Abstract
Homomeric α7 nicotinic acetylcholine receptors represent an important and complex pharmaceutical target. They can be activated by structurally diverse agonists and are highly likely to enter and remain in desensitized states at rates determined by the structures of the agonists. To identify structural elements regulating this function, we introduced reactive cysteines into the α7 ligand-binding domain allowing us to bind sulfhydryl-reactive (SH) agonist analogs or control reagents onto specific positions in the ligand binding domain. We identified four α7 mutants (S36C, L38C, W55C, and L119C) in which the tethering of the SH reagents blocked further acetylcholine-evoked activation of the receptor. However, after selective reaction with SH agonist analogs, the type II allosteric modulator N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl-3-isoxazolyl)-urea (PNU-120596) could reactivate L119C and W55C mutants and receptors with a reduced or modified C-loop. Modified S36C and L38C mutants were insensitive to reactivation by PNU-120596, whether they were reacted with agonist analogs or alternative SH reagents. Molecular modeling showed that in the W55C and L119C mutants, the ammonium pharmacophore of the agonist analog methanethiosulfonate-ethyltrimethylammonium would be in a similar but nonidentical position underneath the C-loop. The orientation assumed by the ligand tethered to 119C was approximately 3-fold more sensitive to PNU-120596 than the alternative pose at 55C. Our results support the hypothesis that a single ligand can bind within the receptor in different ways and, depending on the specific binding pose, may variously promote activation or desensitization, or, alternatively, function as a competitive antagonist. This insight may provide a new approach for drug development.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels, mainly expressed in muscle and neurons, and are also found in glial and non-neuronal tissues (Gahring and Rogers, 2005). In the brain, heteromeric α4β2-containing and homomeric α7 subtypes are the two major types of the nAChRs representing high-affinity binding sites for nicotine and α-bungarotoxin, respectively (Clarke et al., 1986; Gotti et al., 2007). Although α4β2 receptors have been strongly associated with the cognitive and addicting effects of nicotine, α7 nAChRs have been implicated as influential in neuroprotection (Svensson and Nordberg, 1999), attentional and cognitive enhancement (Young et al., 2004), and the regulation of inflammatory signaling (Wang et al., 2003; Giebelen et al., 2007; Pavlov et al., 2007).
Electron microscopy studies of Torpedo californica nAChR (Unwin et al., 2002; Miyazawa et al., 2003) and more recently X-ray analyses of molluscan acetylcholine binding protein (AChBP) have provided high-resolution templates for homology models of the nAChR (Smit et al., 2001; Sixma and Smit, 2003). Each nAChR subunit consists of an extracellular ligand binding domain (LBD), a transmembrane domain consisting of four bundled α-helices, and an intracellular domain with poorly defined structure and function. In the LBD, agonists are bounded by six loop structures, loops A, B, and C from the α subunit (primary face) and loops D, E, and F from the non-α subunit (complementary face) (Sixma and Smit, 2003). Conserved residues on these loops are important for agonist binding and/or activation on the receptor (Brejc et al., 2001).
The nAChR are members of the large “cysteine-loop” superfamily of ligand-gated ion channels, which includes receptors for GABA, glycine, and serotonin (Millar and Gotti, 2009). Ion channel activation is associated with one or more of the conformational states that can be induced by ligand binding; although nonconducting, putatively desensitized states are more stable and predominate after the prolonged binding of agonist. The α7 receptor has been proposed to manifest a distinct form of rapid desensitization, which may be facilitated by high levels of agonist binding-site occupancy (Papke et al., 2000). This form of desensitization can be destabilized by mutations in the ion channel or with a positive allosteric modulator (PAM) (Bertrand et al., 2008).
The discovery of α7-selective PAMs such as N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl-3-isoxazolyl)-urea (PNU-120596) has both suggested new ways to target α7 receptors therapeutically (Grønlien et al., 2007) and provided new tools to study them experimentally. In particular, we have gained new insights into the unique desensitization properties of α7 receptors through the use of this type II PAM (Grønlien et al., 2007; Papke et al., 2009), which not only enhances apparent peak current during agonist application but can also reactivate one or more desensitized states (Papke et al., 2009) by binding in an intrasubunit cavity located between the four α-helical transmembrane domains (Young et al., 2008).
The α7 nAChR is a challenging therapeutic target because of both the structural limitation associated with homology modeling and its propensity to enter and remain in desensitized states in ways that can depend on the structure of the agonist (Papke et al., 2009). An important goal is to decipher the complex structural interplay between the character and disposition of ligands bound to the receptor and the affect of that binding on receptor activation and desensitization.
Cysteine mutagenesis is a commonly used approach for determining the solvent accessibility and functional significance of specific residues in proteins such as ligand-gated ion channels (Karlin and Akabas, 1998; Spura et al., 1999; Sullivan et al., 2002; Barron et al., 2009). Unique cysteines are introduced by point mutations at a series of positions, and each mutant is then reacted with a sulfhydryl-reactive (SH) reagent to determine by functional analyses whether the reaction produced a labeled receptor.
In this article, we report a systematic study of the geometric and spatial requirements for how bound ligands may activate the α7 receptor and, in some cases, promote conversion of the receptor to PNU-120596-sensitive desensitized states. Site-specific cysteine mutants in the LBD of human α7 were expressed in Xenopus laevis oocytes. The cysteine mutants and wild-type receptors with the C-loop disulfide Cys190 to Cys191 reduced were reacted with a panel of SH-reactive agonist analogs and characterized in terms of initial channel activation, blockade of subsequent ACh-evoked responses, and the induction of PNU-120596-sensitive desensitization. Our experimental data provide a useful approach for integrating nAChR homology modeling, including docking and molecular dynamics simulations, with functional analysis of ligand-dependent conformational changes.
Materials and Methods
α7 nAChR Clones and Site-Directed Mutants.
The human α7 clone was obtained from Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA). The human RIC-3 clone, obtained from Dr. Millet Treinin (Hebrew University, Jerusalem, Israel), was coinjected with the α7 constructs to improve the levels and speed of receptor expression. Amino acids are numbered as for human α7 (vicinal C-loop cysteines at positions 190 and 191). Mutations were introduced using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. To obtain a cysteine-null background, a naturally occurring cysteine in α7 was mutated to serine (α7 C116S). As reported previously (Papke et al., 2010), the pharmacology and macroscopic activation properties of this cysteine-null receptor were indistinguishable from those of wild-type α7 in regard to the potency and relative efficacy of diverse agonists including ACh, tetramethylammonium (TMA), quinuclidine, and 3-(2-methoxy,4-hydroxy-benzylidene)anabasine. The mutant also was indistinguishable from wild-type in regard to the rapid concentration-dependent desensitization characteristic of α7 (Papke et al., 2010). The novel cysteine mutants used in these experiments were made in the LBD of α7 C116S. All mutations were confirmed with automated fluorescent sequencing. After linearization and purification of cloned cDNA, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion Inc. (Austin, TX).
Expression in X. laevis Oocytes.
Mature (>9 cm) female X. laevis African frogs (Nasco, Ft. Atkinson, WI) were used as the source of oocytes. Before surgery, frogs were anesthetized by laying the animal in a 1.5g/l solution of 3-aminobenzoic acid ethyl ester (MS222; Sigma-Aldrich, St. Louis, MO) for 30 min. Oocytes were removed from an abdominal incision.
To digest the follicular cell layer, harvested oocytes were treated with 1.25 mg/ml collagenase from Worthington Biochemical Cooperation (Freehold, NJ) for 2 h at room temperature in the Barth's solution without calcium (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, pH 7.6, and 12 g/l tetracycline). After that, stage 5 oocytes were isolated and injected with 50 nl (5–20 ng) of appropriate subunit cRNAs. Recordings were made 2 to 10 days after injection. The experimental response values were normalized to control ACh applications to avoid the variety of the absolute magnitude of the evoked current response over time.
Chemicals.
