Abstract
The signaling capacity of endogenous cannabinoids (“endocannabinoids”) is tightly regulated by degradative enzymes. This Perspective highlights a research article in this issue (p. 996) in which the authors show that genetic disruption of monoacylglycerol lipase (MAGL), the principal degradative enzyme for the endocannabinoid 2-arachidonoylglycerol (2-AG), causes marked elevations in 2-AG levels that lead to desensitization of brain cannabinoid receptors. These findings highlight the central role that MAGL plays in endocannabinoid metabolism in vivo and reveal that excessive 2-AG signaling can lead to functional antagonism of the brain cannabinoid system.
Introduction
The endogenous cannabinoid (endocannabinoid) system modulates a wide range of physiological processes in mammals, including pain and inflammation, feeding and energy regulation, learning and memory, and emotionality (Fowler, 2006; Pacher et al., 2006). Components of this system include the cannabinoid receptors CB1 and CB2, the endocannabinoids N-arachidonylethanolamine (anandamide; AEA) and 2-arachidonylglycerol (2-AG), and the enzymes responsible for endocannabinoid biosynthesis and degradation (Di Marzo et al., 2007; Ahn et al., 2008). Although direct agonists for cannabinoid receptors, such as Δ9-tetrahydrocannabinol, the primary psychoactive constituent of Cannabis sativa, produce well known medicinal effects such as analgesia, they also possess dependence liability and cause detrimental effects on fine motor control and cognition that limit their broad therapeutic potential. Amplifying the actions of endocannabinoids by inhibiting their enzymatic degradation has emerged as an alternative strategy to exploit the endocannabinoid system for possible clinical benefit. Still, much remains unknown about the pharmacological and behavioral impact of disrupting endocannabinoid metabolism. Termination of endocannabinoid signaling is carried out primarily by two hydrolytic enzymes in the nervous system, fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996), which degrades AEA, and monoacylglycerol lipase (MAGL) (Dinh et al., 2002; Blankman et al., 2007), which breaks down 2-AG. A considerable body of research has demonstrated that FAAH inhibitors reduce pain in a wide range of acute, inflammatory, and neuropathic pain models (Lichtman et al., 2004; Chang et al., 2006; Russo et al., 2007). More recently, a MAGL inhibitor has been demonstrated to reduce acute and neuropathic pain (Kinsey et al., 2009; Long et al., 2009).
In this issue of Molecular Pharmacology, Chanda et al. (2010) reveal that targeted disruption of the MAGL (or MGLL) gene leads to severe disturbances in the ability of mice to metabolize the endogenous cannabinoid 2-AG, resulting in dramatic increases of brain 2-AG and compensatory dampening of CB1 receptor function. Although short-term pharmacological inhibition of MAGL produces antinociception (Kinsey et al., 2009; Long et al., 2009), MAGL(−/−) mice displayed normal pain responses in complete Freund's adjuvant, spinal nerve ligation, and acute thermal models of pain. Moreover, these mice did not show any apparent behavioral alterations indicative of cannabinoid activity, such as hypothermia, hypomotility, or catalepsy. Although (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone (WIN55,212-2), a full CB1 receptor agonist, produces analgesia, locomotor depression, catalepsy, and hypothermia in wild-type animals, its actions were greatly curtailed in MAGL(−/−) mice. MAGL(−/−) mice also exhibited a phenotypic decrease in body weight that bears a striking resemblance of the lean phenotype in CB1(−/−) mice or animals treated with a CB1 receptor antagonist (Di Marzo et al., 2001). Consistent with these results that indicate genetic deletion of MAGL causes functional antagonism of CB1 receptors, MAGL(−/−) mice displayed significant decreases in the number of CB1 receptors and functional CB1 receptor activity in brain compared with wild-type mice.
Corroborating the provocative findings of Chanda et al. (2010), we recently reported that MAGL(−/−) mice display normal nociceptive responses to acute thermal stimuli, decreased sensitivity to the antinociceptive and hypothermic effects of WIN55,212-2, and CB1 receptor desensitization (Schlosburg et al., 2010). These effects were phenocopied by long-term treatment with the selective MAGL inhibitor 4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184), which also caused tolerance to the antiallodynic effects of short-term MAGL inhibition, cross-tolerance to the antiallodynic effects of FAAH inhibition, and profound deficits in endocannabinoid-mediated short-term synaptic plasticity. In marked contrast to the consequences of long-term elevation of 2-AG, the analgesic effects of a FAAH inhibitor persisted after long-term administration, despite the fact that equi-effective analgesic doses of JZL184 and the FAAH inhibitor were used. As has been observed previously in FAAH(−/−) mice (Cravatt et al., 2001; Lichtman et al., 2002), CB1 receptor function remained normal after long-term pharmacological inhibition of FAAH (Schlosburg et al., 2010).
