes responsible for the metabolism and elimination of a variety of exogenous compounds including drugs, chemotherapeutic agents, and environmental pollutants and carcinogens via conjugation with glucuronic acid (Tukey and Strassburg, 2000; Nagar and Remmel, 2006). Glucuronidation is also a major mode of metabolism and excretion of steroid hormones including androgens, estrogens, and their metabolites (Tukey and Strassburg, 2000; Lévesque et al., 2001; Bélanger et al., 2003; Guillemette et al., 2004; Lépine et al., 2004; Nagar and Remmel, 2006). Although many enzymes participate in maintaining steroid hormone balance, it is postulated that UGTs are vital enzymes modulating the action of steroid hormones, with virtually all individual UGTs exhibiting some level of activity against estrogens and/or androgens (Bélanger et al., 2003). Although the UGT1A enzymes generally exhibit the highest activity for estrogens, the UGT2B subfamily of enzymes exhibits the highest activity against androgens (Tukey and Strassburg, 2000; Lévesque et al., 2001; Bélanger et al., 2003; Guillemette et al., 2004; Lépine et al., 2004; Nagar and Remmel, 2006).
Several studies have shown that androgens and estrogens regulate the expression of the UGT2B family of enzymes (Beaulieu et al., 1997; Guillemette et al., 1997; Strasser et al., 1997; Bélanger et al., 1998; Hum et al., 1999; Li et al., 1999; Magnanti et al., 2000; Chouinard et al., 2006; Harrington et al., 2006; Hu and Mackenzie, 2009). In human prostate cell lines, the expression of UGT2B10, 2B15, and 2B17 was down-regulated by androgens, whereas UGT2B11 expression was up-regulated by androgen treatment (Chouinard et al., 2006). Harrington et al. (2006) showed that in estrogen receptor-positive human breast cancer cells treatment with 17β-estradiol increased expression of UGT2B15 but not of other UGT2B enzymes. However, using one of the same cell lines (MCF-7), Hu and Mackenzie (2009) demonstrated that expression of UGT2B17, as well as that of UGT2B15, is induced by 17β-estradiol. Despite the fact that many UGT1A genes also metabolize hormones, no studies have examined the effects of androgens and estrogens on expression of these genes in cells. Although no studies of sex differences in UGT gene expression have as yet been performed in human tissues, there have been studies demonstrating that UGT2B15-mediated oxazepam glucuronidation is faster in men than in women (Greenblatt et al., 1980; Court et al., 2002, 2004). In addition, a previous study showed that there were sex differences in the expression of UGT genes in mice and that this effect varied by tissue type and by UGT isoform (Buckley and Klaassen, 2007).
Because UGT2B enzymes metabolize many exogenous compounds including a variety of carcinogens, drugs, and cancer chemotherapeutic agents, a sex difference in UGT2B gene expression and corresponding protein activity could potentially result in different levels of carcinogen detoxification or drug elimination in men versus women. With use of a panel of normal human liver specimens, the goal of the present study was to examine whether differences exist in the expression and activity of UGT2B enzymes by sex.
Materials and Methods
Chemicals.
Exemestane was purchased from Hangzhou HETD Industry Co. Ltd. (Zhejiang, China), and 17-dihydroexemestane (17-DHE) was synthesized in the Organic Synthesis Core at Penn State University College of Medicine. Suberoylanilide hydroxamic acid (SAHA) was also synthesized in this core facility. 3-Hydroxycotinine (3-HC), 3-HC glucuronide, and deuterium-labeled internal standards cotinine-(methyl-D3)-3′-O-glucuronide were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). Structures of all compounds were provided in previous publications (Balliet et al., 2009; Sun et al., 2010; G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication). UDP-glucuronic acid, alamethicin, and β-glucuronidase were purchased from Sigma-Aldrich (St. Louis, MO). Predesigned and preoptimized TaqMan Gene Expression Assays were purchased from Applied Biosystems (Foster City, CA). All other chemicals were purchased from Thermo Fisher Scientific (Waltham, MA) unless specified otherwise.
Tissues.
Total RNA from pathologically normal liver tissue from 103 patients (62 men and 41 women) undergoing surgery for hepatocellular carcinoma was obtained and has been described previously (Wiener et al., 2004). Genomic DNA and total RNA was extracted from the same specimens by standard phenol/chloroform or TRIzol methods, and sufficient liver tissue was also obtained for preparations of human liver microsomes (HLM) and glucuronidation activity assays for these specimens.
Glucuronidation Assays.
