Abstract
Studies in vertebrate neuromuscular synapses have revealed previously that ATP, via P2Y receptors, plays a critical role in regulating postsynaptic gene expressions. An equivalent regulatory role of ATP and its P2Y receptors would not necessarily be expected for the very different situation of the brain synapses, but we provide evidence here for a brain version of that role. In cultured cortical neurons, the expression of P2Y1 receptors increased sharply during neuronal differentiation. Those receptors were found mainly colocalized with the postsynaptic scaffold postsynaptic density protein 95 (PSD-95). This arises through a direct interaction of a PDZ domain of PSD-95 with the C-terminal PDZ-binding motif, D-T-S-L of the P2Y1 receptor, confirmed by the full suppression of the colocalization upon mutation of two amino acids therein. This interaction is effective in recruiting PSD-95 to the membrane. Specific activation of P2Y1 (G-protein-coupled) receptors induced the elevation of intracellular Ca2+ and activation of a mitogen-activated protein kinase/Raf-1 signaling cascade. This led to distinct up-regulation of the genes encoding acetylcholinesterase (AChET variant), choline acetyltransferase, and the N-methyl-d-aspartate receptor subunit NR2A. This was confirmed, in the example of AChE, to arise from P2Y1-dependent stimulation of a human ACHE gene promoter. That involved activation of the transcription factor Elk-1; mutagenesis of the ACHE promoter revealed that Elk-1 binding at its specific responsive elements in that promoter was induced by P2Y1 receptor activation. The combined findings reveal that ATP, via its P2Y1 receptor, can act trophically in brain neurons to regulate the gene expression of direct effectors of synaptic transmission.
Introduction
ATP is both a transmitter and a modulator of signaling in the nervous system (Abbracchio et al., 2006; Filippov et al., 2006; Hussl and Boehm, 2006; Neary and Zimmermann, 2009). In the brain, ATP is present not only at millimolar levels in the cytosol of all cells but is also at significant concentrations in the extracellular space around the neurons. Thus, glial cells have been shown to constantly release ATP, which can modulate local neuronal activity (as discussed below), whereas from neurons, ATP is well known to be coreleased frequently with other neurotransmitters at central and peripheral synapses. Neuronal actions of ATP in the central nervous system (CNS), both presynaptically and postsynaptically, have often been studied, but as short-term effects in exciting P2X ion channels or in modulating neurotransmission via the P2Y family of G protein-coupled receptors (GPCRs) for nucleotides (Bowser and Khakh, 2004; Hussl and Boehm, 2006; Lee et al., 2007). A few long-term effects of extracellular nucleotides in the CNS have also been observed (Liu et al., 2008), but analysis of the molecular mechanisms of those P2Y receptor actions has begun so far only on the astrocyte contribution (Neary and Zimmermann, 2009), not on the neurons and synapses. However, in the peripheral nervous system, as in vertebrate skeletal neuromuscular junctions, we have previously found such a long-term action of ATP or UTP via their P2Y receptors there, namely in regulating and maintaining synaptic maturation. Thus, ATP acts as a trophic factor to induce and maintain the expression of genes for the postsynaptic nicotinic acetylcholine receptors and acetylcholinesterase (AChE): P2Y1 and P2Y2 nucleotide receptors are localized in the synaptic region, and both subtypes act to trigger that action (Choi et al., 2001a, 2003; Tung et al., 2004). The pathway to activation of the ACHE gene, mediated by the P2Y1 receptor (P2Y1R), was shown to involve protein kinase C, intracellular Ca2+ release, and phosphorylation of extracellular signal-regulated kinases (ERKs) (Choi et al., 2003). In developing muscle, this culminates in activation of the transcription factor Elk-1, which acts on the promoter of the ACHE gene. For CNS neuronal synapses, the P2Y1R has been shown to be, exceptionally in the P2Y family, widely expressed on brain neurons, as seen by strong P2Y1R mRNA expression there (Webb et al., 1998; Moore et al., 2001; Rodrigues et al., 2005) and authenticated P2Y1R protein and functional responses there as cited in Results. However, a role of any of this receptor family in the regulation of the gene expression of proteins involved directly in CNS synaptic transmission (effectors) has not been investigated previously.
Proceeding from those results on the neuromuscular synapse, here we study the rodent cerebral cortex for potential long-term actions of P2Y1R. We find that in neurons from the developing cortex, the P2Y1R subtype is, indeed, significantly expressed and functional. Hence we show that it is in an association with postsynaptic density protein 95 (PSD-95), a marker postsynaptic protein known (Kim and Sheng, 2004) to form the major scaffold for a set of brain receptors in excitatory synapses. Finally, we demonstrate the stimulation of postsynaptic gene expressions for neurotransmission (cholinergic and glutaminergic systems) via P2Y1Rs, and the mapping of signal transduction cascade in controlling the gene transcription in the cortex neurons.
There are some reports in the literature regarding a range of molecular pathways that may regulate brain-specific gene transcriptions (Liu et al., 2008; Flavell and Greenberg, 2008; Neary and Zimmermann, 2009), but those identified so far control steps in the proliferation, migration, and differentiation of neurons, and some elements of morphogenesis. Despite the numerous GPCRs active in the CNS, a GPCR that regulates gene transcriptions for transmitter/receptor systems, as here, is truly exceptional. It should encourage a search for other GPCRs with similar dual roles in the brain.
Materials and Methods
Materials.
Sprague-Dawley rats, from the Animal Care Facility of the Hong Kong University of Science and Technology were used. All procedures were conducted under the guidelines for the use and care of laboratory animals in research (Animal Research Panel of The Hong Kong University of Science and Technology). Cell culture media were from Invitrogen (Invitrogen, Carlsbad, CA). P2Y receptor agonists and antagonists were the purest grades available, from Tocris Bioscience (Bristol, UK) or from Sigma-Aldrich (St. Louis, MO). Apyrase (grade VII) and phorbol ester (TPA) were from Sigma-Aldrich. To ensure the purity of nucleotides, 2-MeSADP and ADP stock solutions (1 mM) were preincubated with hexokinase/glucose (Roche Diagnostics, Mannheim, Germany), whereas ATP stock solution (1 mM) was pretreated with creatine phosphokinase/creatine phosphate, all as described and validated elsewhere (Choi et al., 2001a).
Cell Culture and Drug Treatment.
Cultured cortical neurons and human embryonic kidney (HEK) 293T cells were prepared and cultured as described in Xie et al. (2009). In studies on mRNA and protein responses to drug treatments, cultured cortical neurons at 15 DIV were first treated with apyrase (1 U/ml, 1 h) to remove nucleotides in the culture medium, followed by washing once with neurobasal medium. To avoid interference due to the spontaneous neuronal activity in such cells, fresh medium containing tetrodotoxin (TTX) (100 nM, Sigma-Aldrich) was added to the culture and incubated for 3 h to obtain a stable baseline. Each drug was then added in fresh TTX-containing culture medium. Antagonists were present for at least 30 min before an agonist addition and then throughout the treatment. In phosphorylation studies, the cultures were incubated in neurobasal medium only for 3 h before the application of reagents.
mRNA Analyses.
Total cDNA from cortex of adult rats or from cultured cortical neurons was prepared as described in Xie et al. (2009). For qualitative detection of P2Y1R mRNA, total cDNA from the cortex (500 ng) or cultured cortical neurons (200 ng) was amplified in each PCR assay with Taq DNA polymerase by the P2Y1R primers, 5′-CCT GCG AAG TTA TTT CAT CTA-3′ (forward) and 5′-GTT GAG ACT TGC TAG ACC TCT-3′ (reverse). Control samples using rat genomic DNA (5 ng/reaction) or RNAs (50 ng/reaction for cortex and 200 ng/reaction for cultured cortical neurons) lacking reverse transcriptase were processed in parallel.
Real-Time Quantitative PCR.