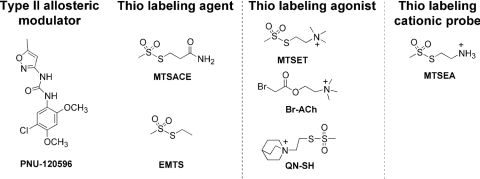
The methanethiosulfonate compounds ethyl methanethiosulfonate (EMTS), 2-(aminocarbonyl)ethyl methanethiosulfonate (MTSACE), 2-(quinuclidinium)ethyl methanethiosulfonate (QN-SH), (2-aminoethyl)methanethiosulfonate (MTSEA), and methanethiosulfonate-ethyltrimethylammonium (MTSET) (Fig. 2) were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). PNU-120596 was obtained from Tocris Bioscience (Ellisville, MO), and all of the other chemicals for electrophysiology including Bromo-acetylcholine (Br-ACh) were from Sigma-Aldrich. EMTS, PNU-120596, and MTSACE stock solutions were made in dimethyl sulfoxide monthly and freshly diluted in Ringer's solution every day. Other SH reagent stock solutions were made daily in Ringer's solution and diluted.
Fig. 2.
The structures of PNU-120596 and the six reagents: Br-ACh, MTSET, MTSEA, QN-SH, EMTS, and MTSACE.
Electrophysiology.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Sunnyvale, CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Cells were automatically bath-perfused with Ringer's buffer, and both the voltage and current electrodes were filled with 3 M KCl. The agonist compounds were delivered from a 96-well plate and applied via disposable tips to eliminate any possibility of cross-contamination. Drug applications alternated between ACh controls and experimental applications. Cells were voltage-clamped at a holding potential of −60 mV. Data were collected at 50 Hz and filtered at 20 Hz. Flow rates were set at 2 ml/min. Unless otherwise indicated, drug applications were 12 s in duration followed by 181-s washout periods.
Experimental Protocols and Data Analysis.
Each oocyte received two initial control applications of ACh, a 60-s 1 mM SH reagent application (initial flow rate of 2 ml/min for 10 s, followed by 0.5 ml/min for 50 s), a follow-up control application of ACh, then an application of 300 μM PNU-120596, and another follow-up ACh control application. Control ACh was 60 μM for α7 wild type, α7 C116S, and α7 L119C; 100 μΜ ACh for α7 L38C; and 300 μM for the other mutants. These concentrations were empirically determined to give robust reproducible responses with repeated ACh applications and reflected intrinsic differences in the ACh potency for the mutants (Papke et al., 2010).
The peak amplitude and the net charge (Papke and Porter Papke, 2002) of experimental responses were calculated relative to the average of the first two ACh control responses to normalize the data and compensate for the varying levels of channel expression among the oocytes. PNU-120596 was capable of allowing agonists to induce enormous and sustained responses, which introduced large variance into the calculated net charge response. Therefore, peak responses were compared after using PNU-120596, whereas treatments before PNU-120596 were compared based on the net charge response. In separate experiments (data not shown), it was determined that one of the primary effects of PNU-120596 is to eliminate the normal separation of peak current and net charge concentration-response relationships seen with α7 nAChR (Papke and Porter Papke, 2002). This suggests that a primary effect of PNU-120596 is to reduce the unique agonist concentration-dependent form of desensitization that we hypothesize is promoted by binding of agonist at multiple sites.
Means and S.E.M. values were calculated from the normalized responses of at least four oocytes for each experimental concentration. Individual oocytes were used for no more than one test concentration because SH reagents are potentially able to form covalent bonds with the receptor. Whenever PNU-120596 was used, the cells were discarded afterward; the bath was cleaned with ethanol and flushed with Ringer's buffer for 20 min.
The protocol for study of the reduced Cys190–Cys191 C-loop disulfide was as above, except that after the initial ACh controls, the α7 C116S nAChRs were treated with 1 mM dithiothreitol (DTT) for 60 s (initial flow rate of 2 ml/min for 10 s followed by 0.5 ml/min for 50 s) plus additional minutes, as indicated, via static bath (i.e., stopping the flow of Ringer's washthrough to retain the reagent in the bath). Then, a follow-up ACh test was applied before a 60-s treatment with an SH reagent, followed by ACh, PNU-120596, and another ACh application. All of the responses were normalized to the average of the two initial ACh control applications and compared as described above.
SH reagent labeling kinetics experiments similarly involved incubating receptors for varied periods of reaction time before washout of reagent. The comparative concentration-response data between wild-type and C116S receptors for Br-ACh, MTSET, MTSEA, and QN-SH were collected from 100 μM to 3 mM, using 300 μM ACh as control before and after each application of the reagent. Data were analyzed as described previously (Papke and Porter Papke, 2002).
Molecular Modeling.
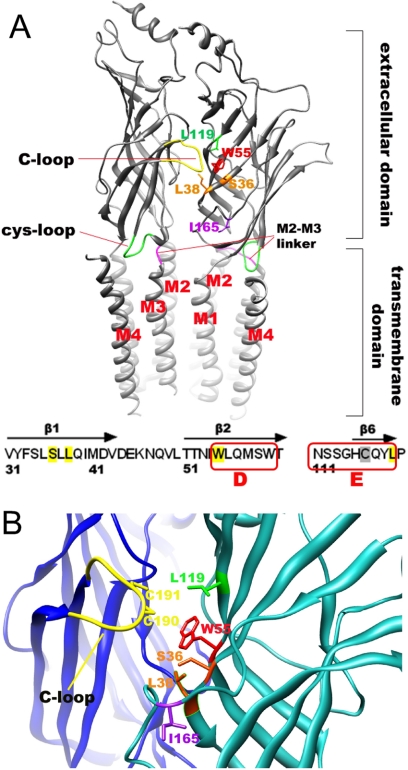
A homology model for the human α7 nAChR was created using the Aplysia californica AChBP structure 2PGZ to select the cysteine mutant candidates (Hansen et al., 2005). A ClustalW (Higgins et al., 1996) alignment of the AChBP and human α7 sequence was generated and submitted to the Swiss Model structure server. The resulting monomeric model was superimposed twice on the A and B chains of the AChBP pentameric crystal structure to generate a dimer model (Fig. 1A). The model was then examined for clashes, which subsequently resolved by variation of side chain rotamer or in combination with constrained minimization using the GROMOS force field resident in the Swiss-PdbViewer (version 4.0; http://spdbv.vital-it.ch/), followed by Amber 10 (http://ambermd.org/) molecular mechanics refinement with the bound 2PGZ ligand (cocaine hydrochloride) included to prevent collapse of the LBD during structural optimization. Docking was performed with the Dock 6.1 program (http://dock.compbio.ucsf.edu/DOCK_6/index.htm) with evaluation of dock scores based on a grid of 0.3-Å spacing.
Fig. 1.
Cysteine mutations in the LBD of α7 nAChR. A, side view of the receptor model as a dimer. The key elements of the α7 receptor were modeled by using the 2BG9 template for the transmembrane domain fused to the ligand binding domain made from 2PGZ template. The plus face C-loop is shown in yellow, the M2 and M3 linker loop is shown in red-pink, the cysteine loop is colored green, and the different residues subjected for mutation are colored variously. All of the cysteine mutants were made in the background of C116S. Free cysteines at Cys190 and Cys191 were generated at the same time by using DTT to reduce the C-loop disulfide bond. The view was made from the outside of the channel pore, and the four transmembrane helixes (M1 to M4) were lined clockwise as shown, putting M2 helix toward the channel pore. The intracellular domain is not shown. B, a close view of the predicted agonist binding site indicating the plus face and minus face of the receptor, colored in blue and light sea green, respectively.
Homology models for MTSET- and MTSEA-labeled cysteine mutants and energy minimization of these structures in AMBER required definition of atoms and force field parameters, which were obtained by computation on model-labeled cysteines. The labeled adduct formed between N-formyl cysteine carboxamide and MTSEA or MTSET was built and its structure minimized, and charges were calculated with MOPAC and AM1 parameters in ANTECHAMBER. Force constants were identified for the structures with the generalized Amber force field, and these values were transferred as required to allow recognition of the labeled residues of the protein model in the xleap routine of Amber 10.
Results
The Location of Cysteine Mutants in the LBD.