Microsomes were prepared and in vitro glucuronidation activity assays against three UGT2B17 substrates were performed as described previously (Balliet et al., 2009; Sun et al., 2010; G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication). In brief, glucuronidation activities of HLM against 17-DHE, 3-HC, or SAHA were determined after an initial incubation of HLM (2.5–25 μg of protein) with alamethicin (50 μg/mg protein) for 15 min in an ice bath. Incubations (10–50 μl) were subsequently performed at 37°C for 1 h in 50 mM Tris buffer (pH 7.5), 10 mM MgCl2, 4 mM UDP-glucuronic acid, and 9.4 μM to 8 mM substrate for rate determinations, whereas 4 to 150 μM 17-dihydroexemestane was used for kinetic analysis by HLM. Reactions were terminated by the addition of the same volume as the initial reaction of ice-cold acetonitrile. For 3-HC, reactions were first spiked with 5 μl of internal standard, cotinine-(methyl-D3)-3′-O-glucuronide, at a concentration of 10 ppm, and 3-HC reactions were terminated by the addition of ice-cold acetonitrile-methanol (75:25%, v/v). Reactions were centrifuged at 12,000 to 16,000g for 10 min at 4°C, and supernatants were collected for analysis by ultrapressure liquid chromatography (UPLC) with ultraviolet detection or MS/MS.
UPLC and UPLC-MS/MS Conditions.
17-DHE glucuronidation was analyzed as described previously (Sun et al., 2010) using an ACQUITY UPLC System (Waters, Milford, MA) with a 1.7-μm ACQUITY UPLC BEH C18 analytical column (2.1 mm × 50 mm; Waters Chromatography Ireland Ltd., Dublin, Ireland) in series with a 0.2-μm assay frit filter (2.1 mm; Waters). The gradient elution conditions, using a flow rate of 0.3 ml/min, were as follows: starting with 19% acetonitrile and 81% buffer A (5 mM ammonium acetate, pH 5.0) for 1 min, a subsequent linear gradient to 75% acetonitrile and 25% buffer over 2 min, and then maintenance at 75% acetonitrile for 2 min. Exemestane-17-O-glucuronide was confirmed by its stability in 1 M NaOH but sensitivity to the treatment of β-glucuronidase. In addition, incubation products (up to 5 μl) were loaded onto the UPLC-MS/MS system for confirmation of exemestane-17-O-glucuronide formation. By using a positive mode, the parent compound [M + H]+ peak and the glucuronide [M − Gluc + H]+ peak were characterized.
SAHA glucuronidation assays were also performed by UPLC, as described previously (Balliet et al., 2009), with a gradient elution starting with 5.6% buffer B (100% acetonitrile) and 94.4% buffer A [10 mM ammonium acetate (pH 5.0) and 10% acetonitrile]; a linear gradient to 72% buffer B over 3 min was used. The flow rate was maintained at 0.3 ml/min. The amount of glucuronide formed was determined on the basis of the ratio of SAHA glucuronide versus unconjugated SAHA after the area under the curve for the SAHA and SAHA glucuronide peaks was calculated using the known amount of SAHA for each reaction as the reference. SAHA glucuronide was confirmed by sensitivity to β-glucuronidase and mass spectrometry.
3-HC glucuronidation was analyzed as described previously (G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication) using an ACQUITY UPLC-MS/MS system (Waters), consisting of an ACQUITY UPLC pump, an autosampler, an ACQUITY UPLC BEH HILIC (2.1 × 100 mm, 1.7-μm particle size; Waters) column at 45°C, and an ACQUITY TQ tandem mass spectrometer (Waters). UPLC was performed at a flow rate of 0.5 ml/min using the following conditions: 1.5 min in 20% solvent A, a linear gradient for 0.5 min to 100% solvent A, and 2 min in 100% solvent A, where solvent A is 5 mM NH4AC (pH 5.7) and 50% acetonitrile (v/v) and solvent B is 5 mM NH4AC (pH 5.7) and 90% acetonitrile (v/v). After each run, the column was washed and reconditioned with 2 min in 100% solvent A and 2 min in 20% solvent A at flow rate of 1 ml/min. The injection volume of each prepared sample was 5 μl. The Waters ACQUITY TQ tandem mass spectrometer was equipped with an electrospray ionization probe operated in the positive ion mode, with capillary voltage at 0.64 kV. Nitrogen was used as both the cone and desolvation gases with flow rates maintained at 20 and 760 l/h, respectively. Ultrapure argon was used as the collision gas with a flow rate of 0.1 l/h for collision-induced dissociation. The source and desolvation gas temperatures were 140 and 450°C, respectively. For the assay of 3-HC Gluc in 3-HC glucuronidation assay samples, the mass spectrometer was operated in the multiple reaction monitoring mode, and the concentration of 3-HC Gluc was determined. The dwell time for each ion was 100 ms with 5 ms of interscan delay. The cone voltage and collision energy were 35 V and 15 V, respectively, for both 369 > 193 (3-HC Gluc) and 372 > 196 (internal standard) ion transitions. Standard curves were constructed by plotting the ratio of the 3-HC Gluc peak area to the peak area of its deuterium-labeled internal standards versus analyte concentration ranging from 0.2 to 25 ppm. 3-HC Gluc concentrations were determined by measuring the peak area ratios of 3-HC Gluc to its internal standard and then calculating the 3-HC Gluc concentration from the standard curve using MassLynx software (Waters). The 3-HC Gluc formation rate was calculated accordingly.