Real-time PCR was performed using SYBR green master mix and Rox reference dye according to the Roche instructions. The primers were: 5′-CTG GGG TGC GGA TCG GTG TAC CCC-3′ (forward) and 5′-TCA CAG GTC TGA GCA GCG TTC CTG-3′ (reverse) for the AChET variant, 5′-AAC GGA TTT GGC CGT ATT GG-3′ (forward) and 5′-CTT CCC GTT CAG CTC TGG G-3′ (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The SYBR green signal was detected on the Mx3000p multiplex quantitative PCR platform (Stratagene, La Jolla, CA). The transcript expression levels were measured using the ΔΔCt value method (Xie et al., 2009), in which values were normalized by the internal control GAPDH in the same sample. PCR products were analyzed as above, plus melting curve analysis to confirm the specific amplification.
cDNA Plasmids, Reporter Gene Constructs, and Transfection.
Rat P2Y1R cDNA was subcloned into the pCMV-Tag-3 vector (Stratagene) to produce N-terminal-Myc-P2Y1R or into the pcDNA4 vector (Invitrogen) for tagging with N-terminal hemagglutinin (HA) peptide by a standard PCR method using Pfx (Invitrogen). Each was confirmed by DNA sequencing. Carboxyl-terminal truncation was made using a reverse primer flanking 985 to 1002 bp in rat P2Y1R cDNA to form P2Y1RΔC-term (Choi et al., 2008). Mutations in the carboxyl tail, changing DTSL to DYSR, were made using the reverse primer 5′-AA CTC GAG TCA CCT ACT GTA GTC TCC-3′ to form the P2Y1RDYSR construct (XhoI site is underlined). The human AChE promoter reporter and Raf constructs, pAChE-Luc, pAChEΔElk-1 [1]-Luc, pAChEΔElk-1 [3]–Luc, pAChEΔElk-1 [1,3]–Luc, RafWT, and RafCAAX were as described by Choi et al. (2003). Cultured cortical neurons cultured for 3 and 14 days for differentiation and drug treatment studies, respectively, were transfected transiently with the purified plasmids (0.5 μg/well in 24-well plates) by Lipofectamine 2000-CD (Invitrogen). The transfection efficiency in the neurons was approximately ∼5%. HEK 293T cells were transfected with calcium phosphate (Choi et al., 2003).
Immunoblotting and Phosphorylation Studies.
The cultured neurons were collected in lysis buffer (100 μl of per well of six-well plate) containing 150 mM NaCl, 10 mM HEPES pH 7.5, 0.5% Triton X-100, 5 mM EGTA, 5 mM EDTA, 1 mg/ml bacitracin, 1 μg/ml leupeptin, and 1 μg/ml aprotinin. Phosphorylation analysis and collection of the lysates were as in Siow et al. (2002). The final lysate supernatant was loaded on gels at 10 μl per lane. The proteins were separated on 8 or 12% SDS-polyacrylamide gels and electroblotted onto nitrocellulose filters for 16 h. Western blot analyses were carried out as described in Siow et al. (2002), the following commercial antibodies were used: anti-AChE antibody (1:2000; BD Biosciences, Franklin Lakes, NJ), anti-P2Y1R antibody (1:1000; Alomone Labs, Jerusalem, Israel), anti-NR1 antibody (1:5000, BD Biosciences, San Jose, CA), anti-NR2A antibody (1:1000; Millipore Corporation, Billerica, MA), anti-choline acetyltransferase (ChAT) antibody (1:2000; Millipore Corporation), anti-muscarinic acetylcholine receptor M1 (M1 mAChR) antibody (1:1000; Sigma-Aldrich), anti-PSD-95 antibody (1:5000; Millipore Corporation), anti-neurofilament-200 antibody (1:2000; Sigma-Aldrich), Cy3-conjugated anti-glial fibrillary acidic protein (GFAP) antibody (1:5000; Sigma-Aldrich), anti-microtubule-associated protein 2 (MAP-2; Sigma-Aldrich) antibody (1:5000; Sigma-Aldrich), anti-GAPDH and anti-α-tubulin antibodies (1:10,000; Abcam Inc., Cambridge, MA), anti-phospho ERK antibody, and anti-ERK antibody (1:5000; Cell Signaling Technology, Danvers, MA). The immunoreactive bands were visualized by chemiluminescence with the ECL protocol (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The labeling intensities of the protein band from the control and from the agonist-stimulated samples, run in the same gel, were compared by densitometry within the range of a calibrated density/response curve. α-Tubulin or GAPDH, as shown, were included as visual loading controls and used to normalize the densities before plotting.
Immunocytofluorescent Staining.
Cultured cortical neurons or cDNA-transfected HEK 293T cells were grown on glass coverslips. They were fixed and stained as described previously (Tung et al., 2004). The following commercial antibodies were used in this study: anti-P2Y1R (1:100; to an epitope in the third intracellular loop of P2Y1R; Alomone Labs); anti-AChE (1:500; BD Biosciences); Cy3-conjugated anti-GFAP (1:2000; Sigma-Aldrich); anti-MAP-2 (1:500; Sigma-Aldrich); goat anti-neurogranin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA); anti-PSD-95 (1:500; Millipore Corporation); or anti-SV48 (1:500; Xie et al., 2009). The cells were washed and stained with the corresponding Alexa 488- or 555-conjugated secondary antibodies (Invitrogen) plus nuclear stain TO-PRO-3 (1:500; Invitrogen), and then dehydrated serially in ethanol and mounted with fluorescence mounting medium (Dako North America, Inc., Carpinteria, CA). Confocal fluorescence microscopy (DMIRE2; Leica, Wetzlar, Germany) was used with excitation (Ex) at 488 nm/emission (Em) at 500 to 535 nm (green), Ex at 543/Em at 560 to 615 nm (red); for nuclear staining, Ex at 640/Em at 660 to 750 nm for TO-PRO-3 or Ex at 405/Em at 465 for 4,6-diamidino-2-phenylindole (both blue pseudocolor).
For colocalization of PSD-95 and P2Y1Rs, PSD-95-green fluorescent protein (GFP) cDNA, (Stratagene) was cotransfected with Myc-P2Y1RΔC-term or Myc-P2Y1RDYSR cDNAs in HEK 293T cells. After transfection (48 h), cultures were and incubated with Cy3-conjugated anti-Myc antibody (1:500; Invitrogen) in blocking solution to recognize the membrane receptors only. The cultures were washed, mounted, and analyzed by confocal microscopy as above.
Fluorometric Measurement of Ca2+ Mobilization.
Cortical neurons on coverslips mounted at the base of the reaction chamber were loaded with 2 μM Fluo-4-acetoxymethyl ester (Invitrogen) in 100 μl of Hanks' balanced salt solution buffered with 20 mM HEPES, pH 7.5 (HBSS), for 1 h at 37°C. After three 5-min washes with HBSS, serial images were captured in Leica confocal microscopy at Ex at 488/Em at 500 to 550 nm, and analyzed by ImageJ software (http://rsbweb.nih.gov/ij/) to measure the change in intracellular Ca2+. To determine the mean response in the cell population, cortical neurons (at 15 DIV) in black-walled, clear-bottomed, 96-well culture plates at confluence were labeled at 37°C as above, followed by medium replacement with 150 μl of HBSS. For each assay, the agonist was added in 50 μl of HBSS. To obtain a stable baseline reading, TTX (100 nM; Sigma-Aldrich) was added to the buffer. Changes in fluorescence were measured in a FlexStation II plate reader (Molecular Devices, Sunnyvale, CA) at Ex 488/Em 520 nm. Data were expressed as changes in fluorescent intensity relative to the basal (untreated) value and analyzed with Prism (ver. 3; GraphPad Software Inc., San Diego, CA).
Immunoprecipitation and Pull-Down Assays.
