Abstract
The voltage-gated sodium channel Nav1.7 plays a crucial role in pain, and drugs that inhibit hNav1.7 may have tremendous therapeutic potential. ProTx-II and huwentoxin-IV (HWTX-IV), cystine knot peptides from tarantula venoms, preferentially block hNav1.7. Understanding the interactions of these toxins with sodium channels could aid the development of novel pain therapeutics. Whereas both ProTx-II and HWTX-IV have been proposed to preferentially block hNav1.7 activation by trapping the domain II voltage-sensor in the resting configuration, we show that specific residues in the voltage-sensor paddle of domain II play substantially different roles in determining the affinities of these toxins to hNav1.7. The mutation E818C increases ProTx-II's and HWTX-IV's IC50 for block of hNav1.7 currents by 4- and 400-fold, respectively. In contrast, the mutation F813G decreases ProTx-II affinity by 9-fold but has no effect on HWTX-IV affinity. It is noteworthy that we also show that ProTx-II, but not HWTX-IV, preferentially interacts with hNav1.7 to impede fast inactivation by trapping the domain IV voltage-sensor in the resting configuration. Mutations E1589Q and T1590K in domain IV each decreased ProTx-II's IC50 for impairment of fast inactivation by ∼6-fold. In contrast mutations D1586A and F1592A in domain-IV increased ProTx-II's IC50 for impairment of fast inactivation by ∼4-fold. Our results show that whereas ProTx-II and HWTX-IV binding determinants on domain-II may overlap, domain II plays a much more crucial role for HWTX-IV, and contrary to what has been proposed to be a guiding principle of sodium channel pharmacology, molecules do not have to exclusively target the domain IV voltage-sensor to influence sodium channel inactivation.
Introduction
Voltage-gated sodium channels play important roles in action potential generation and propagation. Because Nav1.7 is a crucial contributor to pain sensation (Cox et al., 2006; Cummins et al., 2007), drugs that selectively target human Nav1.7 (hNav1.7) could be ideal analgesics. Unfortunately, drugs targeting sodium channels typically have broad-spectrum sodium channel activity and narrow therapeutic windows (Cummins and Rush, 2007). Therefore, there is substantial interest in identifying compounds that selectively target hNav1.7 and determining their molecular mechanisms of action.
ProTx-II and huwentoxin-IV (HWTX-IV) are tarantula toxins that target voltage-gated sodium channels. These toxins belong to the inhibitory cystine knot family and are stabilized by the same disulfide frame (C1–C4, C2–C5, and C3–C6) (Middleton et al., 2002; Peng et al., 2002). However, they show limited sequence similarity (Fig. 1A). Although ProTx-II inhibits multiple sodium channel subtypes (Nav1.1–1.8), it has been reported to be ∼100-fold more selective for Nav1.7 (Smith et al., 2007; Schmalhofer et al., 2008). HWTX-IV preferentially inhibits tetrodotoxin (TTX)-sensitive neuronal subtypes (including Nav1.7), does not inhibit TTX-resistant neuronal subtypes, and has little effect on skeletal muscle (Nav1.4) and cardiac (Nav1.5) subtypes (Xiao et al., 2008). Although these toxins have divergent properties, both are classified as voltage-sensor modifiers (Sokolov et al., 2008; Xiao et al., 2008).
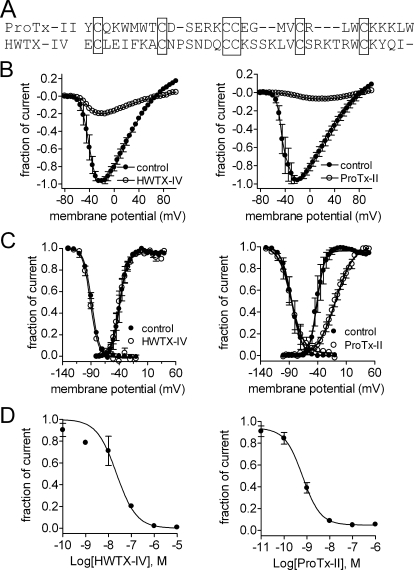
Fig. 1.
Effects of ProTx-II and HWTX-IV on WT Nav1.7 expressed in HEK293 cells. A, sequence alignment of ProTx-II and HWTX-IV. Six conserved cysteines are identified by the encompassing rectangles in the sequence alignment. B, differential effects of two toxins on the current-voltage relationships of WT Nav1.7. Cells were held at −100 mV. Nav1.7 currents were elicited by 50-ms depolarization steps to various voltages ranging from −80 to +100 mV in 5-mV increments. Currents elicited before and after application of 100 nM ProTx-II (left) or 100 nM HWTX-IV (right) were normalized to the maximum amplitude of control peak current. C, effects of the two toxins on normalized steady-state activation and inactivation of WT Nav1.7. Channel conductances before and after application of 100 nM ProTx-II or 100 nM HWTX-IV were calculated with the equation: G(V) = I/(V − Vrev), in which I, V, and Vrev represented inward current elicited as described in B, test potential, and reversal potential, respectively. Data are plotted as a fraction of the maximum conductance. The voltage-dependence of steady-state inactivation was estimated using a standard double-pulse protocol, in which a 20-ms depolarizing test potential of 0 mV followed a 500-ms prepulse at potentials that ranged from −130 to −10 mV with a 10-mV increment. Cells were held at −100 mV. All curves were fit with the Boltzmann equation as described under Materials and Methods. D, concentration-dependent inhibition of WT Nav1.7 by two toxins. Data points (mean ± S.E., each from three to four cells) were fit with the Hill equation as described under Materials and Methods. The values of IC50, slope factor (nH), and fbottom yielded are shown in Tables 1 and 2.
Voltage-sensor modifiers target the voltage sensors of ion channels. The pore-forming sodium channel α-subunit consists of four domains (DI–DIV), each having six transmembrane segments (S1–S6) (Catterall et al., 2003). The S5–S6 segments form the channel pore, and the S1–S4 segments form voltage sensor modules. The S4 segments, rich in positive residues, sense membrane depolarization and move outward to induce channel gating. Scorpion toxins have been extensively characterized as voltage sensor modifiers, and understanding the molecular determinants of their interactions with voltage-gated sodium channels has provided invaluable insight into channel structure-function relationships. Scorpion α-toxins interact with the DIV S3–S4 linker to stabilize DIV S4 in the closed state, impeding fast inactivation. Scorpion β-toxins bind to the DII S3–S4 linker, trapping the DII S4 in the activated state and enhancing channel activation. The binding sites for these scorpion toxins are defined as neurotoxin receptor sites 3 and 4, respectively (Cestèle and Catterall, 2000). These data, in conjunction with other studies, indicate that the S4 segments in DI, DII, and DIII are determinants of channel activation, whereas that of DIV is predominantly involved in channel inactivation (Cha et al., 1999; Cestèle et al., 2001; Sheets and Hanck, 2007). Both ProTx-II and HWTX-IV have been proposed to inhibit activation by trapping the DII voltage sensor in the resting configuration. However, their binding determinants may not be identical. Although HWTX-IV may selectively bind to neurotoxin site 4 (Xiao et al., 2008), ProTx-II may interact with novel binding sites on Nav1.5 (Smith et al., 2007). It has been suggested that ProTx-II inhibits activation of rNav1.2a by interacting with the voltage-sensor “paddles” (S3b–S4 motifs) of DI, DII, and DIV (Bosmans et al., 2008). This finding was somewhat surprising given the presumed role of DIV in inactivation, leading to the proposal that for a toxin to alter inactivation, it must exclusively interact with the voltage sensor paddle of DIV (Bosmans et al., 2008).
We investigated the interactions of ProTx-II and HWTX-IV with the voltage sensor paddles in hNav1.7 DI, DII, and DIV. Our data show that these two tarantula toxins differ substantially in their interactions with hNav1.7. Although they may not be ideal as analgesic drugs, understanding the molecular determinants of their complicated interactions with voltage-gated sodium channels should aid the development of novel hNav1.7 blockers.
Materials and Methods
Toxins.
ProTx-II was recombinantly produced as described by Smith et al. (2007). HWTX-IV was purified from the crude venom of the female tarantula Ornithoctonus huwena as described by Peng et al. (2002). The purity of ProTx-II and HWTX-IV was determined to be more than 99% by high-pressure liquid chromatography and matrix-assisted laser desorption ionization time-of-flight analysis.