Expression and Genotype Assays.
Reverse transcription PCR was performed using a SuperScript II kit (Invitrogen, Carlsbad, CA) on 103 liver RNA samples to obtain cDNA. Real-time PCR gene expression assays were performed on 32 frequently used endogenous control genes for a subset of our data set (>5%) to choose an appropriate control in our liver samples. Because peptidylprolyl isomerase A (PPIA) exhibited the least variability of all 32 endogenous control genes (including β-actin and glyceraldehyde-3-phosphate dehydrogenase), it was used as the endogenous control for further analysis of UGT expression. Predesigned and preoptimized TaqMan Gene Expression Assays were used in real-time PCRs performed on an ABI 7900 HT Sequence Detection System (gene expression assays for UGT2Bs: Hs02383831_s1 for UGT2B4, Hs02556232_s1 for UGT2B7, Hs02556282_s1 for UGT2B10, Hs01894900_gH for UGT2B11, Hs00870076_s1 for UGT2B15, Hs00854486_sH for UGT2B17, and Hs00852540_s1 for UGT2B28). Each reaction was performed in triplicate according to the manufacturer's instructions. SDS 2.2.2 software was used to evaluate real-time PCRs and to determine the threshold cycle (CT), which is defined as the cycle at which PCR amplification reaches a value significantly greater than the baseline. This software was also used to calculate the relative expression of each mRNA by the ΔCT method, which is defined as the value obtained by subtracting the CT value of PPIA mRNA from the CT value of the target mRNA. The amount of target UGT mRNA relative to that of PPIA mRNA was presented as 2−ΔCT, as done traditionally (Nishimura and Naito, 2006). The UGT2B17 deletion polymorphism was genotyped by real-time PCR with allelic discrimination as described previously (Gallagher et al., 2007).
Statistical Analysis.
The nonparametric Mann-Whitney test was used to determine statistical significance of expression of UGT2B genes between men and women. Kinetic constants were determined using the Michaelis-Menten model in GraphPad Prism 5 software. The equation for this model is
where X represents substrate concentration, and Y represents enzyme velocity. All other statistical analyses were conducted using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL).
Results
The levels of mRNA expression of the seven UGT2B genes (UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15, UGT2B17, and UGT2B28) in a series (n = 103) of normal human liver specimens are shown in Table 1. All seven UGT2B genes were expressed at detectable levels in human liver, with UGT2B4 expressed at the highest level, followed in order by UGT2B15 > UGT2B10 > UGT2B7 > UGT2B17 > UGT2B11 > UGT2B28. UGT2B expression was different in livers from men versus women, with the order being UGT2B4 > UGT2B15 > UGT2B10 > UGT2B7 > UGT2B17 > UGT2B11 > UGT2B28 in men and UGT2B4 > UGT2B15 > UGT2B10 > UGT2B7 > UGT2B28 > UGT2B17 > UGT2B11 in women. UGT2B17 exhibited a 3.6-fold higher level of expression in liver specimens from men than in liver specimens from women (p = 0.007) (Table 1). Whereas UGT2B4 expression was observed to be marginally higher in liver specimens from women (1.9-fold; p = 0.074), no other UGT2B gene exhibited significant differences in expression between men and women.
TABLE 1.
Sex differences in UGT2B gene expression in human liver
| Gene | 2−ΔCTa |
p valueb | |
|---|---|---|---|
| mRNA Expression Men | mRNA Expression Women | ||
| n = 62 | n = 41 | ||
| UGT2B4 | 5.14 ± 0.24 | 9.90 ± 2.14 | 0.074 |
| UGT2B7 | 1.16 ± 0.05 | 1.14 ± 0.07 | 0.866 |
| UGT2B10 | 2.28 ± 0.11 | 2.68 ± 0.26 | 0.573 |
| UGT2B11 | 0.014 ± 0.001 | 0.037 ± 0.021 | 0.339 |
| UGT2B15 | 3.71 ± 0.55 | 3.35 ± 0.39 | 0.178 |
| UGT2B17 | 0.424 ± 0.057 | 0.119 ± 0.029 | 0.007 |
| UGT2B28 | 0.006 ± 0.001 | 0.126 ± 0.119 | 0.856 |
Gene expression values are presented as means ± S.E. of each UGT mRNA relative to PPIA mRNA as 2−ΔCT.