To select the cysteine mutants for labeling study, we docked Br-ACh and QN-SH to the wild-type human α7 nAChR dimer. After docking, different poses within 5 kcal/mol difference from the best score were used to predict the potential interacting side chains that could be mutated to cysteine. This set included five on the complementary face: Ser36, Leu38, Trp55, Leu119, and Ile165 (Fig. 1B). We have demonstrated previously the functional competency of receptors with cysteine mutations at these sites and determined that cysteine mutations on the primary face of the LBD are less well tolerated because such mutants were either not well expressed or were poorly responsive to ACh (Papke et al., 2010). The ACh EC50 values for wild-type (C116C), C116S, S36C, L38C, W55C, L119C, and I165C were 27 ± 3 (Horenstein et al., 2008), 29.4 ± 0.7 (Papke et al., 2010), 20 ± 9, 24 ± 5, 180 ± 20, 30 ± 4, and 60 ± 26, μM, respectively.
To evaluate the labeling kinetics for different cysteine mutants, it is important to know the environment of the free cysteine. In general, ionic thiolate (solvent accessible) is a much better nucleophile than nonionic thiolate (solvent exclusive) (Roberts et al., 1986). The environments of the five cysteine mutation sites at positions Ser36, Leu38, Trp55, Leu119, and Ile165 were predicted by evaluating both the solvent-accessible surface and the solvent-exclusive surface in Chimera-1.4 (http://www.cgl.ucsf.edu/chimera/download.html) for the homology model built on the 2PGZ template. Both methods ranked the five amino acids consistently, in an order of Ile165 > Trp55 > Leu119 > Ser36 > Leu38, in regard to relative solvent accessibility.
SH Reagents.
The structures of the six SH reagents used in these experiments are shown in Fig. 2 along with that of the type II α7-selective PAM PNU-120596, which was used as a functional probe of the covalently modified receptors. Two of these agents, MTSET and Br-ACh, are agonist analogs that have been used previously to characterize muscle and/or T. californica-type nAChR (Spura et al., 1999; Stewart et al., 2006). Because quinuclidine is an agonist for neuronal nAChR, we hypothesized that if posed in a favorable orientation in the α7 LBD, QN-SH might also function as tethered agonist once covalently bound to the receptor. We used MTSEA as a fourth cationic SH reagent to test for the potential importance of the positive charge placement in the LBD independent of the other elements of the presumed pharmacophore (Horenstein et al., 2008). The noncationic agents MTSACE and EMTS were used as controls for nonspecific effects of the SH reactions.
Intrinsic Agonist Activity of the Three Agonist Analogs on Cysteine-Null and Wild-Type α7.
As expected, neither MTSEA nor the noncationic SH reagents evoked currents when applied to cells expressing either wild-type α7 or the cysteine-null α7 C116S. The affinity-alkylating agent Br-ACh was a potent and efficacious full agonist for wild-type and C116S α7 (Fig. 3). MTSET, an affinity ligand used previously to study muscle-type and T. californica nAChR subtypes, was a low-potency weak partial agonist for both of the α7 control receptors (Fig. 3). Although quinuclidine is an effective agonist for α7 (Horenstein et al., 2008), QN-SH had no apparent agonist activity above our limits of detection for either wild-type or C116S α7 (data not shown).
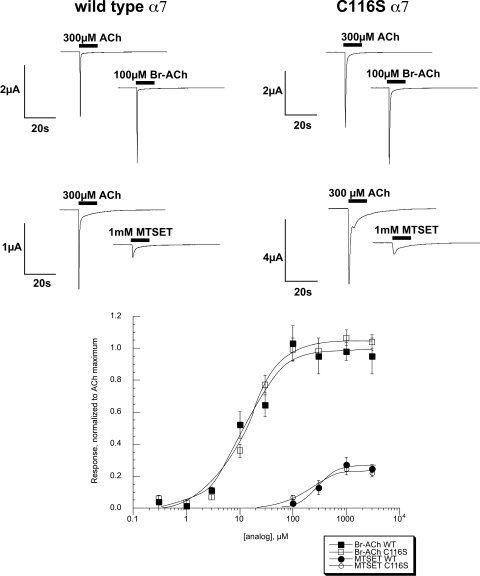
Fig. 3.
Intrinsic agonist activity of SH reagent agonist analogs for wild-type α7 and α7 C116S. Top, representative traces of wild-type α7 and α7 C116S to Br-ACh and MTSET displayed with ACh control responses obtained from the same oocytes. Bottom, concentration-response curves for Br-ACh and MTSET, normalized to the maximal ACh-evoked responses. Each point represents the average response of at least four oocytes (± S.E.M.).
Experimental Design to Characterize the Effects of α7 Receptor Covalent Modifications by SH Reagents.
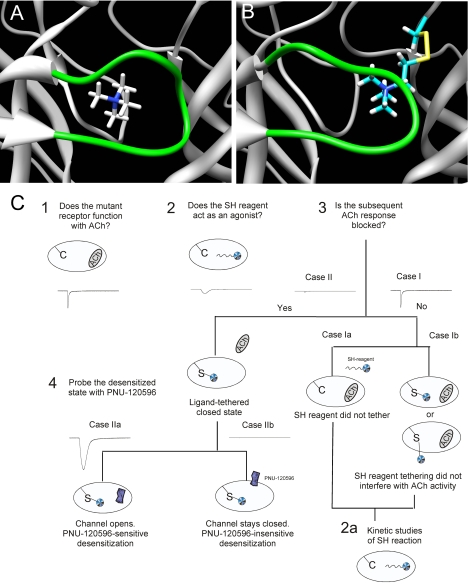
Figure 4A illustrates the docking of TMA in the LBD of α7. TMA is a full agonist of α7, so this pose represents the hypothetical minimal structure able to induce the array of α7 functional states, including the active ion channel state and ligand-bound, nonconducting states commonly associated with “desensitization,” of which one or more can be converted into a conducting state with the application of the PAM PNU-120596. Figure 4B shows the predicted placement of the affinity ligand MTSET covalently bound to a cysteine at the location of Leu119. The model suggests that the agonist analog will be most likely positioned in the same vicinity of the LBD as the docked TMA, but with important differences. Our experimental goal is to determine whether there is functionality to MTSET or alternative agonist analogs tethered in such orientations in regard to ion channel function or the induction and stabilization of functionally relevant nonconducting states.
Fig. 4.
A, the docked pose of TMA in the LBD of α7. B, the predicted placement of the affinity ligand MTSET covalently bound to a cysteine at the location of Leu119. C, experimental design. Schematic of the design and hypotheses related to the possible events in the labeling study with the SH reagents, after the initial characterization of functional cysteine mutants. Case I represents the lack of functional evidence that a covalent bond formed between the cysteine mutant and the SH reagent. This could indicate two possible conditions: case Ia, in which no covalent modification occurred to because of unfavorable reaction conditions; and case Ib, in which the tethered α7 receptor can still accommodate ACh in one or more of the five binding sites, either the same domain where the SH reagent is attached or other free sites. Case II represents the condition in which the SH reagent has become covalently attached, and upon further application of ACh, activation of the ion channel is not observed, because of either SH reagent competitively occupying the agonist binding site or inducing ligand-bound nonconducting (i.e., desensitized) states. Receptors were subsequently challenged with the type II PAM PNU-120596 to determine whether the orientation of the reagent in the binding site was sufficiently agonist-like to induce desensitization that is reversible by PNU-120596 (case IIa) or whether the ligand is functionally indistinguishable from a tethered antagonist (case IIb).
Figure 4C outlines the basic sequence of experiments to be presented in the following sections along with proposed interpretations. After the initial characterization of the cysteine mutants in regard to ACh sensitivity (experiment 1), the mutants were treated with SH reagents (experiment 2). Experiment 2 first served to determine whether the mutants retained the same pharmacological profile as the control receptors (wild-type and C116S α7) in regard to transient activation of the ion channel by the SH reagents. Next, we conducted additional ACh applications (experiment 3) to make an initial determination of whether a covalent modification of the receptors occurred that resulted in perturbation of function. We anticipated two most likely outcomes: that subsequent ACh-evoked responses would remain unchanged (case I), or that they would be reduced, indicative of functionally significant modification of the receptor (case II). Case I represents two possibilities: failure of the SH reagent to react with the receptor (case Ia), or failure of the modification to result in a detectable change in function (case Ib). A third outcome that might also have been anticipated is that receptors would be modified in such a way as to have increased sensitivity to ACh (case III), which is not illustrated in the figure.