Plasmids of Sodium Channels.
The cDNA genes encoding rat (r) Nav1.2, rNav1.3, and rNav1.4 were inserted into the vectors pRC-CMV, pcDNA3.1-mod, and pRBG4, respectively (Ukomadu et al., 1992; O'Leary, 1998; Cummins et al., 2001). The cDNA genes encoding human (h) Nav1.5 and hNav1.7 were subcloned into the vectors pcDNA3.1 and pcDNA3.1-mod, respectively (Klugbauer et al., 1995). Auxiliary subunits hβ1 and hβ2 were inserted into an internal ribosome entry site vector (Lossin et al., 2002).
Site-Directed Mutagenesis of Nav1.7.
All hNav1.7 mutations in this study were constructed using the QuikChange II XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instruction. All constructs were sequenced to confirm that the appropriate mutations were made.
Transient Transfection.
Transient transfections of hNav1.7 wild-type (WT) and mutant constructs into human embryonic kidney (HEK) 293 cells were performed using the calcium phosphate precipitation method. HEK293 cells were grown under standard tissue culture conditions (5% CO2 and 37°C) in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The WT and mutant hNav1.7 channels were cotransfected with the hβ1 and hβ2 subunits to increase the current density. The calcium phosphate-DNA mixture (channel constructs and a green fluorescent protein reporter plasmid) was added to the cell culture medium and left for 3 h, after which the cells were washed with fresh medium. Cells with green fluorescent protein fluorescence were selected for whole-cell patch-clamp recordings 36 to 72 h after transfection. Stably transfected cell lines containing WT rNav1.2, rNav1.3, rNav1.4, and hNav1.5 without any β subunit or green fluorescent protein reporter plasmid were prepared using the method described previously (Xiao et al., 2008).
Whole-Cell Patch-Clamp Recordings.
Whole-cell patch-clamp recordings were carried out at room temperature (∼21°C) using an EPC-10 amplifier (HEKA, Lambrecht/Pfalz, Germany). Data were acquired on a Pentium IV computer using the Pulse program (version 8.31; HEKA). Fire-polished electrodes were fabricated from 1.7-mm capillary glass (VWR, West Chester, PA) using a P-97 puller (Sutter Instrument Company, Novato, CA). The standard pipette solution contained 140 mM CsF, 1 mM EGTA, 10 mM NaCl, and 10 mM HEPES, pH 7.3. The standard bathing solution was 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES, pH 7.3. After filling with pipette solution, the access resistance of electrode pipette ranged from 0.8 to 1.4 MΩ and the average resistance was 0.98 ± 0.02 MΩ (n = 250). The liquid junction potential for these solutions was <8 mV; data were not corrected to account for this offset. The offset potential was zeroed before contacting the cell. After establishing the whole-cell recording configuration, the resting potential was held at −100 mV for 5 min to allow adequate equilibration between the micropipette solution and the cell interior. Linear leak subtraction, based on resistance estimates from four to five hyperpolarizing pulses applied before the depolarizing test potential, was used for all voltage-clamp recordings. Membrane currents were usually filtered at 5 kHz and sampled at 20 kHz. Voltage errors were minimized using 80% series resistance compensation, and the capacitance artifact was canceled using the computer-controlled circuitry of the patch-clamp amplifier. The stock solutions for ProTx-II and HWTX-IV were made at 1 mM using bathing solution containing 1 mg/ml bovine serum albumin, and aliquots were stored at −20°C. Before use, the solution was diluted to the concentrations of interest with fresh bathing solution. Toxin was diluted into the recording chamber (volume of 300 μl) and mixed by repeatedly pipetting 30 μl to achieve the specified final concentration. The extent of the inhibitory effect of toxin was typically assessed approximately 10 to 20 min after toxin treatment.
Data Analysis.
Data were analyzed using the Pulsefit (HEKA) and GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA) programs. All data points are shown as mean ± S.E., and n is presented as the number of the separate experimental cells. Steady-state activation and inactivation curves were fitted using Boltzmann equation: y = 1/(1 + exp((V1/2 − V)/k), in which V1/2, V, and k represented midpoint voltage of kinetics, test potential, and slope factor, respectively. Concentration-response curves to determine IC50 values were fitted using the Hill equation: y = fbottom + (1 − fbottom)/(1 + ([Tx]/IC50) n H ), where nH is the Hill coefficient, IC50 is half-maximal inhibitory concentration, and fbottom is the fraction of current resistant to inhibition at high toxin (Tx) concentration. For HWTX-IV, the nH was set to 1 because our mutagenesis data have shown that the toxin had a single high-affinity binding site in hNav1.7. For ProTx-II slowing fast inactivation, the nH was also set to 1 because only sodium channel DIV is involved in channel inactivation gating.
Results
ProTx-II and HWTX-IV Block hNav1.7 Channels at Nanomolar Concentrations.
Although ProTx-II and HWTX-IV have been tested previously against hNav1.7 channels (Schmalhofer et al., 2008; Xiao et al., 2008), their activity has not been compared directly. Therefore, we first compared the effects of ProTx-II and HWTX-IV on WT hNav1.7 channels expressed in HEK293 cells using whole-cell voltage-clamp recordings. Although both toxins blocked the peak transient sodium currents conducted by hNav1.7, ProTx-II exhibited a 30-fold higher affinity for WT hNav1.7 than did HWTX-IV (Fig. 1D). The IC50 values for ProTx-II and HWTX-IV were determined to be 0.7 and 22.7 nM, respectively. Consistent with previous findings (Smith et al., 2007; Schmalhofer et al., 2008), 100 nM ProTx-II not only shifted channel activation in the depolarizing direction by 31.1 mV but also increased the slope factor by 2-fold. In contrast, in the presence of 100 nM HWTX-IV, neither the activation nor the slope factor was obviously modified in the range of voltages tested in the present study (Fig. 1, B and C; Tables 1 and 2). Because 100 nM is not a saturating concentration of HWTX-IV, it is likely that the residual currents shown in Fig. 1B result from channels that did not bind HWTX-IV. Indeed, in our previous study, we showed that saturating concentrations of HWTX-IV shift the voltage-dependence of activation by at least 200 mV in the depolarizing direction (Xiao et al., 2008).
TABLE 1.
Characterization of activation of hNav1.7 mutants in the presence of 100 nM ProTx-II
Cells were held at −100 mV. Families of currents were induced by 50-ms depolarizing steps to various potential ranging from −80 to +40 mV. Recording currents from WT and mutant Nav1.7 started at ∼20 min after establishing whole-cell configuration. All data come from three to four cells. The half-activation potential (V1/2) and slope factor (k) were determined with Boltzmann fits. The values of IC50, slope factor (nH), and fbottom were determined with Hill equation in Figs. 2A, 6B, and 7B.