The Mann-Whitney test was used to determine statistical significance of expression of UGT2B genes between sexes.
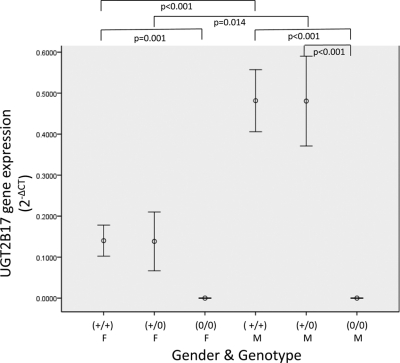
The UGT2B17 gene deletion allele is present at an allelic frequency of 0.30 in whites (Murata et al., 2003; Wilson et al., 2004; Gallagher et al., 2007). When UGT2B17 expression was stratified by UGT2B17 deletion genotype and sex (Fig. 1), liver specimens from men exhibited significantly higher levels of expression of UGT2B17 then liver specimens from women. Male livers exhibited approximately 4-fold higher levels of expression of UGT2B17 than female livers for individuals with either one (p = 0.014) or two (p <0.001) copies of UGT2B17. As expected, liver specimens from both women and men with the UGT2B17 deletion genotype [(0/0)] exhibited no expression of UGT2B17, and specimens from both women and men with the (+/0) or (+/+) UGT2B17 genotypes exhibited significantly higher levels of UGT2B17 gene expression than specimens from individuals with no genomic copies of UGT2B17 (p < 0.001 for both).
Fig. 1.
UGT2B17 gene expression by sex and deletion genotype in human liver specimens. Real-time PCR of UGT2B17 from 103 human liver cDNA specimens was performed in triplicate. The y-axis displays the amount of UGT2B17 gene expression relative to PPIA (presented as 2−ΔCT). Sex [female (F) and male (M)] and UGT2B17 deletion genotype [(+/+), (+/0), and (0/0)] are shown on the x-axis. Only significant comparisons are shown (p < 0.05).
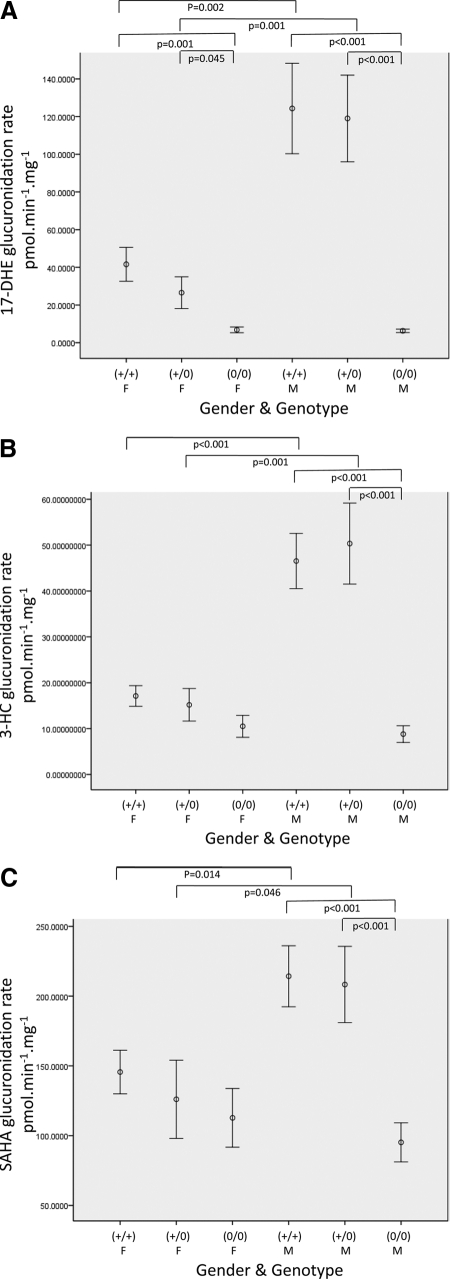
Because UGT2B17 was the only UGT2B gene that displayed significant sex differences in gene expression, glucuronidation activity assays were performed in HLM against UGT2B17 substrates to determine whether the increased expression in men resulted in increases in glucuronidation activity in men. UGT2B17 was shown previously to exhibit the highest glucuronidation activity of any hepatic UGT tested against 17-DHE, 3-HC, and SAHA (Balliet et al., 2009; Sun et al., 2010; G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication), so those substrates were the focus of the glucuronidation studies by sex in this study. When we stratified by sex, significantly higher amounts of exemestane-17-O-glucuronide formation were observed in individuals with either one or two copies of UGT2B17 than in individuals with 0 copies of UGT2B17 for both women (p = 0.045 and 0.001, respectively) and men (p < 0.001 for both) (Fig. 2A). Men exhibited a 4.5-fold higher level of glucuronidation activity against 17-DHE than women for individuals with one copy of UGT2B17 [(+/−); p = 0.001] and a 3.0-fold higher level of glucuronidation activity against 17-DHE than women for individuals for individuals with two copies of UGT2B17 [(+/+); p = 0.002] (Fig. 2A). There was no difference between men and women in the amount of exemestane-17-O-glucuronide formation for individuals who did not have any copies [(0/0)] of UGT2B17 (p = 0.773).