An important caveat to results that appeared intermediate between those anticipated for cases I and II is that there might be significant difference in the reaction rates for the various covalent modifications; therefore, in such cases, kinetic studies were also conducted (experiment 2A). Once receptors were determined to be conditionally induced into a covalently modified state that was unresponsive to further activation by ACh, we hypothesized that (case IIb) the tethered ligand might be situated as a simple antagonist, either occluding the access of the LBD to agonist or holding the receptors in a nonactivatible state or (case IIa) that the tethered agonist might function like an agonist that, after a transient phase of activation, stabilizes the receptor in a desensitized conformation that might be reactivated by the type II PAM PNU-120596. Experiment 4, the application of PNU-120596 was conducted to make this determination. Finally, experiment 5, a follow-up application of ACh, probes the PNU-120596 potentiation of this ACh response as affected by the sulfhydryl-reacted receptors.
The Basic Effects of Cysteine Mutagenesis.
In the human α7 nAChR, a single, nondisulfide bonded free cysteine is found at position 116, which, if left intact, could complicate the interpretation of thiol-specific labeling studies when cysteine is introduced elsewhere in the receptor. Therefore the α7 C116S mutant (Papke et al., 2010) was prepared and used as the background for all additional cysteine mutants. The mutants used for these studies were selected from a total of 44 α7 LBD cysteine mutants reported elsewhere (Papke et al., 2010). The cysteine mutations at Ser36, Leu38, Trp55, Leu119, and Ile165 (Fig. 1B) were selected for further study in these experiments based on their strategic placement within the LBD and also because these cysteine mutations had relatively little impact on receptor function before SH modifications. When control applications of ACh were made to oocytes expressing these mutants (experiment 1 in Fig. 4C), all gave robust responses measuring at least 30% the net charge of oocytes injected with the C116S receptor (Papke et al., 2010) at the same time point after injection.
Intrinsic Activity of the SH Reagents as Agonists for Wild-Type and C116S α7 Receptors.
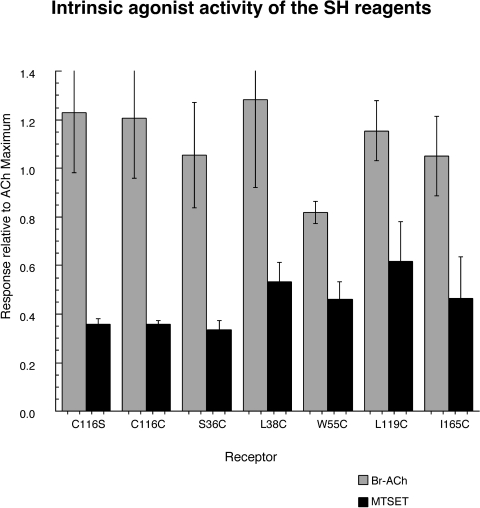
Six SH reagents were used for experimental modification of the cysteine mutants (Fig. 2), four of which we hypothesized would function as agonist analogs. To confirm that hypothesis, they were compared with ACh for their ability to activate wild-type and C116S α7 receptors expressed in oocytes. Our experiments confirmed (Fig. 3, bottom) that Br-ACh is a potent full agonist for α7 control nAChR (EC50 ≈ 12–15 μM), and MTSET was a weak partial agonist (Imax ≈ 26%), with an EC50 values greater than 200 μM for both control constructs (Fig. 3, bottom). The EC50 values for MTSET were 292 ± 62 and 219 ± 104 μM for wild-type (C116C) and C116S, respectively. Although considered a possible agonist analog, QN-SH had no apparent agonist activity above our limits of detection for either wild-type or C116S α7. Likewise, the three other SH reagents (Fig. 2) showed no detectable agonist activity on wild-type or C116S α7 nAChR (data not shown).
Intrinsic Activity of the SH Reagents on the α7 Cysteine Mutants.
The presentation of a SH reagent to a cysteine mutant (experiment 2 in Fig. 4C) is predicted to result in a covalent modification of the receptor, dependent on the accessibility of the specific mutated residue. However, because several of our reagents are agonist analogs, covalent reaction with the receptor might be expected to be preceded by, or coincident to, activation of the receptor. Therefore, we measured current stimulated by the presentation of the SH reagents. Not surprisingly, significant currents were only detected for Br-ACh and MTSET (for representative MTSET responses, see Fig. 5), the two analogs that had the greatest agonist activity with the wild-type and C116S α7 receptors. A summary of evoked responses stimulated by Br-ACh and MTSET is displayed in Fig. 6. Analysis of variance indicated that for all of the receptors tested, Br-ACh was equally as efficacious as ACh and that MTSET was significantly less efficacious than either ACh or Br-ACh (p < 0.001). There were no significant differences in the relative agonist efficacies among the various mutants. It is noteworthy that QN-SH, which showed minimal agonist property with wild-type α7 or any of the mutants tested, did show significant responses during the application to α7 L119C nAChR at 1 mM (21% of the net-charge response relative to the ACh controls; data not shown).
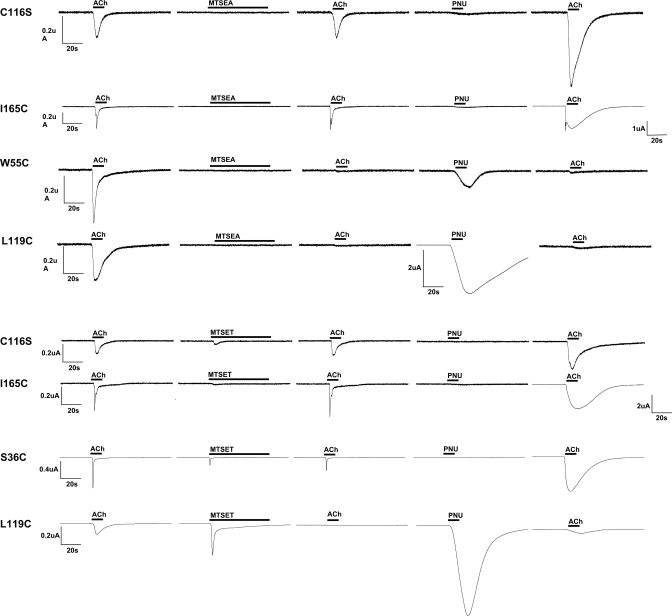
Fig. 5.
Representative traces from voltage-clamp experiments, outlined in Fig. 4C, illustrating the effects of 60-s applications of SH reagents on C116S and cysteine mutant α7 receptors and the subsequent effects of PNU-120596's potentiation. The results are displayed in the order of experiment protocol from left to the right with total time of 5 min between each step: ACh (12 s), SH reagent (1 mM MTSET or MTSEA, as indicated, for 60 s), ACh (12 s), PNU-120596 (300 μM for 12 s), and ACh (12 s). Because some mutants showed reduced sensitivity to ACh, the concentration of ACh applications was 60 μM for C116S and L119C α7 mutants, and 300 μM for α7 S36C, W55C, and I165C. The drug applications are shown above the response curve as boldface black lines. Because of the magnitude of PNU-120596 effects, the ACh-evoked responses of I165C after PNU-120596 application are shown at a less sensitive current scale, and likewise for the PNU-120596-evoked response of α7 L119C after MTSEA. Note that although a 60-s application of MTSET produced a significant reduction in the subsequent ACh-evoked response of the S36C mutant, longer applications were required to produce a maximal effect (Fig. 7).
Fig. 6.
Activation profiles of Br-ACh and MTSET on wild-type and mutant human α7 nAChRs. The responses shown in the graph are net-charge responses obtained during the application of 1 mM Br-ACh or MTSET, normalized to the ACh maximal response determined previously (Papke et al., 2010). Data represent the average responses of at least four oocytes (± S.E.M.).