| Channel | Voltage Dependence of
Activation |
||||||
|---|---|---|---|---|---|---|---|
| Control |
ProTx-II |
||||||
| V 1/2 | k | V 1/2 | k | IC50 | n H | f bottom | |
| mV | mV | mV | mV | nM | |||
| hNav1.7 | −41.5 ± 0.8 | 6.5 ± 0.7 | −10.4 ± 0.9 | 14.5 ± 0.8 | 0.7 ± 0.2 | 1.1 ± 0.2 | 0.05 ± 0.02 |
| L201V/N206D | −42.5 ± 0.4 | 5.3 ± 0.4 | −12.1 ± 1.0 | 17.1 ± 0.9 | 1.0 ± 0.3 | 1.1 ± 0.2 | 0.03 ± 0.01 |
| F813G | −39.6 ± 0.2 | 5.5 ± 0.2 | −23.0 ± 2.7 | 18.7 ± 2.8 | 6.0 ± 2.1 | 1.0 ± 0.2 | 0.09 ± 0.04 |
| E818C | −33.0 ± 1.2 | 8.1 ± 1.1 | −0.6 ± 1.2 | 15.3 ± 1.1 | 2.9 ± 0.1 | 1.0 ± 0.1 | 0.13 ± 0.03 |
| E203K/E818C | −14.2 ± 0.4 | 9.0 ± 0.4 | 14.2 ± 1.8 | 19.2 ± 1.5 | 4.6 ± 0.7 | 1.2 ± 0.2 | 0.16 ± 0.02 |
| F204A/F813G | −41.1 ± 1.3 | 7.2 ± 1.1 | −26.3 ± 1.2 | 14.9 ± 1.2 | 8.2 ± 1.1 | 1.3 ± 0.2 | 0.08 ± 0.03 |
| F813G/E818C | −34.0 ± 1.2 | 8.1 ± 1.1 | −20.5 ± 1.4 | 15.3 ± 1.3 | 29.8 ± 3.6 | 1.0 ± 0.2 | 0.10 ± 0.05 |
| D1586A | −33.0 ± 0.8 | 7.5 ± 0.7 | −7.1 ± 1.2 | 16.6 ± 1.1 | 1.0 ± 0.1 | 1.2 ± 0.4 | 0.08 ± 0.04 |
| D1586E | −40.6 ± 0.7 | 7.0 ± 0.6 | −13.6 ± 1.9 | 17.5 ± 1.9 | 0.4 ± 0.03 | 1.0 ± 0.2 | 0.09 ± 0.02 |
| E1589Q | −40.0 ± 1.6 | 8.4 ± 1.4 | −11.7 ± 1.9 | 17.2 ± 1.8 | 0.5 ± 0.1 | 1.0 ± 0.1 | 0.06 ± 0.02 |
| T1590K | −44.2 ± 0.5 | 5.2 ± 0.4 | −16.2 ± 0.8 | 14.0 ± 0.7 | 0.9 ± 0.4 | 1.1 ± 0.3 | 0.07 ± 0.04 |
| F1592A | −34.1 ± 1.1 | 8.0 ± 0.9 | −5.3 ± 2.1 | 19.3 ± 2.1 | 0.7 ± 0.3 | 1.1 ± 0.4 | 0.04 ± 0.04 |
| D1586A/T1590K | −40.5 ± 0.3 | 6.9 ± 0.2 | −2.5 ± 0.8 | 18.5 ± 0.8 | 0.7 ± 0.4 | 0.8 ± 0.4 | 0.04 ± 0.01 |
| T1590K/F1592A | −35.1 ± 0.2 | 6.9 ± 0.2 | −8.3 ± 2.6 | 25.2 ± 2.9 | 0.8 ± 0.4 | 1.1 ± 0.1 | 0.06 ± 0.01 |
TABLE 2.
Characterization of activation of hNav1.7 mutants in the presence of 100 nM HWTX-IV
Cells were held at −100 mV. Families of currents were induced by 50-ms depolarizing steps to various potentials ranging from −80 to +40 mV. Recording currents from WT and mutant Nav1.7 started at ∼20 min after establishing whole-cell configuration. All data come from three to four cells. The half-activation potential (V1/2) and slope factor (k) were determined with Boltzmann fits. IC50 values were determined with Hill equation in Fig. 2B, in which slope factor was set to 1. The fbottom values yielded were 0 on both wild-type and mutant Nav1.7 channels and therefore are not listed.
| Channel | Voltage Dependence of
Activation |
||||
|---|---|---|---|---|---|
| Control |
HWTX-IV |
||||
| V 1/2 | k | V 1/2 | k | IC50 | |
| mV | mV | mV | mV | nM | |
| hNav1.7 | −38.8 ± 0.8 | 6.8 ± 0.7 | −40.2 ± 0.7 | 7.3 ± 0.6 | 22.7 ± 6.3 |
| L201V/N206D | −40.4 ± 1.3 | 7.9 ± 1.2 | −36.7 ± 1.2 | 9.6 ± 1.1 | 20.8 ± 3.6 |
| F813G | −32.7 ± 1.0 | 6.7 ± 0.9 | −36.3 ± 1.4 | 8.0 ± 1.3 | 28.2 ± 5.7 |
| E818C | −41.6 ± 0.8 a | 7.1 ± 0.7 a | −39.7 ± 0.9 a | 8.9 ± 0.8 a | 9120.1 ± 1400.0 |
| E203K/E818C | −20.5 ± 0.3 a | 8.5 ± 0.3 a | −16.0 ± 1.1 a | 13.8 ± 1.0 a | 8892.0 ± 1135.2 |
| F204A/F813G | −46.7 ± 0.4 | 4.5 ± 0.3 | −42.7 ± 0.4 | 6.1 ± 0.3 | 26.7 ± 6.4 |
The concentration of HWTX-IV was 10 μM.
Comparison of the Electrophysiological Properties of WT and Mutant hNav1.7 Channels.
To explore the molecular determinants of ProTx-II and HWTX-IV interactions with hNav1.7, we made specific mutations in the S3–S4 regions of DI, DII, and DIV. We mainly focused on residues in the S3–S4 regions that previous studies have indicated were important for the inhibitory activity of either ProTx-II or HWTX-IV. The electrophysiological properties of mutant channels, expressed in HEK293 cells, were characterized under the whole-cell recording configuration, and the voltage-dependent properties were compared with WT hNav1.7. The electrophysiological parameters of activation and steady-state inactivation, estimated by fitting the data with Boltzmann equations, are summarized in Supplemental Table S1. These data indicate that acidic residues in extracellular S3–S4 linkers of DI and DII can modulate voltage-dependent activation of hNav1.7. Although the mutations L201V/N206D, F813G, and F204A/F813G shifted the midpoint potentials of activation of hNav1.7 by less than +5 mV, the mutations of acidic residues E203K/E818C, E818C, and F813G/E818C shifted activation by +17.6, +9.8, and +9.2 mV, respectively. This finding is consistent with previous reports that voltage sensors of DI and DII are important for channel activation (Cha et al., 1999; Cestèle et al., 2001). By contrast, consistent with the finding that DIV S4 is mainly responsible for fast inactivation (Cestèle et al., 2001; Sheets and Hanck, 2007), mutations of most residues (D1586A, D1586E, E1589Q, T1590K, F1592A, and D1586A/T1590K) in DIV had limited effect on channel activation. Only the double mutation T1590K/F1592A substantially shifted channel activation by +8.2 mV (Supplemental Table S1). None of the mutations significantly altered steady-state inactivation. The slope factors for steady-state activation and inactivation also did not change compared with WT hNav1.7 (Supplemental Table S1).
Because the inhibition of hNav1.7 by ProTx-II is voltage-dependent in the range of physiological voltages (Smith et al., 2007), the shifting of the current-voltage relationship caused by channel mutation might affect the assessment of toxin affinity when measured at the same test pulse potential. To precisely measure toxin affinity under similar activation conditions, the test pulse potential to activate hNav1.7 WT and mutant construct channels was set between −10 and +10 mV for the various constructs to ensure that ∼90% channel conductance was available.
Mutations in DII S3–S4 Linker Differentially Decreased Toxin Affinities for hNav1.7.