Fig. 2.
Human liver microsome activity against UGT2B17 substrates by sex and genotype. Glucuronidation assays were performed against three UGT2B17 substrates as described previously (Balliet et al., 2009; Sun et al., 2010; G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication). Sex [female (F) and male (M)] and UGT2B17 deletion genotype [(+/+), (+/0), and (0/0)] are shown on the x-axis. The y-axis displays HLM glucuronidation activity against 17-DHE (A), 3-HC (B), and SAHA (C). Only significant comparisons are shown (p < 0.05).
A similar pattern was observed against additional UGT2B17 substrates, 3-HC and SAHA. Whereas no significant difference in 3-HC glucuronide formation was observed between individuals with one copy of UGT2B17 and individuals with zero copies of UGT2B17 in women, this difference was significant in men (p < 0.001). A marginal difference in 3-HC glucuronide formation was observed in HLM from women with two copies of UGT2B17 versus HLM from women with zero copies of UGT2B17 (p = 0.061); this difference was significant in men (p < 0.001). A 3.3-fold higher level of 3-HC glucuronide formation activity was observed in HLM from men than in HLM from women for subjects with one copy of UGT2B17 [(+/−); p = 0.001]. This difference was 2.7-fold higher for HLM from subjects with two copies of UGT2B17 [(+/+), p < 0.001] (Fig. 2B). There was no difference between HLM from men versus women in the amount of 3-HC glucuronide formation for subjects homozygous deleted [(0/0)] for UGT2B17.
For SAHA, significant differences in SAHA glucuronide formation were not observed between HLM from women with one or two copies of UGT2B17 versus HLM from women with zero copies of UGT2B17 (Fig. 2C). However, a significant difference in SAHA glucuronide formation was observed in HLM from men with one or two copies of UGT2B17 versus HLM from men with zero copies of UGT2B17 (p < 0.001 for both). HLM from men exhibited a 1.5-fold higher level of glucuronidation of SAHA than HLM from women for individuals with two copies of UGT2B17 [(+/+), p = 0.014] and a 1.6-fold higher level of glucuronidation activity than HLM from women with one copy of UGT2B17 [(±), p = 0.046]. There was no difference between HLM from men versus HLM from women in the amount of SAHA glucuronide formation in individuals who did not have any copies [(0/0)] of UGT2B17.
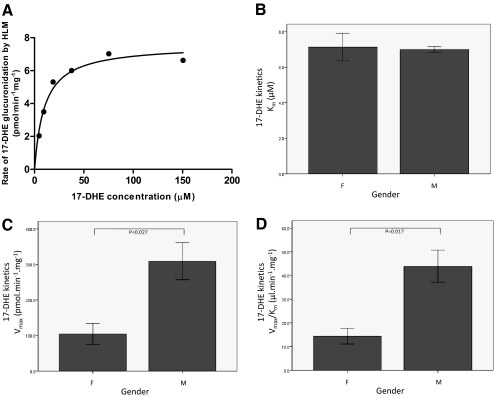
To determine whether the sex difference in glucuronidation activity was also manifested as a change in kinetics, kinetic analysis was performed against 17-DHE as a sample UGT2B17 substrate for three men and three women who each had two copies of UGT2B17 [(+/+)]. A representative concentration curve for exemestane-17-O-glucuronide formation from HLM is shown in Fig. 3A. Similar to the pattern observed in the HLM glucuronidation screening assays, a significant (p = 0.017) 3-fold increase in Vmax/Km was observed for exemestane-17-O-glucuronide formation in UGT2B17 (+/+) HLM from men compared with HLM from women (Fig. 3D). This difference was due to a significantly lower Vmax in HLM from women than from men (p = 0.027) (Fig. 3C); no difference was observed in Km (Fig. 3B).
Fig. 3.