Lysates from cultured cortical neurons or transfected HEK 293T cells in 100-mm plates were prepared in modified radioimmunoprecipitation assay buffer (20 mM sodium phosphate, 1% Triton X-100, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 2 mM sodium orthovanadate, and 50 mM sodium fluoride). The lysates (∼500 μg of protein per lysate) were incubated with protein G-agarose (Roche Diagnostics) suspension (50% slurry, 50 μl) for 2 h at 4°C. The supernatant was then incubated with specific antibodies (∼4 μg) and gently rotated at 4°C overnight. After that, another 50 μl of protein G-agarose suspension was added and gently rotated for 5 h at 4°C. The collected beads were washed three times with modified radioimmunoprecipitation assay buffer and then with 50 mM Tris-HCl, pH 8.0. Finally, the beads were resuspended in SDS sample buffer (25 μl) and heated at 100°C for 5 min. The extracts were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting.
Other Assays.
Protein concentration and AChE enzymatic activity determinations, and the separation of AChE molecular forms on sucrose density gradient, were as described in Xie et al. (2009). The validation of the G4 AChE isoform by anti-proline-rich membrane anchor (PRiMA) antibody was also as described in Xie et al. (2009). Luciferase assay was carried out on the cultures of cells expressing the luciferase constructs noted above, as described in Siow et al. (2002).
Data Analysis.
Gel documentation and relative quantification were performed with the IS1000 Digital Imaging System. For the colocalization ratios, 20 neurons were randomly selected from each double-immunostained coverslip on the basis of healthy morphology using phase contrast. Then the numbers of immunopositive puncta were counted per 50-μm dendrite length. Only puncta larger than 10 pixels were counted to avoid background staining. The colocalization ratio (percentage) of each two immunopositive puncta was calculated: [number of puncta double-immunostained per number of total puncta immune stained with one of the two antibodies] × 100%. On average, 80 puncta were analyzed per neuron.
All statistical analyses were by two-tailed, unpaired, one-way analysis of variance. Data are plotted as mean ± S.E.M. (except where the bars fall within a symbol), for n = 4 independent experiments (unless stated otherwise), each using triplicate samples. Other figures are likewise representative of four or more replicates.
Results
Expression and Localization of P2Y1 Receptors in Neurons of the Cortex.
P2Y1R mRNA was found to be well expressed both in the intact adult rat cerebral cortex and in the cortex neurons in primary culture used in this study (Fig. 1A). Here, we used primary cultures derived from developing rat cortex, in development up to 25 DIV, in conditions in which the neurons could be studied specifically to investigate P2Y1Rs thereon. Our untreated cultures contained neurons and many glia and showed strong reaction both for MAP-2 (a neuronal marker) and for the astrocyte marker GFAP, in immunocytofluorescent staining and in Western blotting (Fig. 1B). A schedule of treatment with the glial-suppressant Ara-C (see Materials and Methods) reduced the astrocyte content to a negligible level, as indicated by selective disappearance of anti-GFAP reactivity (Fig. 1B). In the conditions used, the neuron-rich cortical cultures are fully viable, as has been shown by others (Zhang et al., 2003; Hilgenberg and Smith, 2007). These were used (except where stated) in all of the analyses in culture here. In view of reports (Yang et al., 2003) that the differentiation of brain neurons is retarded if cultured as here in the absence of astrocytes, because of the lack then of the astrocyte-derived activator d-serine, we tested the effect of including d-serine (10 μM) in the neuronal cultures. The expression of three neuronal proteins studied here as reported below, AChE, ChAT, and NR2A, was found to show no change in this medium in their expression levels throughout the culture period, hence validating the culture conditions used here (data not shown).
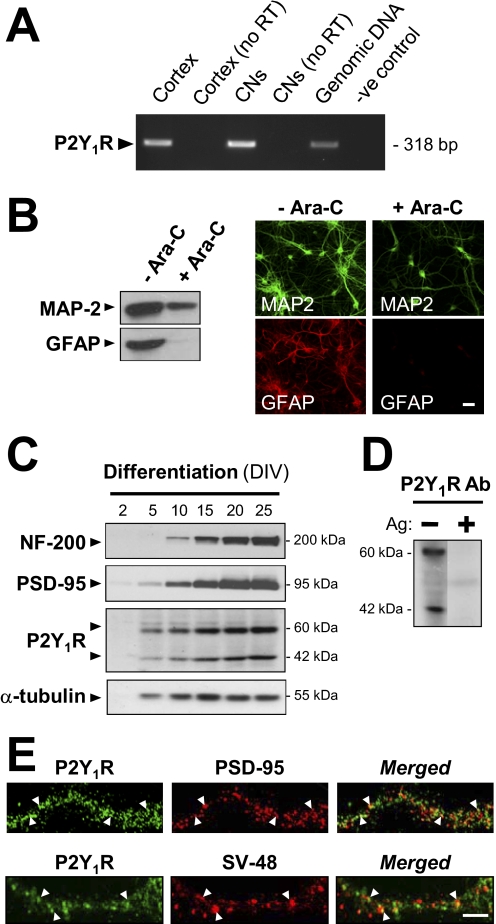
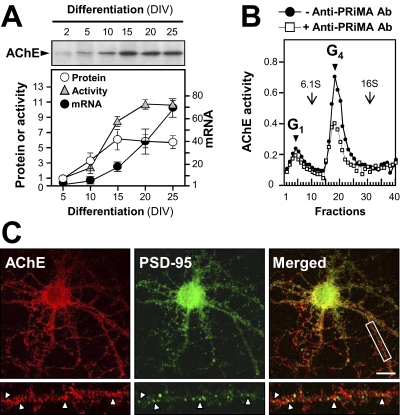
Fig. 1.
Mainly postsynaptic localization of P2Y1Rs in cortical neurons. A, expression of P2Y1R mRNA in rat brain cortex and in cortical neurons (CNs) cultured therefrom. Total RNA isolated from either source was analyzed using reverse transcriptase PCR with primers specific for the rat P2Y1R and gel electrophoresis. Identification of each product was made by extraction from the gels and DNA sequencing. No products are seen from these RNA samples not subjected to reverse transcriptase or where template DNA was omitted (-ve control). Genomic DNA (5 ng per reaction) gives a positive control. B, cortical neuronal cultures at 15 DIV, with or without Ara-C treatment to prevent proliferation of glia, were stained using antibodies to GFAP and to MAP-2 (a neuron-specific marker). After immunoblotting (left) or immunocytofluorescent staining (right), both results indicated that astrocytes were essentially absent from the Ara-C-treated cultures, thereafter routinely prepared thus. Scale bar, 40 μM. C to E were obtained in the presence of Ara-C to prevent glia proliferation. C, immunoblots showing the progressive expression of neurofilament 200 (a neuronal differentiation marker), PSD-95, and P2Y1R during the differentiation of the cultured cortical neurons. α-Tubulin served as an internal control for neuronal protein content. For each time point, all blots were run from the same sample. D, immunoblot, from cultures at 21 DIV, probed with anti-P2Y1R (1 μg/ml). Protein bands of ∼60 kDa (the glycosylated P2Y1R size) and ∼42 kDa (the predicted P2Y1R protein size) were detected as in C. The reaction of both forms is fully blocked by the peptide antigen (Ag, 5 μg/ml). E, the cultures, at 21 DIV, were fixed and doubly stained using anti-P2Y1R (green) plus either anti-PSD-95 (top) or anti-SV48 (bottom, presynaptic marker) antibody (both red), showing only dendrite regions. Punctate loci of P2Y1R clearly colocalized with PSD-95 or with SV-48 are shown by arrowheads: the extent of this for each pair was analyzed across 20 fields (see text). Scale bar, 20 μm.