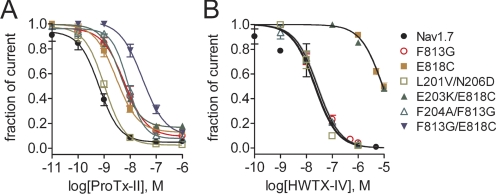
In previous studies, two residues in the DII S3–S4 linker, Phe813 and Glu818 (Supplemental Fig. S1C), were shown to be important for hNav1.7 block by ProTx-II and HWTX-IV, respectively (Schmalhofer et al., 2008; Xiao et al., 2008). However, it is not known whether Phe813 is important for HWTX-IV block or whether Glu818 is important for ProTx-II block of hNav1.7. To determine whether these two tarantula toxins share the same binding site on the DII S3–S4 linker of hNav1.7, we measured the IC50 values of the two toxins on two single mutations, F813G and E818C, using the whole-cell patch-clamp technique. As shown in Fig. 2, the F813G mutation decreased ProTx-II affinity for hNav1.7 by 9-fold with the IC50 value estimated to be 6.0 nM, but the value of HWTX-IV (28.2 nM) for this mutant was close to that (22.7 nM) for WT channels (see Tables 1 and 2). This result indicates that the residue Phe813 in hNav1.7 might interact structurally with ProTx-II but not HWTX-IV. Our previous work demonstrated that the neutralizing mutation E818Q could decrease HWTX-IV affinity by 63-fold (Xiao et al., 2008). It is noteworthy that when this acidic residue was substituted with cysteine (E818C) in our present study, hNav1.7 current became substantially more resistant to HWTX-IV. Even when exposed to the toxin at concentrations up to 10 μM, hNav1.7-E818C current was only inhibited by 50.3 ± 3.0% (n = 3). The IC50 value was estimated to be 9.1 μM (Fig. 2B and Table 2), indicating that the E818C mutation decreased the sensitivity of hNav1.7 to HWTX-IV by at least 400-fold. By contrast, this mutation was found to only decrease ProTx-II affinity by 4-fold, with an IC50 value of 2.9 nM (Table 1). Given the weak decrease of ProTx-II block by E818C and the proximity of Phe813 to Glu818, it is possible that the decrease caused by the E818C mutation results from a change in the orientation of Phe813 within the DII S3–S4 linker. To further examine this possibility, we constructed a double mutant F813G/E818C. The IC50 value of ProTx-II for the double mutant was estimated to be 29.8 nM in Fig. 2A (Table 1). The decrease in ProTx-II affinity (42-fold) for the double mutant F813G/E818C is additive relative to the effects of the two single mutations from which it derived. Overall, these data strongly indicate that although the binding determinants of ProTx-II and HWTX-IV may partially overlap, they are not identical on hNav1.7 DII.
Fig. 2.
Concentration-response inhibitory curves of ProTx-II (A) and HWTX-IV (B) on DI and DII mutant Nav1.7 channels. Sodium current was induced at 5-s intervals by a 20-ms depolarization from a holding potential of −100 mV. The test pulse potentials to activate channels were set to −10 mV (WT and L201V/N206D), −5 mV (F204A/F813G, F204A/F813G, F813G, E1589Q), 0 mV (E818C and F813G/E818C), and +10 mV (E203K/E818C), respectively. The residual current after toxin treatment was plotted as fraction of the control current. Data points (mean ± S.E., each from 3 - 7 cells) were fit with a Hill equation as described under Materials and Methods. The values of IC50, slope factor (nH), and fbottom yielded are shown in Table 1 and 2.
Mutations in DI S3–S4 Linker Did Not Significantly Change Toxin Affinities for hNav1.7.
Because DII mutations only partially reduced ProTx-II block, and a previous study (Bosmans et al., 2008) showed that ProTx-II is likely to interact with multiple voltage sensors of rNav1.2a including DI, we next asked whether the extracellular DI S3–S4 linker contributes to the sensitivity of hNav1.7 for these two toxins. We first focused on Glu203 and Phe204 in DI S3–S4 of hNav1.7 (Supplemental Fig. S1C), because mutation of the corresponding residues in rNav1.2a (Glu207 and Phe208) was reported to reduce the binding affinity of ProTx-II for rNav1.2a by 2.7- and 13.5-fold, respectively (Bosmans et al., 2008). Here, we constructed two double mutants of hNav1.7 E203K/E818C and F204A/F813G, with the expectation that mutations that reduced binding at DII might help to identify the contributions of residues in DI. The IC50 values of ProTx-II for E203K/E818C (4.6 nM) and F204A/F813G (8.2 nM) were not different from those of single mutants E818C (2.9 nM) and F813G (6.0 nM), respectively (Fig. 2A; Tables 1 and 2). These data suggested that although hNav1.7-E203 may have a weak interaction with ProTx-II, hNav1.7-Phe204 does not seem to play a role in ProTx-II inhibition of hNav1.7.
We were somewhat surprised that the E203K and F204A mutations did not significantly alter the effect of ProTx-II on hNav1.7, given the reported effect of the corresponding mutations on rNav1.2a. One possibility was that the difference between the relative impact of the DI S3–S4 substitutions in rNav1.2a and hNav1.7 could be a result of overall sequence differences in the DI S3–S4 linker region. Although this linker region is highly conserved among voltage-gated sodium channel isoforms, rNav1.2a differs from the hNav1.7 construct that we used at several positions in the DI S3–S4 linker. It is noteworthy that this linker is subject to alternative splicing in both rNav1.2 and hNav1.7 (Raymond et al., 2004). Splicing of exon 5 changes Leu201 and Asn206 (present in the variant that we have been testing) to valine and asparagine in the DI S3–S4 linker, respectively (Chatelier et al., 2008). Sequence alignment shows that the Val201 and Asp206 in the alternative splice variant hNav1.7a are conserved at the corresponding positions in rNav1.2a (V204 and D209) (see Supplemental Fig. S1). The effect of alanine substitutions at these residues on rNav1.2a sensitivity to ProTx-II was examined previously and, although the V204A mutation did not affect ProTx-II block of rNav1.2a/Kv2.1 chimeras, the D209A mutation decreased block in the chimeric channels by ∼3-fold (Bosmans et al., 2008). Therefore, we examined whether alternative splicing of DI S3–S4 of hNav1.7 could affect the sensitivity to ProTx-II. Figure 2A shows that the IC50 value of ProTx-II was measured to be 1.0 nM for the variant hNav1.7a (L201V/N206D), which is close to the value for WT hNav1.7. Although our results do not completely rule out the possibility that ProTx-II interacts with DI S3–S4 in hNav1.7, it indicates that such an interaction is less important than in rNav1.2a.
As shown in Fig. 2B, the concentration-dependencies of HWTX-IV inhibition almost completely overlap for the mutants E818C and E203K/E818C, as did the curves for WT, F813G, and F204A/F813G Nav1.7 channels. Our data also indicate that alternative splicing of DI S3–S4 in hNav1.7 does not alter block by HWTX-IV (Fig. 2B and Table 1). Overall, these data suggest that the DI S3–S4 linker is not a major determinant of either ProTx-II or HWTX-IV interactions with hNav1.7.
ProTx-II Preferentially Interacts with hNav1.7 to Increase Sustained Currents.
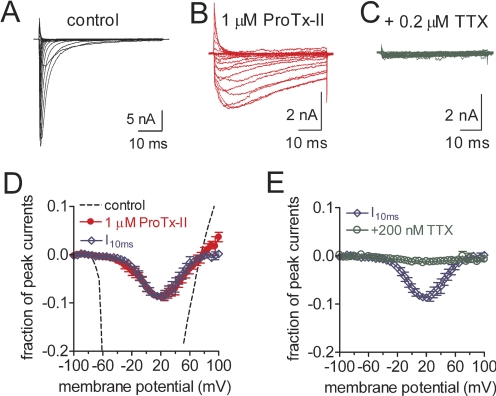
In the presence of 100 nM ProTx-II, 4.9 ± 0.6% of sodium channels could still be activated at −10 mV (Fig. 2A, n = 4). In an attempt to completely eliminate the hNav1.7 sodium current, we increased the ProTx-II concentration to 1 μM. However, no further block was observed, suggesting that the toxin effect on hNav1.7 activation saturates at a concentration of approximately 100 nM. Intriguingly, 1 μM ProTx-II was detected to significantly increase sustained currents generated by WT hNav1.7 (Fig. 3, A and B). The sustained currents did not decay completely during at least 50 ms. By contrast, in the presence of 1 (or even 10) μM HWTX-IV, no alternation of fast inactivation of WT (or mutant E818C) Nav1.7 channels was detected (Supplemental Fig. S2). Because hNav1.7 currents induced at −10 mV inactivate completely within 10 ms in the absence of ProTx-II, we assayed the efficacy of toxin impeding fast inactivation by measuring the I10 ms/Ipeak ratio, which gives an estimate of the probability for the channel to generate sustained currents after 10 ms. Sustained currents induced by 1 μM ProTx-II were detectable at voltages ranging from −40 to +70 mV, but at voltages more positive than +75 mV, sustained currents were not evident (Fig. 3, B and D), indicating that the ProTx-II enhancement of sustained currents in hNav1.7 was voltage-dependent. It is noteworthy that the sustained currents were blocked completely by 200 nM TTX, providing evidence that these sustained ionic currents were indeed fluxing through the hNav1.7 channel pore (Fig. 3, C and E).
Fig. 3.