Kinetic analysis for human liver microsome activity against 17-DHE by sex. A, representative concentration curve for exemestane-17-O-glucuronide formation from HLM. B to D, kinetic analyses of HLMs from six individuals (three females and three males) against 17-DHE (all of whom had two copies of UGT2B17 [(+/+)]). Sex [female (F) and male (M)] is shown on the x-axis. The y-axis displays the mean and S.E. for Km (B), Vmax (C), and Vmax/Km (D) against 17-DHE.
Discussion
Previous studies have suggested that UGT2B genes may be hormonally regulated in human cell lines (Beaulieu et al., 1997; Guillemette et al., 1997; Strasser et al., 1997; Bélanger et al., 1998; Hum et al., 1999; Li et al., 1999; Magnanti et al., 2000; Chouinard et al., 2006; Harrington et al., 2006; Hu and Mackenzie, 2009). In the present study, normal human liver specimens from men exhibited 4-fold higher levels of expression of UGT2B17 than liver specimens from women. This correlation between expression and sex corresponded with microsomal fractions from the same specimens displaying a 1.5- to 4.5-fold increase in glucuronidation activity against three different UGT2B17 substrates in specimens from males compared with specimens from females. Significant male-specific increases in both liver UGT2B17 expression and liver glucuronidation activity against UGT2B17 substrates were observed in specimens from subjects who carried one [(+/0)] or two [(+/+)] genomic copies of UGT2B17; the levels of glucuronidation activity were lower against all three substrates with no sex difference observed for HLM from individuals completely deleted for the UGT2B17 gene [(0/0)]. These findings corresponded with the fact that no expression of UGT2B17 was observed in all specimens from UGT2B17 (0/0) subjects and suggest that hepatic UGT2B17 may be up-regulated in males versus females in subjects with one or more intact copies of UGT2B17.
Previous studies have demonstrated that several UGTs, including UGT2B17, were down-regulated by androgens in human prostate cancer cell lines (Beaulieu et al., 1997; Guillemette et al., 1997; Bélanger et al., 1998; Chouinard et al., 2006). In addition, Harrington et al. (2006) showed that in estrogen receptor-positive human breast cancer cells, treatment with 17β-estradiol increased expression of UGT2B15 but not of other UGT2B enzymes, including UGT2B17. However, using one of the same cell lines (MCF-7), Hu and Mackenzie (2009) demonstrated that the expression of UGT2B17 and UGT2B15 is induced by 17β-estradiol. The data from the present study suggest that men have a higher amount of UGT2B17 mRNA expression and glucuronidation activity then women, which is consistent with a hormonal regulatory mechanism for UGT2B17 in humans. However, there are additional mechanisms that could account for this difference including sex-dependent imprinting via epigenetic mechanisms. Because UGT2B17 has been shown to be down-regulated by androgens in prostate and up-regulated by estrogens in breast, we would expect women to have higher UGT2B17 expression than men. However, in human liver we found that women have lower UGT2B17 expression and activity then men, which may indicate that these regulatory effects vary by tissue type. Consistent with our study in humans, a recent study showed that there were sex differences in the expression of UGT genes in mice and that this effect varied by tissue type and UGT isoform (Buckley and Klaassen, 2007).
In the present study, no significant differences in expression of UGT2B enzymes other than UGT2B17 were observed in liver specimens from males versus females. However, Court et al. (2004) detected a sex difference in the glucuronidation of a UGT2B15 substrate, oxazepam. Although there is no difference in UGT2B15 mRNA levels in the present study, it is possible that previously unexamined variations in environmental factors, such as drugs or dietary compounds, could be involved in UGT2B15 regulation.
The primers and probes used in the gene expression assay for UGT2B28 (Applied Biosystems) are located on exon 1 of the UGT2B28 gene. One of the two inactive variants of the UGT2B28 gene is lacking much of exon 1; however, the other inactive variant still contains exon 1 (Lévesque et al., 2001). Therefore, the gene expression assay performed for UGT2B28 in this study is probably detecting one active (type I) and one inactive (type II) UGT2B28 variant but not the other inactive variant of UGT2B28 (type III), which could result in aberrant expression findings for the active UGT2B28 variant in the present study, depending on the relative expression of type I versus type II variant UGT2B28 in liver in different individuals. Further studies examining the relative expression of UGT2B28 variants may be required to better assess this possibility.