In immunoblots from such treated cortical neurons when probed with Alomone's anti-P2Y1R antibody (Fig. 1C), bands of proteins migrating at ∼60 and ∼42 kDa were detected [specificity being shown by their elimination by the presence (Fig. 1D) of the corresponding peptide antigen]. The product at ∼42 kDa agrees with the predicted size of the P2Y1R protein. The band at ∼60 kDa was also seen by Moore et al. (2001) in immuno-blots (with a different anti-P2Y1R antibody) derived from whole rat brain and is attributable to post-translational glycosylation. These blots allowed us to see that the relative expression of the P2Y1R protein increased markedly during neuronal differentiation in culture, starting at 5 DIV. The expression was at a plateau after 20 days of culture (Fig. 1C). In parallel, the expressions of neurofilament 200 at ∼200 kDa and PSD-95 at ∼95 kDa, both being differentiation markers for neurons, increased similarly then, again reaching a plateau near 20 DIV. The expression of the neuronal “housekeeping” α-tubulin gene was little changed from 10 DIV onward, serving as an internal loading control (Fig. 1B).
To investigate distributions of P2Y1Rs in the intact neurons, their immunocytochemistry was required, but under the usual conditions for that technique, the risk of nonspecificity in some anti-GPCR antibodies is significant. We have shown (Tung et al., 2004) that in immunocytochemistry, the above-noted commercial anti-P2Y1R antibody can give a cross-reaction with some of the other P2YR subtypes and that this can be blocked by applying it in medium containing relatively high bovine serum albumin levels, maintained before and during the reaction. This medium was used here. For certainty, the specificity for P2Y1R of the antibody used in the immunoblots and in immunocytochemistry was confirmed by a stringent criterion (i.e., showing identical results) in a subsequent test comparison with a new anti-P2Y1R antibody recently validated in testing on brain of P2Y1R-knockout mice (shown in Supplemental Data S1).
It was thus shown in well differentiated cortical neurons that the P2Y1Rs are relatively strongly expressed on most of the dendrite-like neuritis as well as on the cell bodies (data not shown). On the dendrites, the P2Y1Rs showed a punctate localization (Fig. 1E). Of the P2Y1R puncta (on 20 neurons counted), 54.2 ± 2.9% were localized at the postsynaptic densities, as shown by their marker protein PSD-95, relative to the total PSD-95-expressing puncta on those dendrites. A similar overall association with PSD-95 was seen for P2Y1Rs present at cell body membrane regions, but those, being less punctate, could not be quantitated accurately. In contrast, the receptor on the dendritic puncta showed less colocalization with a presynaptic marker, anti-synaptotagmin (SV48) antibody [i.e., 26.3 ± 2.1% (on 20 neurons)], relative to the total SV48-containing puncta (Fig. 1E). The difference in these values is significant at p < 0.001, and at this confocal resolution, level the percentages show that the P2Y1Rs on cortical neuron dendrites are mainly postsynaptic. The apparently presynaptic minority would not be well resolved here from the astrocyte processes carrying P2Y1Rs when those processes are closely apposed to the excitatory synapses, as is common in the forebrain (Perea et al., 2009): there may be still fewer, if any, P2Y1Rs on the neuronal presynaptic membranes. Although these analyses were performed in confocal microscopy, further study at higher resolution was not made here because extensive quantitative electron-microscopic analysis became available of immunogold labeling of forebrain P2Y1Rs by identical antibody (applied in a serum albumin-enriched medium similar to ours) in rat hippocampal sections (Tonazzini et al., 2007). That study has shown clearly the predominant localization of P2Y1Rs at neuronal postsynaptic densities, fully consistent with our results on cortex cultures; it could show, furthermore, that the neuronal P2Y1Rs are associated with the glutamatergic class of synapses. Tonazzini et al. (2007) also found that the astrocyte cell membranes there showed the second highest density of the P2Y1R, in accord with its activity seen in functional studies on astrocytes in brain tissue (Zhang et al., 2003; Bowser and Khakh, 2004; Perea et al., 2009).
P2Y1 Receptors Interact with PSD-95 by PDZ Association.
The carboxyl terminus of all known P2Y1R sequences ends in the conserved motif DTSL, a canonical binding motif for class I PDZ domains (Bockaert et al., 2003). This tetrapeptide in P2Y1R has been shown (Fam et al., 2005) to interact with a PDZ domain from the scaffold protein Na+/H+ exchanger regulatory factor type 2 (NHERF-2). In CNS neurons, PSD-95 is a more generally occurring postsynaptic scaffold protein and contains three PDZ domains and two of other types of binding domain (Kim and Sheng, 2004). We found using extracts of cultured cortical neurons, the native P2Y1Rs could be coimmunoprecipitated with PSD-95 and vice versa (Fig. 2A). The NR2A subunit of N-methyl-d-aspartate (NMDA) receptors served as a positive control because of its known association with PSD-95 (Kim and Sheng, 2004). It is noteworthy that the NR2A subunit could also be pulled down by anti-P2Y1R antibody (Fig. 2A). These specific pull-down experiments show protein interaction in cortical neurons of PSD-95 with P2Y1R, and of NMDA receptors with P2Y1Rs. Because the latter pair differs in containing no PDZ domains, we presume that each of those receptor types attaches directly to PSD-95 in situ.
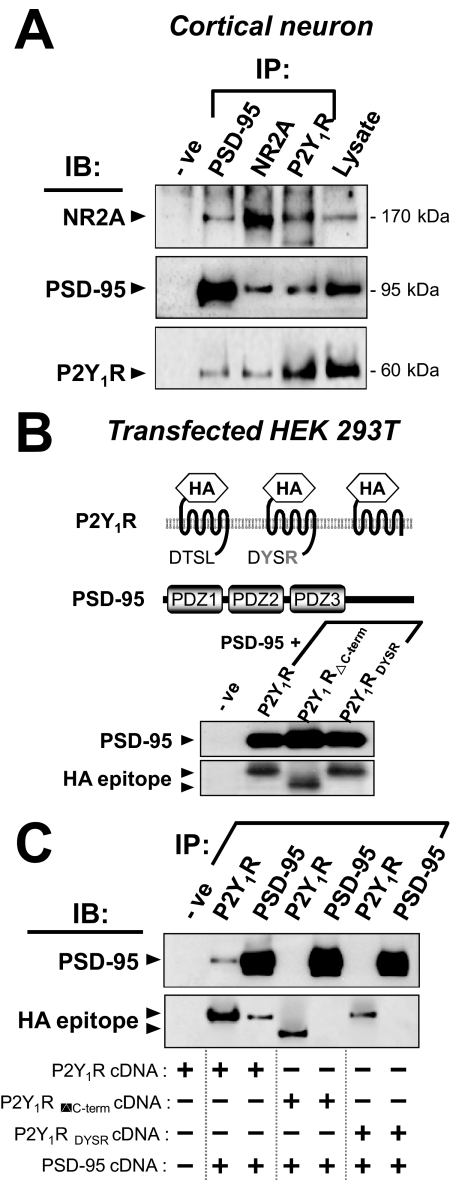
Fig. 2.
The P2Y1R interacts via its C-terminal DTSL motif with the scaffold PSD-95. A, immunoprecipitates (IPs) from lysates of cultured cortical neurons (21 DIV) were obtained (see Materials and Methods) using antibodies to NR2A or PSD-95 or P2Y1R. Blots derived therefrom were probed [immunoblotted (IB)] separately with anti-NR2A antibody (top row), anti-PSD-95 antibody (middle row), or anti-P2Y1R antibody (bottom row). As controls, the corresponding normal serum replaced the IP antibody in the first lane (-ve), and total lysate (20 μg) alone was loaded in the last lane. In both directions in the grid, some P2Y1R and NR2A are seen to be associated with PSD-95. B, HEK 293T cells were cotransfected by PSD-95 cDNA plus P2Y1R cDNA or plus cDNA of a C-terminally mutated construct (P2Y1RDYSR and P2Y1RΔC-term) as illustrated. Each P2Y1R carried an N-terminal HA epitope tag. Lysates (20 μg of protein per lane) of each of these three cell types were tested in immunoblots using anti-HA antibody or anti-PSD-95 antibody, showing bands of the predicted sizes. Control blots with lysate from empty vector-transfected cells did not react (-ve lane). C, HEK 293T cells were cotransfected with PSD-95 and one of those 3 P2Y1R constructs (all HA-tagged) as shown in the columns below the gel; IP and blotting were then performed on their lysates as in A. IB was with anti-PSD-95 or anti-HA antibodies. The control (-ve lane) was as in B control. P2Y1R is seen to be associated with PSD-95, but only when the DTSL motif is intact.