ProTx-II significantly impeded fast inactivation of WT Nav1.7 expressed in HEK293 cells. Cells were held at −100 mV. Families of current traces before (A) and after application of 1 μM ProTx-II (B) or 200 nM TTX (C) were induced by 50-ms depolarizing steps to various potentials ranging from −100 to +100 mV in 5-mV increments. D and E, effects of 1 μM ProTx-II (D) or 200 nM TTX (E) on the current voltage (I-V) relationship of WT Nav1.7. All currents induced before and after toxin treatment were plotted as fraction of the maximum amplitude of control peak current. The dotted line indicates the control I-V curve. The red filled circles indicate the peak I-V curves after application of 1 μM ProTx-II. I10ms (blue open diamond) was shown as the current inactivated at 10 ms after application of 1 μM ProTx-II. E, effects of 200 nM TTX on the current-voltage (I-V) relationship of WT Nav1.7 sustained currents induced by ProTx-II. All currents induced before and after toxin treatment were plotted as fraction of the maximum amplitude of control peak current. The dotted line indicates the control I-V curve. The green open circles indicate the I-V curve after application of 200 nM TTX. I10ms (blue open diamond) was shown as the current inactivated at 10 ms after application of 1 μM ProTx-II.
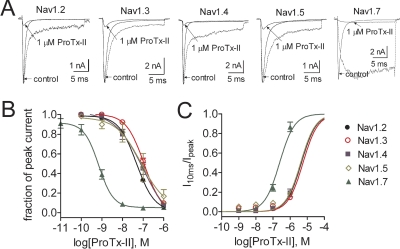
We next wanted to estimate the concentration-response relationship of the apparent ProTx-II effect on the sustained current on multiple sodium channel subtypes expressed in HEK293 cells. Figure 4A shows representative current traces for five subtypes (rNav1.2a, rNav1.3, rNav1.4, hNav1.5, and hNav1.7) before and after application of 1 μM ProTx-II. As reported previously (Smith et al., 2007; Schmalhofer et al., 2008), the toxin IC50 for inhibition of activation of hNav1.7 was ∼70-fold higher than for the other four subtypes rNav1.2a-hNav1.5 (Fig. 4B). In addition, although the sustained currents induced by 1 μM ProTx-II in hNav1.7 was 85.3 ± 3.4% of the peak current (n = 7), in rNav1.2a, rNav1.3, rNav1.4, and hNav1.5, it was only 18.0 ± 2.5, 14.5 ± 1.1, and 19.2 ± 4.0% (n = 3–4) of the peak current, respectively. As can be seen in Fig. 4C, fitting the data on the relative amplitude of the sustained currents induced by ProTx-II with the Hill equation yielded apparent IC50 values of 4.5, 5.6, 4.2, 4.1, and 0.24 μM for rNav1.2a, rNav1.3, rNav1.4, hNav1.5, and hNav1.7, respectively (Supplemental Table S2). It is difficult to accurately determine the IC50 values for apparent inhibition of inactivation. Comparisons of the apparent IC50 values are further complicated here because ProTx-II inhibits activation to different degrees for the different sodium channel isoforms. Despite these caveats, our data clearly indicate that ProTx-II preferentially induces sustained currents in hNav1.7 in addition to the preferential inhibition of hNav1.7 activation.
Fig. 4.
ProTx-II differentially inhibited both activation and inactivation of sodium channel subtypes expressed in HEK293 cells. Cells were held at −100 mV. A, currents through WT Nav1.2, Nav1.3, Nav1.4 and Nav1.7 were induced by a 20-ms depolarizing potential of −10 mV. Nav1.5 current was elicited at −30 mV. The dotted lines show the residual current in the presence of 1 μM ProTx-II after normalization to the maximum amplitude of control current. B, concentration-response inhibitory curves of ProTx-II on the activation of five sodium channel subtypes (Nav1.2, Nav1.3, Nav1.4, Nav1.5, and Nav1.7). The residual current after ProTx-II treatment was plotted as a fraction of control current. Data points (mean ± S.E., each from three to seven cells) were fit with Hill equation as described under Materials and Methods. The IC50 values were estimated to be 52.9 ± 1.1 (Nav1.2a), 109.9 ± 7.1 (Nav1.3), 107.6 ± 7.7 (Nav1.4), and 79.4 ± 40.7 (Nav1.5) nM, respectively. The slope factor (nH) ranged from 0.9 to 1.1. C, concentration-response inhibitory curves of ProTx-II on the fast inactivation of five sodium channel subtypes. The I10ms was plotted as a fraction of the residual current after ProTx-II treatment. Inhibition of fast-inactivation increases the ratio of I10ms/Ipeak. Data points (mean ± S.E., each from three to seven cells) were fit with Hill equation as described under Materials and Methods. The IC50 values are shown in Supplementary Table 2.
The voltage-dependent induction of sustained currents in hNav1.7 by ProTx-II is somewhat similar to the voltage-dependent inhibition of activation by ProTx-II. One explanation for the increased sustained currents is that ProTx-II might induce what seems to be sustained current by variably prolonging the latency to activation. However, the sustained currents are also similar to those induced by scorpion α-toxins that inhibit inactivation of voltage-gated sodium channels (Strichartz and Wang, 1986). Therefore, an alternative explanation is that ProTx-II, in addition to its ability to inhibit activation, also inhibits inactivation, possibly by interacting with the hNav1.7 DIV voltage-sensor associated with inactivation in a manner similar to that of scorpion α-toxins (Rogers et al., 1996). Although all of the previous studies on ProTx-II have indicated that ProTx-II inhibits only activation of voltage-gated sodium channels (Middleton et al., 2002; Smith et al., 2007; Bosmans et al., 2008; Schmalhofer et al., 2008; Sokolov et al., 2008), Bosmans et al. (2008) reported that ProTx-II could interact with the DIV voltage sensor of rNav1.2a. Surprisingly, they found that specific substitutions in the S3–S4 linker region of DIV of rNav1.2a substantially reduce the ability of ProTx-II to inhibit activation of rNav1.2a channels expressed in Xenopus laevis oocytes. Our results suggested that, at least for sodium channels expressed in mammalian cells, ProTx-II might be inducing sustained currents by interacting with DIV. If this effect is due to the binding of DIV, the apparent affinity of ProTx-II binding for hNav1.7 DIV may be ∼17-fold higher than for other subtypes.
Mutations in DIV Alter the Effect of ProTx-II on Sustained Currents Generated in hNav1.7.
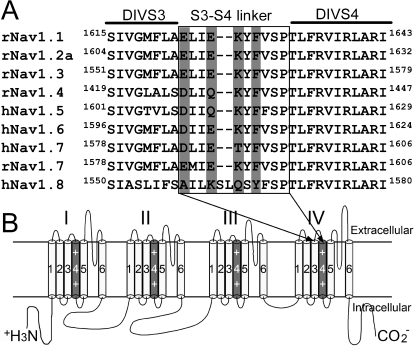
To further investigate how ProTx-II preferentially induced sustained sodium currents in hNav1.7, we compared the amino acid sequences of DIV S3–S4 linkers from eight sodium channel subtypes Nav1.1 to Nav1.8 (Fig. 5). The most striking difference in this region is the unique presence of Thr1590 in hNav1.7; the residue at the corresponding position in rNav1.1-hNav1.6 is lysine and glutamine in hNav1.8. The residue at this position can be important in modulating the effect of scorpion α-toxins on sodium channels (Leipold et al., 2004). It is noteworthy that Asp1586, Glu1589, and Phe1592 in hNav1.7 are conserved at the corresponding positions among six other subtypes (Nav1.1–Nav1.6). The residues at these positions are interesting because previous studies have determined that the first is crucial for the ability of site 3 scorpion α-toxins and sea anemone toxins to modify sodium channel inactivation and the latter two have been implicated in rNav1.2a interactions with ProTx-II (Rogers et al., 1996; Bosmans et al., 2008). Therefore, we next investigated whether single substitutions at these four residues in hNav1.7 (D1586A, E1589Q, T1590K, and F1592A) might be important determinants of ProTx-II preferentially inducing sustained currents in hNav1.7.
Fig. 5.