In addition to hormone metabolism, UGT2B17 also metabolizes many exogenous compounds including cancer chemotherapeutic agents such as SAHA (Balliet et al., 2009), a major metabolite of the aromatase inhibitor exemestane, 17-DHE (Sun et al., 2010), and metabolites of nicotine such as 3-HC (G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication). The sex difference in glucuronidation activity was more pronounced when activity against 17-DHE and 3-HC rather than against SAHA was assessed. In addition, the difference in glucuronidation activity due to the UGT2B17 deletion genotype in both males and females was most pronounced against 17-DHE > 3-HC > SAHA. This result is consistent with the fact that UGT2B17 plays a more primary role in 17-DHE and 3-HC glucuronidation then in SAHA glucuronidation (Balliet et al., 2009; Sun et al., 2010; G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication). Kinetic studies have indicated that there is only one additional hepatic enzyme that glucuronidates 17-DHE (UGT1A4) and that UGT2B17 exhibits a 17-fold higher level of activity than that enzyme (Sun et al., 2010). In contrast, there are two other hepatic enzymes that glucuronidate both 3-HC and SAHA, with UGT2B17 exhibiting a 5- to 8-fold higher level of activity than UGT2B7 or UGT1A9 against 3-HC (G. Chen, N. Giambrone, R. M. Balliet, and P. Lazarus, submitted for publication) and only a 2-fold greater glucuronidation activity than UGT1A9 against SAHA (Balliet et al., 2009).
This is the first study to determine whether there are sex differences in the expression of UGT enzymes in human tissue. Unlike the hormonal regulation observed previously in vitro, UGT2B17 was the only UGT that exhibited sex-specific differences in expression in human liver, a difference that was substantiated in studies examining hepatic glucuronidation activities against three UGT2B17 substrates from the same series of liver specimens. These data are consistent with a hormonal regulatory mechanism for UGT2B17 in humans and suggest that men have a higher amount of UGT2B17 glucuronidation activity then women. This sex difference in hepatic gene expression and activity for UGT2B17 could have significant pharmacogenetic implications in terms of the metabolism of UGT2B17 substrates including carcinogens as well as drugs including cancer therapeutic agents. Studies of metabolite levels in vivo will be required to better assess this possibility.
Acknowledgments.
We thank the Tissue Procurement Facility at the H. Lee Moffitt Cancer Center for tissue specimen acquisition and the Functional Genomics Core and Organic Synthesis Core Facilities at Penn State University College of Medicine for their technical assistance.
This work was supported by the National Institutes of Health National Cancer Institute [Grant K99-CA131477] (to C.J.G.); the National Institutes of Health National Institute of Dental and Craniofacial Research [Grant R01-DE13158] (to P.L.); and the Pennsylvania Department of Health Research Formula Funding Program [Grants SAP#4100038715, SAP#4100038714] (State of PA, Act 2001-77, part of the PA Tobacco Settlement Legislation) (to P.L.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.035345.
- UGT
- UDP-glucuronosyltransferase
- 17-DHE
- 17-dihydroexemestane
- SAHA
- suberoylanilide hydroxamic acid
- 3-HC
- 3-hydroxycotinine
- HLM
- human liver microsome(s)
- UPLC
- ultrapressure liquid chromatography
- MS/MS
- mass spectrometry/mass spectrometry
- Gluc
- glucuronide
- PCR
- polymerase chain reaction
- PPIA
- peptidylprolyl isomerase A
- CT
- threshold cycle.
References
- Balliet RM, Chen G, Gallagher CJ, Dellinger RW, Sun D, Lazarus P. (2009) Characterization of UGTs active against SAHA and association between SAHA glucuronidation activity phenotype with UGT genotype. Cancer Res 69:2981–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Lévesque E, Tchernof A, Beatty BG, Bélanger A, Hum DW. (1997) Chromosomal localization, structure, and regulation of the UGT2B17 gene, encoding a C19 steroid metabolizing enzyme. DNA Cell Biol 16:1143–1154 [DOI] [PubMed] [Google Scholar]
- Bélanger A, Hum DW, Beaulieu M, Lévesque E, Guillemette C, Tchernof A, Bélanger G, Turgeon D, Dubois S. (1998) Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J Steroid Biochem Mol Biol 65:301–310 [DOI] [PubMed] [Google Scholar]
- Bélanger A, Pelletier G, Labrie F, Barbier O, Chouinard S. (2003) Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab 14:473–479 [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35:121–127 [DOI] [PubMed] [Google Scholar]