The interaction of PSD-95 with P2Y1R was further demonstrated in HEK 293T cells heterologously expressing both of those proteins. Considering the above-noted requirement for the C-terminal DTSL motif, cDNAs encoding one of three forms of the P2Y1R were constructed: N-terminal HA-tagged wild-type P2Y1R (HA-P2Y1R), HA-tagged P2Y1R with a mutated C terminus (HA-P2Y1RDYSR), and HA-tagged P2Y1R with the deletion of the cytoplasmic tail (HA-P2Y1RΔc-term) (Fig. 2B). These cDNAs were expressed to provide immunoblots using (alone) each of the corresponding antibodies (Fig. 2B). These cells showed a band at ∼95 kDa for PSD-95 and at ∼42 kDa (bottom gel, with anti-HA) for P2Y1R, except for the shorter HA-P2Y1RΔc-term giving a lower band (∼37 kDa). In the cDNA-transfected HEK 293T cells, the P2Y1R was coimmunoprecipitated with PSD-95 and vice versa (Fig. 2C). Hence, the result was similar to the situation in the brain extract. However, the mutants, HA-P2Y1RDYSR and HA-P2Y1RΔc-term, were unable to coimmunoprecipitate with PSD-95 (Fig. 2C). Thus, the P2Y1R-PSD-95 association is mediated by the DTSL motif of the receptor.
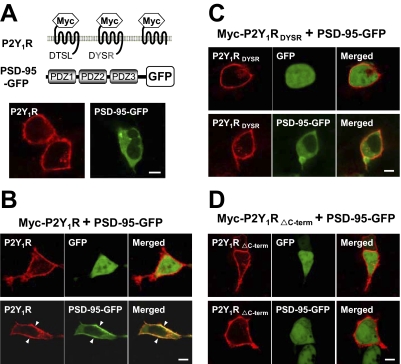
To confirm the association of P2Y1R with PSD-95 in intact cells, HEK 293T cells were cotransfected to express PSD-95 (carrying a GFP label downstream) plus one of the alternative P2Y1R forms as in Fig. 2B. In this case, the receptors were N-terminally tagged with the Myc peptide in place of the HA tag and visualized by the red fluorescence of Cy3-labeled anti-Myc antibody (Fig. 3A). In all cases in which P2Y1R was expressed, it was seen as clearly localized at the cell membrane: its internal fraction (expected in growing cultures) was avoided in the imaging because the directly labeled anti-Myc antibody used for it is to an N-terminal tag, and no permeabilization was given here. In contrast, when the labeled PSD-95 was expressed alone, it was seen to be present throughout the cytosol (Fig. 3A). However, in cells expressing both P2Y1R and PSD-95, most of the PSD-95 became colocalized with membrane P2Y1Rs (Fig. 3B, bottom). In controls cotransfected to express P2Y1R and free (unconjugated) GFP, GFP remained throughout the cytosol and nucleus (Fig. 3B, top). Thus, the P2Y1R is one of the partners of PSD-95, which facilitates its association with the cell membrane. However, alteration at the C terminus of P2Y1R, both in the P2Y1RDYSR and P2Y1RΔc-term constructs removed this ability. Thus, in cells coexpressing PSD-95 with P2Y1RDYSR or with P2Y1RΔc-term, PSD-95 was found in the cytosol and did not colocalize with the receptor, even though the mutant receptor was still maintained at the membrane (Fig. 3, C and D, bottom). In summary, the C terminus of the P2Y1R interacts at its DTSL motif with PSD-95, and formation of this complex can recruit intracellular PSD-95 to the cell membrane. A mechanism for such PSD-95 migration is known (Kim and Sheng, 2004), because another of its PDZ domains binds strongly to the C terminus of a kinesin molecular motor to stimulate translocation of its complexes along microtubule tracks to the cell membrane region.
Fig. 3.
The C-terminal DTSL motif is required to link the P2Y1R to PSD-95 at the plasma membrane. PSD-95 was tagged with GFP, whereas the P2Y1R and its two mutated constructs (A, top) were N-terminally Myc-tagged, and each was expressed by transfection in HEK 293T cells. A, Myc-P2Y1R and PSD-95-GFP were expressed separately, and the former was reacted (without permeabilization) with Cy3-conjugated anti-Myc antibody. Confocal microscopy shows the wild-type P2Y1R is at the cell membrane, whereas PSD-95-GFP is largely intracellular. B, when Myc-P2Y1R was coexpressed with PSD-95-GFP, subsequent staining shows that the PSD-95-GFP has become colocalized with the receptor on the cell membrane (bottom), with high coincident staining at the regions of high P2Y1R content (arrowheads). This migration did not occur when PSD-95-GFP was coexpressed with Myc-P2Y1RDYSR (C, bottom) or with P2Y1RΔC-term (D, bottom). In each case (B–D, top), a control with the receptor cotransfected with the same vector expressing GFP alone shows that the GFP itself does not associate with P2Y1R. Scale bar, 10 μm.
P2Y1 Receptor-Mediated Intracellular Ca2+ Mobilization in Neurons.
The P2Y1R is well established to be in the family of Gq/11-linked receptors and thus is expected to signal upon agonist activation primarily via phospholipase C, inositol trisphosphate, and mobilization of Ca2+ from intracellular stores (Abbracchio et al., 2006). With rat cortex mixed-cell cultures, Bennett et al. (2003) found that only 9 to 15% of the neurons mobilized Ca2+ in response to P2Y1R agonists (but all glia did so) in testing at an earlier stage than ours (E14 cortex, cultured only for 8 days, and with ∼50% glia present). Therefore, our cortex cultures, being more mature and with glial suppression, were compared here in this response.
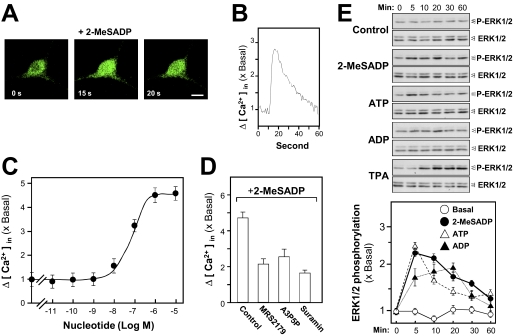
We have demonstrated previously in the case of cultured myotubes (Choi et al., 2003; Tung et al., 2004) that activation by adenosine tri- or diphosphates of P2Y1R or P2Y2 R mobilizes intracellular Ca2+, and furthermore, this leads to an increase in the phosphorylation of the ERK1/2 kinases. Earlier work on transfected cell (Sellers et al., 2001) has indicated that this pathway for P2Y1R involves the mitogen-activated protein kinase kinase-1 and phosphatidylinositol-3-kinase. Here, cortical neurons in culture were preloaded with the Ca2+ indicator Fluo-4, and the mobilization of intracellular Ca2+ upon agonist stimulation was monitored in real time with the confocal microscope. The intracellular Ca2+ sharply increased across the neuron in less than 20 s after the application of 2-MeSADP, a highly potent P2Y1R agonist (Fig. 4, A and B). A control application of buffer alone induced no Ca2+ mobilization (data not shown). In quantitative measurements, all of the neurons tested gave this response. The mean EC50 is 71.3 ± 2.8 nM (Fig. 4C). The 2-MeSADP-induced intracellular Ca2+ accumulation could be blocked (Fig. 4D) by coincubation with the antagonists 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium salt (MRS2179) (P2Y1R-specific: Camaioni et al., 1998) or adenosine-3′, 5′-bisphosphate (A3P5P; P2Y1R-selective) or with the general P2 antagonist suramin. The activation of endogenous P2Y1Rs in cultured cortical neurons by three agonists (2-MeSADP, ATP, and ADP) led to the strong phosphorylation by each agonist of the mitogen-activated protein kinase ERK1 (∼44 kDa) and (at a low level) ERK2 (∼42 kDa). A phorbol ester activator of protein kinase C (PKC), TPA, served as a positive control, inducing a strong (∼4-fold basal) phosphorylation of ERK1/2 (Fig. 4E, top). The ERK1 and ERK2 total protein content remained invariant. The scanned data showed that the phosphorylation of ERK1/2 evoked by the more selective P2Y1R agonist 2-MeSADP is strong and highly significant (Fig. 4E, bottom). Owing to the evidence for interaction of PSD-95 and P2Y1R at the plasma membrane (Fig. 3), it was interesting to know whether PSD-95 would affect the receptor signaling response in terms of Ca2+ mobilization and ERK phosphorylation. Therefore, a differential expression test in HEK293T cells was used for that purpose. The results indicated that there was no significant difference between P2Y1R alone and P2Y1R plus PSD-95 in terms of both Ca2+ mobilization and ERK phosphorylation (Supplemental Data S2). These results suggest that the role of PSD-95 here is to serve as a scaffold protein to concentrate P2Y1R at sites on the plasma membrane rather than to regulate the receptor signaling.