Amino acid sequence alignment of the DIV S3–S4 linkers of seven α subunit isoforms from human. A, crucial determinants of neurotoxin receptor 3 are located in the S3–S4 linker of sodium channel domain II. The positions of amino acid residues of interest are shaded in gray. B, schematic diagram of sodium channel α subunit. The voltage sensor (the fourth segment) of each domain is shaded in gray and marked with “++.” The amino acid sequence of DIV S3–S4 linker is shown in the square frame of A as indicated by arrows.
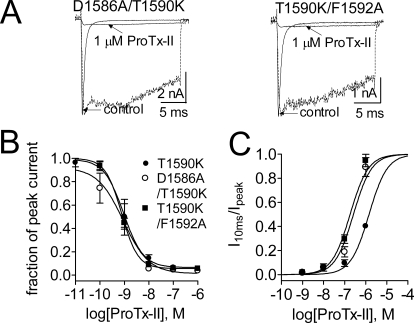
Current traces for these mutant channels were elicited by a 20-ms depolarizing potential of −10 or −5 mV from a holding potential of −100 mV (Fig. 6A). It is important to note that none of the four mutations altered the ability of ProTx-II to block hNav1.7 activation. As shown in Fig. 6B, the fit of the Hill equation yielded the IC50 values for ProTx-II inhibition of activation to be 1.0 (D1586A), 0.5 (E1589Q), 0.9 (T1590K), and 0.7 nM (F1592A). In contrast, these mutations had distinct effects on the ability of ProTx-II to induce hNav1.7 sustained currents (Fig. 6A and Supplemental Fig. S4). Two of the mutations significantly decreased the enhancement of sustained currents by ProTx-II. The sustained currents induced by ProTx-II (1 μM) in E1589Q and T1590K channels were 40.3 ± 1.7% (n = 4) and 37.2 ± 10.0% (n = 3) of the peak current, respectively. The other two DIV mutations that we tested increased the ability of ProTx-II to enhance sustained currents. When treated only with 100 nM toxin, the sustained currents generated in D1586A and F1592A channels was 74.5 ± 2.6% (n = 4) and 61.1 ± 12.8% (n = 4) of the peak current, respectively, which are close to the value obtained for 1 μM ProTx-II on WT hNav1.7. Because ProTx-II inhibits activation of the DIV mutant channels and WT channels to the same extent, we can confidently compare the relative effect on inhibition of inactivation by measuring the current amplitude 10 ms into the depolarizing pulse (I10ms) and calculating the ratio (I10ms/Ipeak), where Ipeak is the peak current remaining after ProTx-II treatment. In Fig. 6C, the apparent IC50 values for ProTx-II inhibition of fast inactivation were estimated to be 48.3 nM, 1.6 μM, 1.4 μM, 70.1 nM, and 240 nM for D1586A, E1589Q, T1590K, F1592A, and WT hNav1.7, respectively (Supplemental Table S2). Therefore, our data show that although two mutations, E1589Q and T1590K, selectively decreased ProTx-II ability to induce sustained currents in hNav1.7 DIV by ∼6-fold, the other two mutations, D1586A and F1592A, selectively increased ProTx-II ability to induce sustained currents by ∼4-fold.
Fig. 6.
Mutations in DIV S3–S4 linker differentially alter the effect of ProTx-II on fast-inactivation of hNav1.7 channels expressed in HEK293 cells. A, representative current traces for five mutant (D1586A, D1586E, E1589Q, T1590K, and F1592A) hNav1.7 channels. The test pulse potential was −10 mV (D1586A and D1586E) and −5 mV (E1589Q, T1590K and F1592A), respectively. Cells were held at −100 mV. The dotted line shows the residual current in the presence of 0.1 or 1 μM ProTx-II after normalization to the maximum amplitude of control current. B, concentration-response inhibitory curves of ProTx-II on the activation of WT and five mutant (D1586A, D1586E, E1589Q, T1590K, and F1592A) Nav1.7 channels. Residual current after toxin treatment was plotted as a fraction of control peak current amplitude. Data points (mean ± S.E., each from three to six cells) were fitted with Hill equation as described under Materials and Methods. The calculated values of IC50, slope factor (nH), and fbottom are shown in Table 1. C, concentration-response inhibitory curves of ProTx-II on fast inactivation of WT and five mutant (D1586A, D1586E, E1589Q, T1590K, and F1592A) Nav1.7 channels. The I10ms was plotted as a fraction of the residual current after ProTx-II treatment. Data points (mean ± S.E., each from three to six cells) were fitted with the Hill equation as described under Materials and Methods.
These findings are very different from those of a previous study, which found that the conserved mutations E1614A and F1620A in rNav1.2a decreased ProTx-II inhibition of rNav1.2a activation by more than 6-fold and decreased ProTx-II affinity for the rNav1.2a DIV paddle motif by more than 10-fold (Bosmans et al., 2008). Again, in these previous studies carried out with rNav1.2a, ProTx-II reportedly had no effect on sustained currents or inactivation. As can be seen in Fig. 5, there are only two differences in the DIV S3–S4 linkers of hNav1.7 and rNav1.2a: Asp1586 and Thr1590 of hNav1.7 are substituted with glutamic acid and lysine, respectively, in rNav1.2a. When we replaced Asp1586 with glutamic acid in hNav1.7, the ability of ProTx-II to inhibit channel activation or induce sustained currents was not changed compared with WT channels (Fig. 6, B and C; Table 1), indicating that the sequence difference at this residue is not important in determining the differences between our observations and those of Bosmans et al. (2008). However, it has been shown clearly that DIV residues can have substantial combinatorial effects on the interaction of scorpion α-toxins with specific sodium channel isoforms (Leipold et al., 2004). Therefore, we next asked whether the residue at position 1590 (threonine versus lysine) was the key factor for reversing the structural interactions of Asp1586 and Phe1592 with ProTx-II. Using the mutant T1590K as a model, we constructed two double mutations D1586A/T1590K and T1590K/F1592A. As seen in Fig. 7, the IC50 values for ProTx-II inducing sustained currents were estimated to be 0.26 μM for D1586A/T1590K and 0.18 μM for T1590K/F1592A, which were 5- and 7-fold smaller than the value for the single mutation T1590K, respectively (Supplemental Table S2). Therefore, together with the data on two single mutations, D1586A and F1592A (Fig. 7C), these results suggest that the threonine to lysine exchange at position 1590 could not reverse the structural interaction of Asp1586 and Phe1592 with ProTx-II. In addition, neither double mutation altered the ability of ProTx-II to inhibit channel activation (Fig. 7B and Table 1).
Fig. 7.
Two mutations (D1586A and F1592A) enhanced the ProTx-II slowing fast inactivation of hNav1.7 in the presence of K1590. A, representative current traces for two double mutant (D1586A/T1590K and T1590K/F1592A) hNav1.7 channels. The test pulse potential was −5 mV for D1586A/T1590K and 0 mV for T1590K/F1592A, respectively. Cells were held at −100 mV. The dotted lines show the residual current in the presence of 1 μM ProTx-II after normalization to the maximum amplitude of control current. B, concentration-response inhibitory curves of ProTx-II on the activation of three mutant (T1590K, D1586A/T1590K, and T1590K/F1592A) Nav1.7 channels. Residual current after toxin treatment was plotted as a fraction of control peak current amplitude. The calculated values of IC50, slope factor (nH), and fbottom are shown in Table 1. C, concentration-response inhibitory curves of ProTx-II on fast-inactivation of three mutant (T1590K, D1586A/T1590K, and T1590K/F1592A) Nav1.7 channels. The I10ms was plotted as a fraction of the residual current after ProTx-II treatment. The IC50 values are shown in Supplementary Table 2. In B and C, data points (mean ± S.E., each from three to four cells) were fitted with a Hill equation as described under Materials and Methods.
Finally, we sought to determine whether decreasing the inhibition of action of hNav1.7 by ProTx-II would alter the apparent effects of ProTx-II on inactivation. Therefore, we used the F813G/E818C double mutation, which reduces inhibition of activation by approximately 42-fold, and examined the effect of the E1589Q and F1592A mutations (Supplemental Fig. S5). In the F813G/E818C background, the E1589Q and F1592A mutations still have no effect on the inhibition of activation by ProTx-II, but the E1589Q mutation decreased the inhibition of inactivation by 5-fold and the F1592A mutation increased the inhibition of inactivation by 8-fold (Supplemental Fig. S5C). Thus, these DIV mutations have nearly identical effects on ProTx-II inhibition of inactivation for WT and F813G/E818C mutant Nav1.7 channels. Together, our data show that although mutations in DIV of hNav1.7 do not alter the inhibition of activation by ProTx-II, they do substantially modulate the ability of ProTx-II to induce sustained sodium currents in hNav1.7. This is similar to the DIV-dependent inhibition of inactivation by scorpion α-toxins, indicating that ProTx-II can inhibit both activation and inactivation of voltage-gated sodium channels.