ACh-Mediated Responses Subsequent to the Application of SH Reagents.
The ACh-evoked responses of C116S and I165C α7 receptors were unchanged relative to their initial ACh control responses by the application of any of the SH reagents (Fig. 5). Given the lack of free cysteines, it is unlikely that there was any covalent modification of α7 C116S, and if there were any reactions with either the wild-type (C116C; data not shown) or the I165C α7 receptors (Fig. 5), they seemed to be functionally neutral, at least in regard to ACh activation (Fig. 4C case I). In contrast, there were functional aftereffects identified in the other four receptors (W55C, L119C, S36C, and L38C) that contained mutations in the complementary face of the LBD. Representative traces are shown in Fig. 5, and a summary of the results is shown in Fig. 7.
Fig. 7.
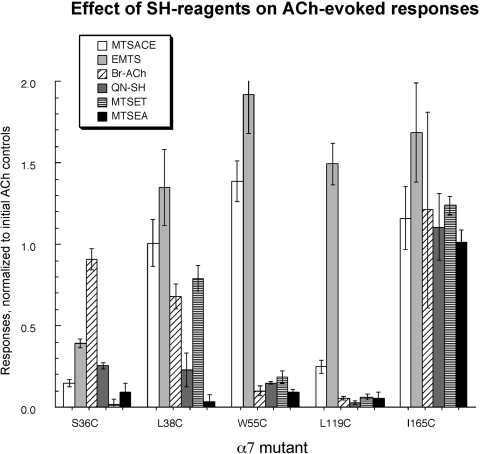
ACh responses after SH reagent application. In initial experiments, the oocytes were treated with the SH reagents at 1 mM for 60 s and washed with Ringer's buffer for 3 min before the application of ACh. This protocol produced only partial effects for Br-ACh and for MTSET on subtypes other than α7 L119C (see Fig. 5). Therefore, to compensate for the relatively slow reaction rates of Br-ACh and MTSET (on receptors other than L119C), the applications of these SH reagents were extended to 5 min, which was sufficient to produce optimal effects. The net charge responses of the post-SH reagent ACh-evoked responses were normalized to the average of the two pre-SH reagent ACh controls. A response of 1.0 represents no detectable change made by SH reagents (reflecting case I in Fig. 4C), whereas a response of 0 represents complete blocking of subsequent ACh activation by covalently attached SH reagents (reflecting case II in Fig. 4C).
As shown in Fig. 7, not all of the reagents were equally effective at inhibiting the ACh-evoked responses of the cysteine mutants. Indeed, each mutant had a distinct profile for the six reagents. Reaction of the free cysteine at the Ser36 position produced decreases in the ACh response with all of the five methanethiosulfonate SH reagents but not the alternative reactive agonist analog Br-ACh. However, treatments of α7 S36C with MTSACE, EMTS, QN-SH, and MTSEA produced only partial inhibition of α7 S36C ACh-evoked responses, whereas after MTSET treatment, no significant ACh responses could be detected. The α7 L38C mutant was very sensitive to MTSEA and showed ≈77% inhibition of ACh-evoked responses after QN-SH. Only the positively charged SH reagents and Br-ACh were able to inhibit the α7 W55C mutant.
As reported previously (Papke et al., 2010), the ACh-evoked responses of α7 L119C were very efficiently blocked by all of the cationic SH reagent treatments. After 60-s treatments with QN-SH, MTSET, or MTSEA, ACh-evoked responses decreased essentially to 0. A 60-s MTSACE application also blocked 75% of the α7 L119C ACh-evoked response. The α7 L119C mutant was also sensitive to Br-ACh; however, a 3-min application was required for the complete blocking effect (data not shown). It is noteworthy that the ACh response of the EMTS-treated α7 L119C receptor seemed enhanced relative to the response of that mutant to ACh before EMTS.
Although MTSEA and QN-SH produced maximal effects with just 60-s treatments, the agonist-like reagents Br-ACh and MTSET required treatments of up to 5 min for maximal effects on subsequent ACh responses (experiment 3a in Fig. 4C), and even after 5-min treatment with MTSET, L38C remained responsive to subsequent applications.
As shown in Fig. 7, the L119C and W55C α7 mutants were similar in their sensitivity to Br-ACh block of ACh-evoked responses. A full 3-min incubation was required to achieve this effect. However, there were no significant effects of Br-ACh applications on the ACh-evoked responses of S36C or L38C α7 mutants, even with Br-ACh applications of up to 7 min.
Activation of SH-Modified α7 Mutants by PNU-120596.
The type II PAM PNU-120596 does not produce significant activation of wild-type or C116S α7 receptors when applied alone (Table 1, Fig. 5). However, when ACh or other agonists are coapplied with PNU-120596 or applied to receptors primed previously with PNU-120596 (Papke et al., 2009), agonist-evoked responses are substantially increased (Table 1 and Fig. 5). The α7 W55C, I165C, and S36C mutants showed no significant response to applications of 300 μM PNU-120596 alone in the absence of agonist or before treatments with SH reagents. After the application of SH reagents to wild-type, C116S, or I165C α7 receptors, there were no responses to subsequent applications of PNU-120596 in the absence of agonist (representative traces in Fig. 5). It is noteworthy that the α7 L38C and L119C mutants, as well as C116S receptors previously treated with DTT, did show small but significant responses to subsequent applications of PNU-120596 alone (Table 1). Nonetheless, PNU-120596 was still a very effective potentiator of these receptors. Applications of 60 μM ACh after priming with 300 μM PNU-120596 produced responses that were 9- to 31-fold larger than the responses measured during the prior application of ACh alone (Table 1).
TABLE 1.
PNU-120596 effects: responses to PNU-120596 applied alone, and subsequent potentiation of ACh-evoked responses
To conduct these experiments, cells were typically used with 24 h of injection, when the control responses to 60 μM were very low, otherwise the potentiated responses became too large to maintain voltage clamp. Under these conditions, PNU-120596 applications produced small stimulus artifact baseline deflections which, when normalized to small initial controls, gave values for peak deflections of <10%, which we take as our limit for detection. Values are peak currents relative to the peak currents evoked by 60 μM ACh before the application of 300 μM PNU-120596.
| Receptor | Response to PNU-120596 Applied Alone | ACh Response after Application of PNU-120596 |
|---|---|---|
| % | ||
| α7 | N.S. | 3000 ± 40 |
| C116S | N.S. | 2000 ± 500 |
| 1165C | N.S. | 1250 ± 240 |
| S36C | N.S. | 1200 ± 450 |
| L38C | 35 ± 9% | 910 ± 63 |
| W55C | N.S. | 1200 ± 40 |
| L119C | 54 ± 4% | 3100 ± 460 |
| C116SDTTa | 26 ± 16% | 1700 ± 500 |
N.S., no significant response.
These are the data for α7C116S receptors after a 60-s treatment with 1 mM DTT intended to reduce the vicinal disulfide in the C-loop.
In addition to priming and/or potentiating agonist-evoked responses, PNU-120596 can be used to convert previously desensitized receptors into a conducting state (experiment 4, Fig. 4C). Therefore, we hypothesized that if the treatment of the cysteine mutant α7 receptors with our SH reagents inhibited subsequent ACh-evoked responses because the receptors were being locked into a PNU-120596-sensitive form of desensitization, then they should be directly activatable by PNU-120596 after SH treatments. We hypothesized that this activity would depend on both the agonist character of the reagent and the specific orientation of the covalently bound reagent in the LBD. Although such effects as seen with W55C and L119C (Fig. 5) most likely are due to exclusion of ACh from the covalently modified agonist binding sites, they might alternatively indicate the conversion of receptors into PNU-120596-insensitive nonconducting states.