Collectively, the studies by Chanda et al. (2010) and Schlosburg et al. (2010) point to a model in which long-term disruption of MAGL leads to heightened 2-AG levels that cause tonic activation and eventual desensitization of the brain CB1 system (Fig. 1). As an interesting contrast, CB2 receptors in spleen remain unperturbed in MAGL(−/−) mice (Chanda et al., 2010). The underlying mechanisms for the differential down-regulation between CB1 and CB2 receptors in MAGL(−/−) mice remain an open question. However, one can speculate that a difference between brain and spleen 2-AG levels may be a driving force for the distinct adaptations in the two cannabinoid receptors. Spleen 2-AG levels are, for instance, elevated by “only” 2- to 3-fold in MAGL(−/−) mice (in contrast to the >10-fold elevations in brain 2-AG levels in these animals) (Chanda et al., 2010; Schlosburg et al., 2010). Under this hypothesis, one might expect that after long-term elevations of 2-AG, both CB1 and CB2 receptors would be down-regulated in the CNS, whereas neither receptor would be altered in peripheral tissues. Alternatively, CB1 receptors may be more likely to undergo internalization and down-regulation in response to 2-AG than CB2 receptors. Based on these differential effects, one might expect that long-term administration of MAGL inhibitors would have minimal effects on CB2 receptor-mediated actions.
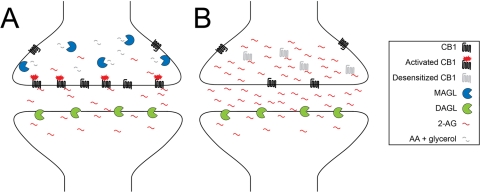
Fig. 1.
Model for 2-AG signaling at CB1 receptors in the nervous system. A, under normal conditions, 2-AG is synthesized postsynaptically by diacylglycerol lipase (DAGL), traverses across the synapse to activate CB1 receptors located presynaptically, and then is rapidly inactivated by MAGL. B, after long-term MAGL blockade, elevated 2-AG levels cause tonic activation and eventual internalization and down-regulation of CB1 receptors.
We have learned from the results of Chanda et al. (2010) and Schlosburg et al. (2010) not only that MAGL is the primary catabolic enzyme responsible for terminating 2-AG function in the nervous system but also that maximal elevations in 2-AG signaling overload CB1 receptor pathways, leading to their down-regulation and dampened responses to endogenous and exogenous cannabinoids. Do these results negate the possibility of using MAGL inhibitors for chronic pain conditions? Perhaps, but another consideration is that partial MAGL blockade will retain analgesic efficacy without resulting in CB1 down-regulation. Other interesting questions include: why does disrupting FAAH activity lead to robust analgesic effects that do not induce tolerance? Might this reflect different modes of CB1 receptor activation by AEA and 2-AG? Finally, might certain neural circuits maintain an intact CB1 receptor system even in the presence of tonically elevated 2-AG signaling, and, if so, what neurophysiological processes will these circuits regulate? Answers to these exciting questions are likely to keep endocannabinoid researchers busy for many years to come.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA009789, DA017259, DA026161] and the Skaggs Institute for Chemical Biology.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069427.
Please see the related article on page 996.
- AEA
- N-arachidonylethanolamine (anandamide)
- 2-AG
- 2-arachidonylglycerol
- FAAH
- fatty acid amide hydrolase
- MAGL
- monoacylglycerol lipase
- WIN55,212-2
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone
- JZL184
- 4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate.
References
- Ahn K, McKinney MK, Cravatt BF. (2008) Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev 108:1687–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P, Gao Y, Mark L, Btesh J, Strassle B, Lu P, Piesla M, Zhang MY, Bingham B, Uveges A, et al. (2010) Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol 78:996–1003 [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. (2006) Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol 148:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 98:9371–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L. (2007) Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol 14:741–756 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, et al. (2001) Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825 [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. (2002) A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids 121:149–158 [DOI] [PubMed] [Google Scholar]
- Fowler CJ. (2006) The cannabinoid system and its pharmacological manipulation–a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam Clin Pharmacol 20:549–562 [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. (2009) Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330:902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. (2002) Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther 302:73–79 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. (2004) Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther 311:441–448 [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. (2009) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, et al. (2007) The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther 322:236–242 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, et al. (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]