Discussion
We investigated the molecular determinants of the interactions of two tarantula toxins with hNav1.7. ProTx-II and HWTX-IV have a similar cysteine knot structure and both exhibit higher affinities for blocking hNav1.7 than for other subtypes (Schmalhofer et al., 2008; Xiao et al., 2008). However, these two toxins differ quite substantially in their noncysteine sequence, and our data indicate that they are very different in the extent and functional consequences of their interactions with hNav1.7.
Differential Interactions of ProTx-II and HWTX-IV with hNav1.7 Voltage Sensors.
ProTx-II and HWTX-IV are classified as voltage-sensor modifiers, and both have been proposed to selectively inhibit channel activation by trapping the DII voltage sensor in the closed state (Sokolov et al., 2008; Xiao et al., 2008). Our results reveal that a single mutation (E818C) reduced the sensitivity of hNav1.7 for HWTX-IV by more than 400-fold (Table 1). Our results further show that mutations of residues in the S3–S4 linkers of DI and DIV did not alter the inhibition of hNav1.7 by HWTX-IV. The DII voltage sensor is clearly the main determinant of action for HWTX-IV inhibition of activation of hNav1.7.
In contrast, ProTx-II is likely to interact with multiple regions of sodium channels. In a study of ProTx-II interactions with Nav1.5, we concluded that ProTx-II may not make critical interactions with extracellular linker regions of Nav1.5, suggesting the existence of a novel toxin binding site (Smith et al., 2007). On the other hand, two studies with rNav1.2a (Sokolov et al., 2008) and hNav1.7 (Schmalhofer et al., 2008) suggested that ProTx-II might specifically interact with the DII voltage sensor. However, using chimeric Kv2.1 channels containing the voltage sensor paddle regions of rNav1.2a, Bosmans et al. (2008) found that ProTx-II can interact with the voltage sensors from three domains (DI, DII, and DIV) of rNav1.2a. Consistent with this finding, we demonstrate that ProTx-II interacts with the voltage sensors of two domains (DII and DIV) in hNav1.7. However, our data suggest that the DI S3–S4 linker is less important in determining hNav1.7 sensitivity to ProTx-II than might be predicted by the chimeric paddle approach and raises the possibility that other regions of the DI voltage sensor (such as the S1–S2 linkers) may influence the isoform-specific interactions.
The Binding Determinants of ProTx-II and HWTX-IV Partially Overlap on hNav1.7 DII.
ProTx-II is at least 70-fold more selective for hNav1.7 over other subtypes that we tested, consistent with previous findings (Smith et al., 2007; Schmalhofer et al., 2008). Our data support the assertion (Schmalhofer et al., 2008) that this selectivity might result from the higher sensitivity of hNav1.7 DII for ProTx-II. In DII, Phe813 is unique in hNav1.7. When Phe813 is substituted with glycine, the corresponding residue in most other sodium channel subtypes, we found that ProTx-II affinity for hNav1.7 is decreased by 9-fold. This decrease is smaller than that reported previously, in which the F813G mutation completely abolished selectivity of ProTx-II for hNav1.7. It is not clear what accounts for this quantitative difference; however, in the previous study, the reverse substitution in hNav1.2 (G839F) did not significantly increase ProTx-II inhibition of hNav1.2 (Schmalhofer et al., 2008), indicating that the residue at this position is not the only determinant of hNav1.7's high sensitivity to ProTx-II. It is noteworthy that Phe813 is specific for ProTx-II's interaction and does not seem to play any role in HWTX-IV's interactions. Furthermore, we show that although Glu818 interacts with both toxins, it is much more important for HWTX-IV. Therefore, our data indicate that the DII S3–S4 linker is important in determining the higher selectivity of these toxins for hNav1.7, but the binding determinants of ProTx-II and HWTX-IV only partially overlap in this region. This observation, in combination with the striking lack of identity between ProTx-II and HWTX-IV, suggests that DII of hNav1.7 may be an excellent target for development of hNav1.7 specific inhibitors.
Molecular Mechanism for ProTx-II Inhibition of Fast Inactivation.
We observed that ProTx-II induced sustained hNav1.7 currents. Because ProTx-II dissociates from hNav1.7 at depolarized potentials, one explanation for the sustained current could have been that ProTx-II simply prolonged the latency to channel opening without any effect on inactivation. However, mutations in hNav1.7 DIV that substantially modulated the sustained current induced by ProTx-II had no effect on the inhibition of activation by ProTx-II, in both the WT and F813G/E818C backgrounds. It is noteworthy that no effect was seen on the voltage-dependence of steady-state inactivation with ProTx-II (Fig. 1C); however, it should be noted that disparate effects on the voltage-dependence of steady-state inactivation and the rate of open-state fast inactivation are frequently observed for DIV manipulations (Yang et al., 1994; Bendahhou et al., 1999). Scorpion α-toxins often have no effect on the voltage-dependence of steady-state fast inactivation but dramatically inhibit open-state fast inactivation of voltage-gated sodium channels (Maertens et al., 2006), and this action is dependent on the DIV S3–S4 linker (Rogers et al., 1996). Based on these previous studies and our data showing that the DIV mutations selectively alter the ability of ProTx-II to induce sustained currents in hNav1.7, we conclude that ProTx-II not only has the ability to inhibit activation but also inhibits fast inactivation of sodium channels through a mechanism similar to neurotoxin receptor site 3 toxins such as α-toxins.
Our data indicate that the effects on activation and inactivation are independent. Mutations in domain IV that substantially alter the effect on the inhibition of inactivation have no effect on the inhibition of activation. Mutations in domain II preferentially affect the inhibition of activation. This suggests that ProTx-II can simultaneously interact with two independent sites, one in DIV and the other possibly in DII. The estimated IC50 for inhibition of activation is ∼400-fold smaller than the apparent IC50 for inhibition of inactivation, providing additional evidence that there are probably two independent interaction sites. Despite this differential in IC50 values, the interaction with DIV is able to induce measurable sustained currents because the inhibition of activation is incomplete, even at relatively high concentrations.
These findings have some important implications. First, they point out potential limitations in the usefulness of ProTx-II as a research tool. Although ProTx-II may help determine whether Nav1.7 contributes to peak transient sodium currents in neurons, our results indicate that it would be problematic to use ProTx-II to determine the contribution of Nav1.7 to sustained sodium currents. Second, it must also be used with care when examining the contribution of Nav1.7 to action potential firing properties. Excitatory toxins such as scorpion α-toxins and the tarantula toxin Jingzhaotoxin-I that impede fast inactivation can prolong action potential duration and increase repetitive action potential firing (Rogers et al., 1996; Xiao et al., 2005, 2007). Sustained Nav1.7 currents induced by ProTx-II, even small ones, could be problematic. Sensory neurons that express Nav1.7 often express other sodium channel isoforms such as Nav1.8 that are less sensitive to ProTx-II (Middleton et al., 2002), and the persistent Nav1.7 currents induced by ProTx-II in conjunction with Nav1.8 currents could have complicated effects on excitability. The multiple effects of ProTx-II on hNav1.7 activation and inactivation would be expected to complicate, if not contraindicate, the use of ProTx-II administration to inhibit pain.