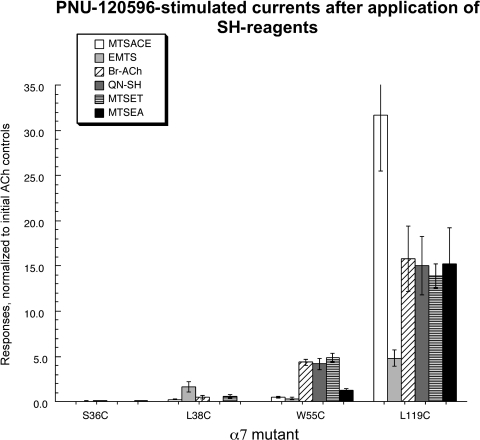
The PNU-120596 response profiles of the different mutants after the SH reagent treatments are shown in Fig. 8, and some representative traces are shown in Fig. 5. The S36C and L38C mutants had no apparent PNU-120596-evoked responses after application of any of the cationic SH reagents. L38C yielded a small PNU-120596-evoked response after treatment with EMTS; however, this PNU response was intrinsic to L38C (Table 1) and did not depend on reaction with an SH reagent. In contrast, W55C showed greatly enhanced responses to applications of PNU-120596 alone after treatment with Br-ACh, QN-SH, and MTSET but no PNU-120596 responses at all after treatment with MTSACE or EMTS. These results suggest that once reacted with the agonist analogs, the α7 W55C receptors were at least partially converted into a PNU-120596-sensitive desensitized state. Consistent with the previous observation that reactions with either MTSACE or EMTS failed to block the subsequent ACh-evoked responses of α7 W55C, these agents clearly did not induce PNU-120596-sensitive desensitization either. It is noteworthy that PNU-120596 did reactivate the MTSEA-treated α7 W55C but less than it did after the true agonist analogs (Fig. 8). This observation may suggest that the core pharmacophore for PNU-120596 reactivation may be simpler than the core agonist structure for activation of the unmodified wild-type receptors.
Fig. 8.
Responses to the type II PAM PNU-120596 after SH reagent treatment. After SH reagent and ACh treatments, 300 μM PNU-120596 was applied for 12 s (see Fig. 5 for sample traces). Because of the prolonged duration of responses consequent and subsequent to the application of PNU-120596 (beyond our normal window for net-charge measurements), data for these experiments are presented as peak current amplitudes, normalized to the peak currents of the initial controls. The treatment of α7 W55C and α7 L119C with some of the SH reagents induced the PNU-120596-sensitive desensitized state (case IIa in Fig. 4C), whereas the same treatments of α7 S36C and α7 L38C gave results as shown for case IIb in Fig. 4C.
To varying degrees, application of PNU-120596 stimulated responses from α7 L119C receptors after treatments with each of the SH reagents. Note that the PNU-120596-evoked response after EMTS treatment was relatively small, suggesting that this reagent was ineffective at converting receptors to the PNU-120596-sensitive desensitized state. This observation provides further insight into what may be the core pharmacophore for PNU-120596 reactivation, suggesting the requirement for only a charged nitrogen or perhaps other cationic center.
PNU-120596 Potentiation of ACh Responses in SH-Reacted Receptors.
Furthermore, although activation by PNU-120596 alone after SH treatments provides a probe for induced desensitization, further applications of ACh to PNU-120596-primed SH-reacted receptors could also indicate forms of receptor blockade through decreases in the ACh potentiation. To compare the effects of SH reagent modifications of the cysteine mutants on ACh responses after treatment with PNU-120596, we made additional ACh applications after the SH reagent and PNU-120596 applications. Responses were compared with baseline ACh-evoked responses, and this value was compared with the PNU-120596 potentiation observed in the absence of SH reactions (Table 1). Some representative traces are shown in Fig. 5, and the results are summarized in Tables 2 and 3. The α7 S36C receptors showed largely diminished post-PNU-120596 ACh peak current values, except after Br-ACh. The post-PNU-120596 ACh peak responses of α7 L38C were most strongly affected by QN-SH and MTSEA and were totally unaffected by EMTS treatment. The PNU-120596-potentiated ACh responses of α7 W55C were most strongly affected by QN-SH and MTSEA, whereas MTSET, Br-ACh, and MTSACE had intermediate effects. EMTS, which seemed to potentiate α7 W55C and L119C ACh-evoked responses (Fig. 7), also increased the PNU-120596-potentiated ACh currents of L119C, suggesting that EMTS, once reacted with L119C, may itself be an allosteric potentiator, possibly working in an additive or synergistic manner with PNU-120596.
TABLE 2.
Effects of SH reagents on PNU-120596-primed ACh-evoked responses
Data are expressed relative to initial ACh control responses. The durations of the SH reagent reactions were those required to give maximal effects in the absence of PNU-120596 treatments.
| Receptor | MTSACE | EMTS | Br-ACh | QN-SH | MTSET | MTSEA |
|---|---|---|---|---|---|---|
| % | ||||||
| S36C | 40 ± 20 | 210 ± 40 | 810 ± 210 | 130 ± 40 | 23 ± 5 | 8 ± 1 |
| L38C | 450 ± 180 | 990 ± 200 | 690 ± 110 | 58 ± 14 | 630 ± 21 | 30 ± 16 |
| W55C | 460 ± 70 | 1760 ± 40 | 220 ± 40 | 56 ± 12 | 440 ± 140 | 7 ± 1 |
| L119C | 160 ± 100 | 2140 ± 260 | 760 ± 340 | 21 ± 10 | 81 ± 10 | 71 ± 52 |
TABLE 3.
The effects of SH reagents on the relative PNU-120596 potentiation of ACh responses
Values are the ratios of PNU-120596 potentiation in Table 1 (before SH treatments) and 1B (after SH treatments).
| Receptor | MTSACE | EMTS | Br-ACh | QN-SH | MTSET | MTSEA |
|---|---|---|---|---|---|---|
| S36C | 0.033 | 0.175 | 0.675 | 0.108 | 0.019 | 0.007 |
| L38C | 0.495 | 1.09 | 0.758 | 0.063 | 0.692 | 0.033 |
| W55C | 0.383 | 1.467 | 0.183 | 0.046 | 0.367 | 0.006 |
| L119C | 0.052 | 0.690 | 0.246 | 0.007 | 0.026 | 0.023 |
The PNU-120596 potentiation of α7 L119C ACh responses was strongly reduced by all of the reagents except for EMTS and Br-ACh, which produced only 30 and 75% reductions, respectively. In general, all of the SH reagent effects on the PNU-120596-potentiated ACh responses were consistent with the inhibition of the control ACh-evoked responses, as shown in Fig. 7. One striking disparity was seen in the effects of MTSACE on α7 W55C. Whereas MTSACE did not block the control ACh-evoked responses, it did decrease the PNU-120596 potentiation. In addition, for the W55C α7 receptor, both MTSET and MTSEA reduced the ACh response (Fig. 7), but after MTSET, PNU-120596 greatly potentiated the ACh response, and after MTSEA, it greatly inhibited it (Tables 1–3).
The Modification of the Reduced C-Loop and Potentiation of PNU-120596.
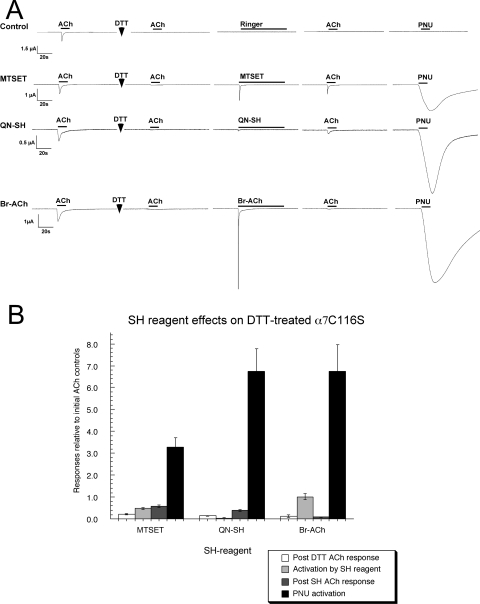
To study the potential effects of SH modifications to the C-loop, a key structure in the LBD (Fig. 1), we attempted to reduce the vicinal disulfide at positions 190 and 191 in the α7 C116S receptor by treatment with 1 mM DTT. We found that when exposed to DTT for increasing periods of time, the receptors became progressively less responsive to activation by ACh, barely showing any response after 5 min of DTT treatment. It is noteworthy that even when the receptors were no longer able to be activated by ACh, the reduced receptors could be activated by Br-ACh or MTSET (Fig. 9). We then conducted a series of ACh and PNU-120596 applications to determine whether the agonist analogs showed evidence of covalently modifying the receptors and inducing PNU-120596-sensitive desensitization.