Fig. 4.
Activation of P2Y1Rs in cortical neurons induces Ca2+ mobilization and ERK phosphorylation. In all plots, the values are normalized as multiples of the basal (no agonist) level. A, a cortical neuron in a culture at 15 DIV, preloaded with Fluo-4, shows activation by the P2Y1R agonist 2-MeSADP (100 μM) with a transient increase (B) in cytosolic Ca2+ (internal green fluorescence). Scale bar, 10 μm. C, in a population of such neurons in 96-well plate format, the potency of 2-MeSADP in Ca2+ activation was determined from readings (n = 4.5 × 104 cells) as in B. D, the specificity of the Ca2+ mobilization induced by 2-MeSADP (10 μM) was confirmed by pretreatment with the P2Y1R antagonists MRS2179 (100 μM) or A3P5P (100 μM), or with the general P2 antagonist suramin (100 μM). E, immunoblotting of extracts from the neuronal cultures after activation by three P2Y1R agonists (each 100 μM) or by TPA (100 nM, as a positive control). The transient phosphorylation of ERK-1 and -2 proteins is shown. Total ERK-1 and -2 proteins in each sample are shown as a loading control. Bottom, quantitation from a set of such blots.
It can be shown that this finding of functional P2Y1Rs on the embryonic cortical neurons in culture here is representative of native embryonic cortex in situ and also of differentiated neurons in the postnatal forebrain. Similar results were found in a recent study of P2Y1Rs on acute slices of day E16 mouse neocortex (Liu et al., 2008). There, abundant identified postmitotic neurons responded to fast-applied ATP with strong Ca2+ transients, which could be fully blocked by MRS2179 or suramin, as was seen here. Furthermore, the responsive receptors on native cortical neurons were confirmed as P2Y1Rs by the suppression of the transients after electroporation of P2Y1R short hairpin RNA into those cells. In addition, in postnatal development, strong electrophysiological activity identified with P2Y1Rs has been shown on pyramidal cells and interneurons of the hippocampus, either in 2-week culture from rat postnatal day P3 (Filippov et al., 2006) or in acute slices from days P7 to P15 (Kawamura et al., 2004). The latter case was confirmed by complete loss of those responses in transgenic P2Y1R-deleted mice. Furthermore, from 6-week-old rats, single-cell PCR analysis of pyramidal neurons in hippocampal sections has confirmed that strong expression of P2Y1Rs in maintained thereon (Rodrigues et al., 2005).
Release of ATP onto Cortical Neurons.
Although functional P2YRs were shown thus to be available here, the extents of endogenous release of ATP onto cultures of brain neurons and of glia have not been reported previously. The concentration of ambient ATP in the bulk phase above the monolayer of cortical neurons in our conditions was measured and found to be low, being limited by the high surface ectonucleotidase activity, but it was increased ∼6-fold when the cultures contained freely growing glial cells (Supplemental Data S3). In agreement, we have found a similar endogenous release of glial ATP from the NG108-15 neuroblastoma/glioma cells cultured alone (Ling et al., 2005). For comparison, activation of P2YRs on cultured cortical astrocytes strongly mobilizes their intracellular Ca2+ to release glutamate, which, when occurring in situ, would activate adjacent neuronal NMDA receptors (Lee et al., 2007). The actual local concentration of agonists at the P2Y1Rs at the surface of the cultured cortical neurons is predicted to be in reality much higher than those measured in the bulk medium above them and to be sufficient to activate them (for details, see Supplemental Data S3). The relationship of these findings to the treatments that we make with ATP and its derivatives is considered in the Discussion.
ATP Induces Neuronal Gene Expressions.
At vertebrate neuromuscular junctions, activation of P2Y1Rs leads to a marked increase in transcriptional activity at the ACHE gene (encoding the catalytic subunit of AChE), with some of the elements responsible for that gene activation identified (Choi et al., 2003). This suggests a potential role of P2Y1R in regulating the ACHE gene in brain neurons. Hence, neuronal AChE was the first synaptic protein analyzed thus in this study. Different forms of AChE (based on the same catalytic subunit and ACHE gene) have been identified at various locations (e.g., globular and collagen-tailed forms) (Xie et al., 2007), and these were distinguished here.
The enzymatic activity of AChE increased during the neuronal differentiation process and reached a maximum activity after 20 DIV (Fig. 5A). The time course of expression of the AChE catalytic subunit protein at ∼68 kDa, revealed by Western blotting and of its mRNA measured by real-time PCR, was in accordance with the increase of activity (Fig. 5A). In cultured cortical neurons, a globular form of AChE with a sedimentation constant of 11S was the most abundant form identified (Fig. 5B). This AChE type corresponds to a known 11S form, G4, a tetrameric enzyme linked to PRiMA (Perrier et al., 2002), an anchoring transmembrane 20-kDa protein. This identification was supported by observing that the 11S form of AChE was selectively depleted by reaction with anti-PRiMA antibody (Fig. 5B).
Fig. 5.
Temporal and spatial expressions of AChE in cortical neurons. A, the AChE mRNA content and AChE enzymatic activity during differentiation were determined on extracts of cultured cortical neurons. The expression of the AChE protein was measured in parallel from a set of its immunoblots (example shown; it produced 1 band at ∼68 kDa). Data are expressed as multiples of the basal activity (at 5 DIV). B, molecular forms of AChE in cortical neurons (at 25 DIV) were revealed by sucrose density gradient analysis of their extracts: the predominant form is G4 AChE (●). This form of AChE was depleted specifically by reaction with anti-PRiMA antibody (□). Data are expressed in arbitrary units. One of four representative plots is shown. C, neurons (21 DIV) were fixed and double-stained with anti-AChE antibody (red) and anti-PSD-95 antibody (green), seen in confocal microscopy. Bottom a higher magnification of the area marked with a rectangle is shown; on dendrites there, AChE is partially colocalized with PSD-95 (arrowheads). Scale bar, 20 μm.
The localization of AChE in cultured cortical neurons was revealed using an AChE-specific antibody. By using immunocytofluorescent staining, AChE showed a punctate localization on the dendrites, which was similar to that revealed for P2Y1R. When costained with PSD-95 antibody, 51.9 ± 5.0% of these AChE puncta (counted on 20 neurons, and relative to the total number of PSD-95 puncta) were found to be colocalized with PSD-95 (Fig. 5C). The results therefore suggest a mainly postsynaptic localization of AChE in cortical neurons.