A previous report concluded that although ProTx-II interacts with the voltage-sensor paddles of DI, DII, and DIV, ProTx-II only inhibited rNav1.2a activation, not inactivation (Bosmans et al., 2008). Because mutations in rNav1.2a DI, DII, and DIV paddles all affected the extent of inhibition of activation of rNav1.2a, it was concluded that drugs targeting any of the paddle motifs in the first three domains would only influence channel activation, regardless of any interaction with DIV. Furthermore, because DIV mutations altered the ability of ProTx-II to inhibit the activation of rNav1.2a channels expressed in X. laevis oocytes, it was concluded that toxins need to exclusively interact with the voltage sensor of DIV to alter the inactivation of voltage-gated sodium channels (Bosmans et al., 2008). Our results challenge the broad applicability of these conclusions. It is not entirely clear what accounts for the seemingly opposite actions of ProTx-II on the DIVs of rNav1.2a and hNav1.7. We found that ProTx-II could impede fast inactivation in multiple isoforms expressed in HEK293 cells, including rNav1.2a. Because the effects on fast inactivation are much smaller in rNav1.2a, rNav1.3, rNav1.4, and hNav1.5 than in hNav1.7, they might have been overlooked in previous studies. On the other hand, the differential effects of ProTx-II could result from differences between X. laevis oocytes and mammalian cells. Differences in the lipid composition of the membrane could be a factor as ProTx-II exhibits substantial lipid binding activity (Smith et al., 2005). Indeed, the sensitivity of potassium channels to tarantula toxins can be modulated by differences in the composition of the lipid bilayer (Schmidt and MacKinnon, 2008). Differences in posttranslational modifications, such as glycosylation, might also modulate the sensitivity of sodium channels to ProTx-II.
In summary, in this study, we extensively investigated the interaction of two tarantula toxins with hNav1.7. HWTX-IV selectively inhibits activation of hNav1.7, and this is specifically determined by residues in the DII voltage sensor. ProTx-II also interacts with DII, but our data indicate that ProTx-II inhibits both activation and inactivation of hNav1.7. These data show that contrary to what has been proposed previously to be a guiding principle of sodium channel pharmacology, toxins do not have to exclusively target the DIV voltage sensor to influence sodium channel inactivation. Molecules that interact with the multiple voltage sensors of sodium channels can impede both activation and inactivation, and these complex interactions need to be carefully considered when targeting the voltage sensors of sodium channels.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS054642, NS053422]; the 973 Research Program of China [Contract 2010CB529800]; and the Program for New Century Excellent Talents in University [Contract NCET-07-0279].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.066332.
- HWTX-IV
- huwentoxin-IV
- TTX
- tetrodotoxin
- WT
- wild type
- HEK
- human embryonic kidney
- DI
- domain I
- DII
- domain II
- DIII
- domain III
- DIV
- domain IV
- S
- transmembrane segment.
References
- Bendahhou S, Cummins TR, Kwiecinski H, Waxman SG, Ptácek LJ. (1999) Characterization of a new sodium channel mutation at arginine 1448 associated with moderate Paramyotonia congenita in humans. J Physiol 518:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclaire MF, Swartz KJ. (2008) Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 456:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SGInternational Union of Pharmacology (2003) International Union of Pharmacology. XXXIX. Compendium of voltage-gated ion channels: sodium channels. Pharmacol Rev 55:575–578 [DOI] [PubMed] [Google Scholar]
- Cestèle S, Catterall WA. (2000) Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 82:883–892 [DOI] [PubMed] [Google Scholar]
- Cestèle S, Scheuer T, Mantegazza M, Rochat H, Catterall WA. (2001) Neutralization of gating charges in domain II of the sodium channel alpha subunit enhances voltage-sensor trapping by a beta-scorpion toxin. J Gen Physiol 118:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, Ruben PC, George AL, Jr., Fujimoto E, Bezanilla F. (1999) Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron 22:73–87 [DOI] [PubMed] [Google Scholar]
- Chatelier A, Dahllund L, Eriksson A, Krupp J, Chahine M. (2008) Biophysical properties of human Na v1.7 splice variants and their regulation by protein kinase A. J Neurophysiol 99:2241–2250 [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444:894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Aglieco F, Renganathan M, Herzog RI, Dib-Hajj SD, Waxman SG. (2001) Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci 21:5952–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Rush AM. (2007) Voltage-gated sodium channel blockers for the treatment of neuropathic pain. Expert Rev Neurother 7:1597–1612 [DOI] [PubMed] [Google Scholar]
- Cummins TR, Sheets PL, Waxman SG. (2007) The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain 131:243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. (1995) Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J 14:1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipold E, Lu S, Gordon D, Hansel A, Heinemann SH. (2004) Combinatorial interaction of scorpion toxins Lqh-2, Lqh-3, and LqhalphaIT with sodium channel receptor sites-3. Mol Pharmacol 65:685–691 [DOI] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL., Jr (2002) Molecular basis of an inherited epilepsy. Neuron 34:877–884 [DOI] [PubMed] [Google Scholar]
- Maertens C, Cuypers E, Amininasab M, Jalali A, Vatanpour H, Tytgat J. (2006) Potent modulation of the voltage-gated sodium channel Nav1.7 by OD1, a toxin from the scorpion Odonthobuthus doriae. Mol Pharmacol 70:405–414 [DOI] [PubMed] [Google Scholar]
- Middleton RE, Warren VA, Kraus RL, Hwang JC, Liu CJ, Dai G, Brochu RM, Kohler MG, Gao YD, Garsky VM, et al. (2002) Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry 41:14734–14747 [DOI] [PubMed] [Google Scholar]
- O'Leary ME. (1998) Characterization of the isoform-specific differences in the gating of neuronal and muscle sodium channels. Can J Physiol Pharmacol 76:1041–1050 [DOI] [PubMed] [Google Scholar]
- Peng K, Shu Q, Liu Z, Liang S. (2002) Function and solution structure of huwentoxin-IV, a potent neuronal tetrodotoxin (TTX)-sensitive sodium channel antagonist from Chinese bird spider Selenocosmia huwena. J Biol Chem 277:47564–47571 [DOI] [PubMed] [Google Scholar]
- Raymond CK, Castle J, Garrett-Engele P, Armour CD, Kan Z, Tsinoremas N, Johnson JM. (2004) Expression of alternatively spliced sodium channel alpha-subunit genes. Unique splicing patterns are observed in dorsal root ganglia. J Biol Chem 279:46234–46241 [DOI] [PubMed] [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. (1996) Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem 271:15950–15962 [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT. (2008) ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol 74:1476–1484 [DOI] [PubMed] [Google Scholar]
- Schmidt D, MacKinnon R. (2008) Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci USA 105:19276–19281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. (2007) Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol 582:317–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Alphy S, Seibert AL, Blumenthal KM. (2005) Differential phospholipid binding by site 3 and site 4 toxins. Implications for structural variability between voltage-sensitive sodium channel domains. J Biol Chem 280:11127–11133 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Cummins TR, Alphy S, Blumenthal KM. (2007) Molecular interactions of the gating modifier toxin ProTx-II with NaV 1.5: implied existence of a novel toxin binding site coupled to activation. J Biol Chem 282:12687–12697 [DOI] [PubMed] [Google Scholar]
- Sokolov S, Kraus RL, Scheuer T, Catterall WA. (2008) Inhibition of sodium channel gating by trapping the domain II voltage sensor with protoxin II. Mol Pharmacol 73:1020–1028 [DOI] [PubMed] [Google Scholar]
- Strichartz GR, Wang GK. (1986) Rapid voltage-dependent dissociation of scorpion alpha-toxins coupled to Na channel inactivation in amphibian myelinated nerves. J Gen Physiol 88:413–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukomadu C, Zhou J, Sigworth FJ, Agnew WS. (1992) muI Na+ channels expressed transiently in human embryonic kidney cells: biochemical and biophysical properties. Neuron 8:663–676 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Bingham JP, Zhu W, Moczydlowski E, Liang S, Cummins TR. (2008) Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain ii voltage sensor in the closed configuration. J Biol Chem 283:27300–27313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Li J, Deng M, Dai C, Liang S. (2007) Characterization of the excitatory mechanism induced by Jingzhaotoxin-I inhibiting sodium channel inactivation. Toxicon 50:507–517 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Tang J, Hu W, Xie J, Maertens C, Tytgat J, Liang S. (2005) Jingzhaotoxin-I, a novel spider neurotoxin preferentially inhibiting cardiac sodium channel inactivation. J Biol Chem 280:12069–12076 [DOI] [PubMed] [Google Scholar]
- Yang N, Ji S, Zhou M, Ptácek LJ, Barchi RL, Horn R, George AL., Jr (1994) Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Natl Acad Sci USA 91:12785–12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.