Fig. 9.
The modification of and PNU-120596's potentiation on α7 C116S after DTT treatment. A, representative traces illustrating the strong agonist activity of MTSET, QN-SH, and Br-ACh for 5-min DTT-treated α7 C116S, and the induction of a PNU-120596-sensitive desensitization. B, the SH reagent and PNU-120596 activation of DTT-treated α7 C116S relative to the average of two ACh controls before DTT treatment. The data represent the average responses of at least four oocytes (± S.E.M.).
After the treatment of the reduced receptors with MTSET or QN-SH, there was a small recovery of sensitivity to ACh, whereas after Br-ACh, the receptors remained unresponsive to ACh (Fig. 9A). However, treatment of the reduced receptors with each of the agonist analogs seemed to very effectively induce the PNU-120596-sensitive desensitized state, because large currents were stimulated by the application of PNU-120596 alone (Fig. 9B).
Modeling Modifications of the Agonist and the Binding Site.
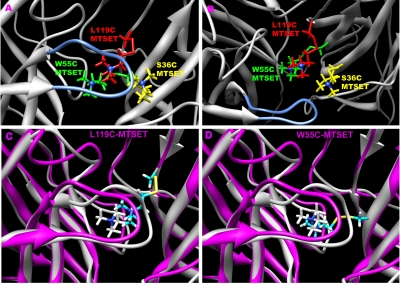
We modeled the potential effects of bound SH reagents by introducing non-natural amino acids into the α7 homology model that would be equivalent to the covalently modified amino acid residues of the receptor (Fig. 10, A and B). For example, after MTSET reacted with the free cysteine in the LBD, a trimethylamino ethylthio group was attached to the sulfur of the cysteine mutant. After molecular mechanics minimization, the structures of the MTSET-modified mutants (S36C, W55C, and L119C) were superimposed. The side view of the receptor (Fig. 10A) shows that MTSET-labeled 36C is farther away from the C-loop, whereas MTSET-55C and MTSET-119C place the ammonium pharmacophore further underneath the C-loop. MTSET-labeled 36C can also place the ammonium group of the label proximate to Glu189, possibly excluding the ammonium group from the LBD by electrostatic attraction.
Fig. 10.
Homology models of α7 covalently modified by the SH-reagents. A and B, A superposition of three labeled receptor models: S36C (yellow), W55C (green), and L119C (red) after covalent reaction with MTSET. The structures were optimized with Amber 10. A, side view from outside the channel pore; B, top view facing the ion channel pore from the extracellular domain. The C-loop is shown in blue, whereas the ammonium of MTSET is shown as a dark blue ball in both. C and D, comparisons of modified receptor to a putative ligand-bound complex. C, the overlay of the α7-TMA complex (white) with the L119C-MTSET (magenta) label. D, the overlay of the α7-TMA complex (white) with the W55C-MTSET (magenta) label.
Discussion
It is unclear how structurally different agonists bind to nAChRs and initiate the channel gating or, alternatively, ion channel desensitization. We traditionally associate the efficacy of specific agents with their relative effectiveness for inducing the transitions of the receptors among multiple conformational states subsequent to binding in a single preferred conformation. However, we know from crystallography of the ACh-binding protein that specific ligands will stabilize these ligand binding domain analogs in distinct low-energy conformations (Celie et al., 2004), which in native receptors are likely to be associated with different conformational states. However, for low Popen receptors such as α7, the lifetime of the open channel conformation is far too brief to expect that particular ligand-bound conformational state to be stable in a low-energy structure. Because of the transient low-probability nature of the open state in an α7 receptor that has not been primed by an allosteric modulator such as PNU-120596, it is reasonable to hypothesize that the ligand-bound state that precedes ion channel opening is itself not stable and will be conformationally distinct from the more stable bound states that are most likely to induce desensitization.
In our studies, we used SH-reactive agonist analogs and the PAM PNU-120596 to probe the α7 LBD for the functional consequences of tethering agonist-like molecules in specific orientations. Our results support the hypothesis that a single ligand can bind within the receptor in different ways and, depending on the specific binding pose, may variously promote activation or desensitization, or, alternatively, function as a competitive antagonist.
One limitation to the use of ACh responsiveness as a sole reporter of covalent modification is that it provides little or no insight into the underlying basis for the loss of function, for example, whether the binding site is simply occluded or whether the receptor is being held in specific nonfunctional states, such as those associated with desensitization. This distinction could be of particular importance for examining the effects of SH reagents that may be agonist analogs. Therefore, we used the type II PAM PNU-120596 as an additional probe for conformational effects after SH modification.
With regard to basic block of ACh responsiveness, each mutant tested had a distinct profile for the six reagents. In the case of I165C, either the cysteine was inaccessible or, more likely, modification at this site was neutral for ACh and PNU activation. We found that MTSET was slow to react with α7 S36C, and the more efficacious agonist Br-ACh did not seem to react at all with this mutant. This slowness or failure to react might have been indicative of a dependence of the labeling reaction on the receptor state or a favored binding pose that was not optimal for the covalent reaction. There were also differences among the reagents for effects on α7 L38C. This mutant seemed to be only sensitive to QN-SH and MTSEA, reagents with little or no agonist activity for receptors in their native state.
It was interesting to note that only cationic reagents seemed able to react with α7 W55C in such a way as to decrease ACh responses. All three of the putative agonist analogs were able to partially stabilize this mutant in the PNU-120596-sensitive state, even QN-SH, which had no detectable agonist activity under control conditions. One striking anomaly was seen in the effects of MTSACE on α7 W55C. Curiously, although MTSACE seemed to enhance the control ACh-evoked responses on the α7 W55C mutant, it did decrease the PNU potentiation. This suggests that MTSACE was at least partially reactive with α7 W55C and perhaps shifted the probability of receptor activation or increased the stability of a PNU-120596-insensitive desensitized state.
As reported previously, the α7 L119C mutant was very sensitive to blockade of ACh-evoked responses by a variety of SH reagents (Papke et al., 2010). However, this mutant also seemed to be more readily activated by weak partial agonists. Based simply on a lack of ACh-blockade, α7 L119C seemed insensitive to EMTS. This agent, which was partially effective on α7 S36C, showed no apparent effect on any of the rest of the mutants. Although EMTS treatment did not seem to inhibit the ACh-evoked responses of α7 L119C, it did seem to increase responses to PNU-120596 alone from 54% ACh peak (Table 1) to approximately 500% ACh peak. This suggests that this agent did at least partially react and increased the reactivity of the mutant receptor to both ACh and PNU-120596. Moreover, EMTS may itself be an allosteric modulator reacting in a manner distinct from that of PNU-120596.
After reduction by DTT, the C-loop vicinal cysteines became potential targets for SH reagents, as demonstrated by the effects of MTSET and Br-ACh. Before DTT treatment, these agents did not have evoked responses larger than the initial ACh controls, but after reduction of the vicinal cysteines, the channels activated strongly during the process of covalent modification. Successful modification was further confirmed by the large subsequent PNU-120596-evoked responses.
Our results show that a single agent, such as MTSET, covalently bound at different sites, can either convert the receptors to the PNU-120596-sensitive desensitized state, as in α7 W55C or α7 L119C, or may simply block ACh activation, as in α7 S36C and α7 L38C. The block of ACh-evoked responses for α7 S36C and α7 L38C, when observed, was consistent with simple occlusion of the binding site or the induction of PNU-120596-insensitive desensitization (Grønlien et al., 2007).
For α7 W55C, and especially α7 L119C, there is good evidence for the induction of the PNU-120596-sensitive desensitized state, but it is unclear how complete that conversion was for the two mutants. Are the differences in the PNU-120596 responses, which were larger for α7 L119C than for α7 W55C, indicative of more incomplete conversion for α7 W55C or just a higher P-open for the modified α7 L119C? The results suggest the possibility that there may be multiple forms of PNU-120596-sensitive and insensitive desensitization, based on similar activity with multiple agents and effective reactions at α7 W55C, α7 L119C, and the reduced vicinal cysteines.