- Chouinard S, Pelletier G, Bélanger A, Barbier O. (2006) Isoform-specific regulation of uridine diphosphate-glucuronosyltransferase 2B enzymes in the human prostate: differential consequences for androgen and bioactive lipid inactivation. Endocrinology 147:5431–5442 [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, Guillemette C, Journault K, Krishnaswamy S, Von Moltke LL, Greenblatt DJ. (2002) Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos 30:1257–1265 [DOI] [PubMed] [Google Scholar]
- Court MH, Hao Q, Krishnaswamy S, Bekaii-Saab T, Al-Rohaimi A, von Moltke LL, Greenblatt DJ. (2004) UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of oxazepam glucuronidation by human liver. J Pharmacol Exp Ther 310:656–665 [DOI] [PubMed] [Google Scholar]
- Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer AM, Chase GA, Richie J, Lazarus P. (2007) The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev 16:823–828 [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. (1980) Oxazepam kinetics: effects of age and sex. J Pharmacol Exp Ther 215:86–91 [PubMed] [Google Scholar]
- Guillemette C, Bélanger A, Lépine J. (2004) Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res 6:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette C, Lévesque E, Beaulieu M, Turgeon D, Hum DW, Bélanger A. (1997) Differential regulation of two uridine diphospho-glucuronosyltransferases, UGT2B15 and UGT2B17, in human prostate LNCaP cells. Endocrinology 138:2998–3005 [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sengupta S, Katzenellenbogen BS. (2006) Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology 147:3843–3850 [DOI] [PubMed] [Google Scholar]
- Hu DG, Mackenzie PI. (2009) Estrogen receptor α, fos-related antigen-2, and c-Jun coordinately regulate human UDP glucuronosyltransferase 2B15 and 2B17 expression in response to 17β-estradiol in MCF-7 cells. Mol Pharmacol 76:425–439 [DOI] [PubMed] [Google Scholar]
- Hum DW, Bélanger A, Lévesque E, Barbier O, Beaulieu M, Albert C, Vallée M, Guillemette C, Tchernof A, Turgeon D, et al. (1999) Characterization of UDP-glucuronosyltransferases active on steroid hormones. J Steroid Biochem Mol Biol 69:413–423 [DOI] [PubMed] [Google Scholar]
- Lépine J, Bernard O, Plante M, Têtu B, Pelletier G, Labrie F, Bélanger A, Guillemette C. (2004) Specificity and regioselectivity of the conjugation of estradiol, estrone, and their catecholestrogen and methoxyestrogen metabolites by human uridine diphospho-glucuronosyltransferases expressed in endometrium. J Clin Endocrinol Metab 89:5222–5232 [DOI] [PubMed] [Google Scholar]
- Lévesque E, Turgeon D, Carrier JS, Montminy V, Beaulieu M, Bélanger A. (2001) Isolation and characterization of the UGT2B28 cDNA encoding a novel human steroid conjugating UDP-glucuronosyltransferase. Biochemistry 40:3869–3881 [DOI] [PubMed] [Google Scholar]
- Li YQ, Prentice DA, Howard ML, Mashford ML, Desmond PV. (1999) The effect of hormones on the expression of five isoforms of UDP-glucuronosyltransferase in primary cultures of rat hepatocytes. Pharm Res 16:191–197 [DOI] [PubMed] [Google Scholar]
- Magnanti M, Giuliani L, Gandini O, Gazzaniga P, Santiemma V, Ciotti M, Saccani G, Frati L, Aglianò AM. (2000) Follicle-stimulating hormone, testosterone, and hypoxia differentially regulate UDP-glucuronosyltransferase 1 isoforms expression in rat Sertoli and peritubular myoid cells. J Steroid Biochem Mol Biol 74:149–155 [DOI] [PubMed] [Google Scholar]
- Murata M, Warren EH, Riddell SR. (2003) A human minor histocompatibility antigen resulting from differential expression due to a gene deletion. J Exp Med 197:1279–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar S, Remmel RP. (2006) Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene 25:1659–1672 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. (2006) Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet 21:357–374 [DOI] [PubMed] [Google Scholar]
- Strasser SI, Smid SA, Mashford ML, Desmond PV. (1997) Sex hormones differentially regulate isoforms of UDP-glucuronosyltransferase. Pharm Res 14:1115–1121 [DOI] [PubMed] [Google Scholar]
- Sun D, Chen G, Dellinger RW, Sharma AK, Lazarus P. (2010) Characterization of 17-dihydroexemestane glucuronidation: potential role of the UGT2B17 deletion in exemestane pharmacogenetics. Pharmacogenet Genomics 20:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616 [DOI] [PubMed] [Google Scholar]
- Wiener D, Fang JL, Dossett N, Lazarus P. (2004) Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res 64:1190–1196 [DOI] [PubMed] [Google Scholar]
- Wilson W, 3rd, Pardo-Manuel de Villena F, Lyn-Cook BD, Chatterjee PK, Bell TA, Detwiler DA, Gilmore RC, Valladeras IC, Wright CC, Threadgill DW, et al. (2004) Characterization of a common deletion polymorphism of the UGT2B17 gene linked to UGT2B15. Genomics 84:707–714 [DOI] [PubMed] [Google Scholar]