A potential regulatory function of the P2Y1R, to induce the expression of the AChE catalytic subunit (as noted above for muscle) was explored in cultured cortical neurons. After the addition to the culture medium of 2-MeSADP or ATP at 50 μM for 24 h, the expression of transcript encoding AChE increased to a maximum of 4.5-fold compared with the level in the ligand-free control culture (Fig. 6A). The addition of TPA (10 nM), activating PKC, served as a positive control, increasing AChE transcript expression to ∼3-fold. The content of AChE catalytic subunit protein (∼68 kDa) was increased to a maximum of ∼3-fold when P2Y1R agonists (2-MeSADP, ADP, and ATP) were applied. The extents (Fig. 6, A and B) are consistent with their known relative potencies (Abbracchio et al., 2006) at mammalian P2Y1R. This P2Y1R agonist-induced AChE expression was blocked by coincubation with MRS2179, a P2Y1R receptor-specific antagonist (Fig. 6B).
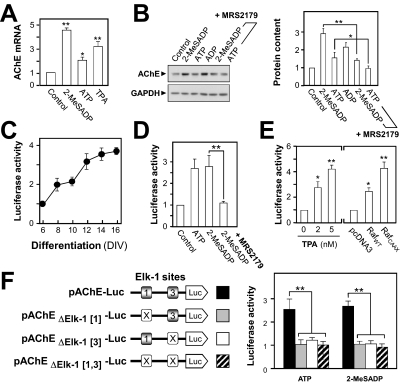
Fig. 6.
Transcriptional regulation of AChE by the P2Y1R. In all plots (except for C), the values are normalized as multiples of the basal level (medium-treated control). A, cortical neurons (15 DIV) were treated with 50 μM 2-MeSADP, 50 μM ATP, or 10 nM TPA for 12 h, and the AChE mRNA expression was analyzed by real-time PCR. B, immunoblot showing the AChE protein content of such neurons before or after treatment for 24 h with the P2Y1R agonists shown (50 μM) alone or (last two lanes) plus MRS2179 (250 μM), a P2Y1R-specific antagonist. GAPDH is the protein loading control. Quantitation from a set of four such blots (standardized for gel loading) is shown at the right. C, a human AChE promoter sequence was tagged with the luciferase reporter gene (pAChE-Luc) and transfected into cultured cortical neurons (at 3 DIV) to provide measurements of the transcriptional activity during differentiation. The values are shown relative to the activity at 6 DIV. D, such pAChE-Luc-expressing neurons were exposed to ligands as in B, followed by assay. E, the pAChE-expressing neurons were exposed to the PKC activator TPA for 24 h and then assayed (left). Right, wild-type Raf (RafWT) or its constitutively active mutant (RafCAAX) or the empty vector (pcDNA3) was cotransfected with pAChE-Luc for similar assay. F, the sites of the mutations (marked X) made at two Elk-1 sites in the AChE promoter as described previously (Choi et al., 2003). Right, each of these Luc-tagged constructs was transfected into cortical neurons, followed by exposure to 50 μM ATP or 2-MeSADP for 24 h and a final transcriptional activity measurement. Differences marked are significant at p < 0.05 (*) or 0.01 (**).
The promoter element of the human ACHE gene has been cloned (Ben Aziz-Aloya et al., 1993) and well characterized. (Siow et al., 2002; Choi et al., 2003; Gao et al., 2009). The DNA (∼2.2 kilobases) encompassing the human ACHE promoter was subcloned into pGL3 vector immediately upstream of a luciferase gene, designated as pAChE-Luc (Choi et al., 2001b; Siow et al., 2002). In cortical neurons transfected by pAChE-Luc, the promoter activity increased with the period of culture (Fig. 6C); this increase was parallel to that seen for the expression of endogenous AChE protein in these cells (Fig. 5A). In transiently transfected cortical neurons, application of 2-MeSADP induced ACHE-promoter-driven luciferase activity by ∼2.7-fold. Again, coincubation with MRS2179 blocked this P2Y1R response (Fig. 6D). This initial stage of promoter activation is, not surprisingly, more sensitive to such regulation than the downstream stages of mRNA and protein production (Fig. 6, A and B) so that even the weaker agonist ATP can then (Fig. 6D) evoke the maximum effect seen.
The downstream activation of this P2Y1R-induced gene expression was also probed. Thus, a PKC activator, TPA, potently induced the promoter activity in a dose-dependent manner: at 5 nM TPA, the induction was 4-fold that in untreated cultures (Fig. 6E). Testing a known pathway via the kinase Raf-1, two cDNA constructs were used: wild-type (RafWT) and a constitutively active, membrane-targeted mutant (RafCAAX) (Schönwasser et al., 1998). When these cDNAs were cotransfected with pAChE-Luc into cultured neurons, they each produced an increase in ACHE gene promoter activity. A greater and robust induction, to ∼6-fold, was revealed in the RafCAAX-expressed neurons (Fig. 6E), suggesting that a Raf signaling pathway is involved in regulating the transcriptional activity of the ACHE gene promoter.
Elk-1 is one of the transcription factors that can be phosphorylated through Raf/mitogen-activated protein kinase kinase/ERK pathway; this phosphorylation of Elk-1 subsequently activates the gene transcription of the ACHE gene (Choi et al., 2003). A search in the promoter region of the human ACHE gene sequence for potential binding sequences for Elk-1 had revealed two functional sites downstream of the 5′-untranslated exon 1; these 2 sites have been demonstrated to be responsive to P2Y1 receptor activation in muscle (Choi et al., 2003). They are located in the first intron, at −1431 to −1412 bp and at −1102 to −1083 bp upstream of the ATG start site, designated as Elk-1[1] and Elk-1[3] respectively, as shown in Fig. 6F. Association of these Elk-1 binding sites in the ACHE gene promoter with P2Y1R activation was further demonstrated here. The ∼2.2-kilobase upstream sequence of human ACHE gene, which includes the promoter region, was mutated at those Elk-1[1] and Elk-1[3] elements, singly or together, to change six consecutive nucleotides in each. These mutant promoters were then tagged downstream with the luciferase reporter to give the constructs pAChEΔElk[1]-Luc, pAChEΔElk[3]-Luc, and pAChEΔElk[1,3]-Luc (Fig. 6F). When these DNA constructs were transfected into the cultured neurons, the mutated constructs were found to have lost all of the nucleotide-induced activation of the ACHE promoter activity (Fig. 6F, right).
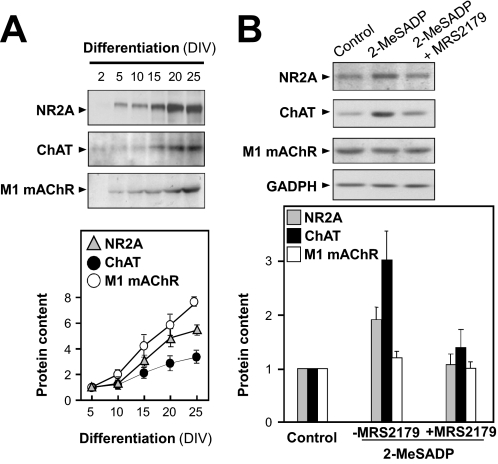
To investigate whether this P2Y1R-mediated gene regulation extends to other components of neuronal signaling, we selected for study some genes involved in the NMDA-glutamatergic and cholinergic systems. By immunoblot analysis, the native expression was demonstrated in the cultured neurons of at least 1 subunit of the NMDA receptor (NR2A at ∼170 kDa), of the acetylcholine-synthesis enzyme ChAT at ∼65 kDa, and of the mAChR subtype M1 at ∼50 kDa. The protein expressions of these components increased during neuronal differentiation (Fig. 7A). By comparing the differentiated and undifferentiated neurons, an increase to 3- to 7-fold was revealed for them (Fig. 7A). Upon treatment of the neuronal cultures with 2-MeSADP (50 μM for 24 h), the gene expressions of NR2A and ChAT were induced significantly, and coincubation with the P2Y1-specific antagonist MRS2179 blocked this response (Fig. 7B). The greatest effect was seen for ChAT, up to ∼3-fold. No significant effects were seen at the muscarinic AChR M1 subunit gene (Fig. 7B). These data, taken with the preceding results, indicate that activation of the native P2Y1Rs on neurons of the cortex can differentially regulate the gene expression of some of the effector components of glutamatergic and cholinergic CNS synapses.