The covalent reactions of MTSET with either W55C or L119C are predicted to put the agonist-like portion of MTSET close to but still at some distance from the predicted preferred position of the minimal agonist TMA in the LBD (Fig. 10, C and D). Notice that the predictions for the two complexes are nonidentical, although both are associated with similar functional results. Whereas the L119C complex puts the MTSET ammonium against the lip of the C-loop, the W55C-MTSET ammonium sits almost perfectly where the TMA ammonium sits. These may both represent intermediate states that relax to an equivalent PNU-120596-sensitive state. Alternatively, both of these intermediate states may, themselves, be stable in the absence of PNU-120596, but, as suggested by the data in Fig. 8, the L119C-MTSET intermediate state is more readily converted into a stable conducting state.
It is reasonable that an agonist-like molecule covalently bound within the LBD of the nicotinic α7 receptors could have the effect of stabilizing some of the same ligand-bound nonconducting (i.e., desensitized) states that predominate the state function of the native receptor in the prolonged presence of agonist. Our most basic concept of ligand-gated ion channel function is that the receptors have evolved to react rapidly and transiently to an activating signal and then are prevented from excessive activation by the absorbing character of the desensitized states. However, although this model fits our perception of synaptic ion channels, it is unclear that it is applicable to receptors such as α7 nAChR, which perform important functions in non-neuronal cells, suggested in some cases to not require ion channel activation (de Jonge and Ulloa, 2007; van Maanen et al., 2009). It has also been shown that some forms of α7-mediated cytoprotection require long periods of treatment with concentrations of agonist lower than the threshold concentrations required for transient activation of the ion channel (Li et al., 1999). In the same cells, strong transient activation of the α7 ion channel is cytotoxic within seconds (Li et al., 1999). These alternative modes of signaling suggest that stable, ligand-bound, nonconducting states, such as those revealed by the effects of PNU-120596, may represent additional functional states for the α7 receptor.
In conclusion, our results support the hypothesis that a single ligand may adopt multiple binding poses within the ligand binding pocket and that the topology of resulting functional and nonfunctional states depends on both the ligand and the way in which it binds. The nonstationary behavior of a ligand-gated ion channel after a jump in agonist concentration may therefore be due to the relative stability of various binding poses and not the relative probability of specific conformational transitions arising from a single binding mode. Our data suggest that a new approach for drug development can be implemented, taking advantage of developing structure models and targeting ligands with more comprehensive consideration for where they bind, how they bind, and most importantly what is likely to happen next.
Acknowledgments
We thank Lynda Cortes, Sara Braley, and Shehd Abdullah Abbas Al Rubaiy for technical assistance.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM057481].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.066662.
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- LBD
- ligand-binding domain
- PAM
- positive allosteric modulator
- PNU-120596
- N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
- TMA
- tetramethylammonium
- Br-ACh
- bromoacetylcholine
- MTSET
- methanethiosulfonate-ethyltrimethylammonium
- MTSEA
- (2-aminoethyl)methanethiosulfonate
- QN-SH
- 2-(quinuclidinium)ethyl methanethiosulfonate
- EMTS
- ethyl methanethiosulfonate
- MTSACE
- 2-(aminocarbonyl)ethyl methanethiosulfonate
- DTT
- 1,4-dithiothreitol
- SH
- sulfhydryl-reactive
- AChBP
- acetylcholine binding protein
- MS222
- 3-aminobenzoic acid ethyl ester
- DTT
- dithiothreitol.
References
- Barron SC, McLaughlin JT, See JA, Richards VL, Rosenberg RL. (2009) An allosteric modulator of alpha7 nicotinic receptors, N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol Pharmacol 76:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, Gopalakrishnan M. (2008) Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2–M3 segment. Mol Pharmacol 74:1407–1416 [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276 [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41:907–914 [DOI] [PubMed] [Google Scholar]
- Clarke PB, Hamill GS, Nadi NS, Jacobowitz DM, Pert A. (1986) 3H-nicotine- and 125I-alpha-bungarotoxin-labeled nicotinic receptors in the interpeduncular nucleus of rats. II. Effects of habenular deafferentation. J Comp Neurol 251:407–413 [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring LC, Rogers SW. (2005) Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J 7:E885–E894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. (2007) Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock 28:700–703 [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74:1102–1111 [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. (2007) Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724 [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y.(2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24:3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DG, Thompson JD, Gibson TJ. (1996) Using CLUSTAL for multiple sequence alignments. Methods Enzymol 266:383–402 [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL. (2008) Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol 74:1496–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. (1998) Substituted-cysteine accessibility method. Methods Enzymol 293:123–145 [DOI] [PubMed] [Google Scholar]
- Li Y, Papke RL, He YJ, Millard WJ, Meyer EM. (1999) Characterization of the neuroprotective and toxic effects of α7 nicotinic receptor activation in PC12 cells. Brain Res 830:218–225 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955 [DOI] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. (2009) Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329:791–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, Uteshev VV. (2000) alpha7 receptor-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol 393:179–195 [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Williams DK, Wang J, Horenstein NA. (2010) Cysteine accessibility analysis of the human alpha7 nicotinic acetylcholine receptor ligand binding domain identifies L119 as a gatekeeper. J Neuropharmacol doi: 10.1016/j.neuropharm.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35:1139–1144 [DOI] [PubMed] [Google Scholar]
- Roberts DD, Lewis SD, Ballou DP, Olson ST, Shafer JA. (1986) Reactivity of small thiolate anions and cysteine-25 in papain toward methyl methanethiosulfonate. Biochemistry 25:5595–5601 [DOI] [PubMed] [Google Scholar]
- Sixma TK, Smit AB. (2003) Acetylcholine binding protein (AChBP): a secreted glial protein that provides a high-resolution model for the extracellular domain of pentameric ligand-gated ion channels. Annu Rev Biophys Biomol Struct 21:21. [DOI] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. (2001) A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411:261–268 [DOI] [PubMed] [Google Scholar]
- Spura A, Russin TS, Freedman ND, Grant M, McLaughlin JT, Hawrot E. (1999) Probing the agonist domain of the nicotinic acetylcholine receptor by cysteine scanning mutagenesis reveals residues in proximity to the alpha-bungarotoxin binding site. Biochemistry 38:4912–4921 [DOI] [PubMed] [Google Scholar]
- Stewart DS, Chiara DC, Cohen JB. (2006) Mapping the structural requirements for nicotinic acetylcholine receptor activation by using tethered alkyltrimethylammonium agonists and antagonists. Biochemistry 45:10641–10653 [DOI] [PubMed] [Google Scholar]
- Sullivan D, Chiara DC, Cohen JB. (2002) Mapping the agonist binding site of the nicotinic acetylcholine receptor by cysteine scanning mutagenesis: antagonist footprint and secondary structure prediction. Mol Pharmacol 61:463–472 [DOI] [PubMed] [Google Scholar]
- Svensson AL, Nordberg A. (1999) Beta-estradiol attenuate amyloid beta-peptide toxicity via nicotinic receptors. Neuroreport 10:3485–3489 [DOI] [PubMed] [Google Scholar]
- Unwin N, Miyazawa A, Li J, Fujiyoshi Y. (2002) Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J Mol Biol 319:1165–1176 [DOI] [PubMed] [Google Scholar]
- van Maanen MA, Papke RL, Koepke J, Bevaart L, Clark R, Lamppu D, Vervoordeldonk MJ, LaRosa GJ, Tak PP. (2009) Therapeutic Effect of Stimulating the Nicotinic Acetylcholine Receptor in the Collagen-Induced Model of Rheumatoid Arthritis: a Role for Ion Channel Activity and Penetration of the Central Nervous System. Thesis, pp 77–97, University of Amsterdam, Amsterdam, Netherlands [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388 [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. (2004) Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology 29:891–900 [DOI] [PubMed] [Google Scholar]