Fig. 7.
Activation of P2Y1Rs differentially induces synaptic gene expressions. A, immunoblots show the profiles of the expression in the cultured neurons of the glutamate receptor NR2A (∼170 kDa) subunit and of the cholinergic components ChAT (∼65 kDa) and M1 mAChR (∼50 kDa). Quantitations from a set of four such blots are shown at the bottom as multiples of the value at 5 DIV. B, neuronal cultures were treated with 2-MeSADP (50 μM, 24 h) alone or plus MRS2179 (250 μM). GAPDH is the protein loading control. Quantitation (n = 4 blots) is shown at the bottom.
Discussion
Expression of P2Y1R (mRNA and protein) was clearly seen on cortex neurons, in adult brain, and in culture (Fig. 1). This extends the previous findings on P2Y1R mRNA in the cortex. Thus, Moore et al. (2001) found it is strongly expressed in total extracts of the adult human cortex, and Bennett et al. (2003) showed that it is abundant in extracts of E14 rat cortex and in mixed-cell cultures derived from it, and in glia cultured alone. Specifically, in the multiprocess-bearing astrocytes freshly isolated from the rat cortex or hippocampus, 10 to 30% of the cells have been found to contain P2Y1R mRNA and to show P2Y1R functional responses, with this proportion increasing to ∼90% of astrocytes after 2 weeks in culture (Zhu and Kimelberg, 2001). P2Y2R mRNA was absent initially. Webb et al. (1998) mapped P2Y1R mRNA by its quantitative in situ hybridization in the chick brain: it is strongly expressed in the avian precursor of the mammalian cortex and in the hippocampus, cerebellum, and the striatal complex and has a strong presence there in the neurons. These lines of evidence raised our interest in an investigation of the functional role of P2Y1R in the brain. We had shown previously the presence of P2Y1R at skeletal nerve-muscle synapses and its role there in the control of gene expression of cholinergic-effector proteins (see Introduction). This is extended here to the very different neuron-neuron synapses of the brain and to noncholinergic transmission.
A recent consensus has developed that ATP is constantly released onto brain neurons, in part from other neurons but largely from astrocytes (Zhang et al., 2003; Bowser and Khakh, 2004; Perea et al., 2009). Most synapses of forebrain neurons in situ are tightly enwrapped by astrocyte processes, with most astrocytic ATP release focused onto synapses (Perea et al., 2009). In our cultures, ATP release was indeed greatly increased by glial presence (Supplemental Data S3). Most released ATP is rapidly hydrolyzed at neuronal surfaces, but mainly to ADP rather than adenosine by the triphosphate-preferring nucleoside-triphosphate-diphosphohydrolase-2, which is the main ecto-nucleotidase thereon (Neary and Zimmermann, 2009). ADP has a much higher agonist potency than ATP at mammalian P2Y1Rs (Simon et al., 2001; Abbracchio et al., 2006); hence, its action is significant at submicromolar levels: levels of ADP plus ATP predicted in the unstirred surface layer (Joseph et al., 2003) from constant ATP release, further stimulated via glutamate released in brain activity (Zhang et al., 2003), would be ample to elicit P2Y1R responses on those neurons in situ (Supplemental Data S3).
Signaling Steps in the ATP-Induced ACHE Gene Expressions.
The pathway of gene regulation by P2Y1R in cortical neurons was explored for one target, the ACHE gene. AChE is a multifunctional protein well known for its classic role as a hydrolytic enzyme for synaptic acetylcholine and its nonclassic roles such as an adhesion protein to promote cell-to-cell recognition or to induce signal transduction events under different physiological conditions. Thus, several neuronal binding partners of AChE are known, including β-amyloid (Alvarez et al., 1997), RACK1 (Birikh et al., 2003), laminin-1 (Paraoanu and Layer, 2004), and α7 nicotinic acetylcholine receptor (Greenfield et al., 2008). Hence, the finding of a regulation of the brain AChE gene activation via a transmitter has a wider significance.
Activation of P2Y1R in cortical neurons induced mobilization of intracellular Ca2+ and ERK1/2 expression, as is known for P2Y1Rs in recombinant expression (Sellers et al., 2001), and likewise when endogenous, as in muscle cells (Choi et al., 2003). This activation induced increases in AChE mRNA and protein and in ACHE gene promoter activity, involving protein kinase C and Raf-1. This leads to the phosphorylation of Elk-1 and its action at a responsive element within the ACHE gene promoter, already known (Choi et al., 2003) to act thus in muscle.
Regarding the P2Y1R-induced AChE expression, the AChE enzymatic activity (attributed to the AChET variant) remained unchanged (data not shown). That phenomenon occurred likewise in our previous studies in muscle (Choi et al., 2001a, 2003) and in other findings (Rotundo, 2003), in which the AChET protein existed as both active and inactive pools. It is noteworthy that such an observation in the nervous system was different from that in the immune system; the change of mRNA/protein expression of AChER variant corresponded to the change of AChE activity in splenocytes (Shaked et al., 2009), suggesting that the control of enzymatic activity would be isoform-specific.
The predominant form (11S) of AChE in mammalian brain is known to differ from that in skeletal muscle. The catalytic subunit (AChET) is the same in both, but in brain, it assembles as a tetramer anchored at the neuronal membrane through another (PRiMA) subunit, whereas muscle subunits assemble differently as multimers around a collagen-tail subunit and insert thereby into the extracellular matrix (Perrier et al., 2002). It was hitherto unknown whether the regulation of ACHE gene expression in brain neurons would operate as seen (Choi et al., 2003) in muscle, but we find here that its P2Y1R-initiated pathway is common to both. However, a clear distinction in this between the two tissues is known at the promoter level (Camp et al., 2005): a deletion in transgenic mice of a 255-bp region of ACHE intron-1 suppressed its expression in muscle but not in brain. That region contains an N-box and an E-box motif, which exert ACHE gene promoter activity in muscle by binding other trophic factors (Schaeffer et al., 2001), but those motifs are distant from the Elk-1 sites involved here. Hence, the AChE promoter responsive to Elk-1 via nucleotide/P2Y1R activation is distinct, both by its location in the gene and its operation in both muscle and brain expression. Such distinguishing characteristics suggest that this nucleotide activation plays a significant role in brain neuronal function.
Neuronal P2Y1 Receptors Are in a Postsynaptic Microdomain.
We found that in well differentiated neuronal cultures, the major fraction of the P2Y1Rs is located either in the cell body membrane region or at punctate sites (developing spines) on the dendrites, strongly colocalized there with the postsynaptic protein PSD-95. As we noted, this agrees with electron-microscopic evidence (Tonazzini et al., 2007) that P2Y1Rs in hippocampal sections are predominantly localized at the PSD. Coanchorage of different receptors plus their effectors in microdomains via such scaffolds is currently seen as integrating much Gq/11-based signaling (Bockaert et al., 2003; Delmas et al., 2004; Kim and Sheng, 2004). This is now extended to P2Y1R, as concluded from that colocalization plus several lines of additional evidence (Figs. 1–3). First, P2Y1Rs were coimmunoprecipitated from lysates of native cortex with PSD-95 (and vice versa) or with NR2A subunits from cortical neuron extracts by antibody to any one of those three proteins; NR2A is already known (Kim and Sheng, 2004) to bind to PSD-95. Second, after PSD-95 was expressed in HEK 293T cells, it was cytosolic but could largely be recruited to the cell membrane by coexpressed P2Y1R. Third, because such PSD-95 attachments occur at one of its PDZ domains through the C-terminal tetrapeptide of each ligand protein (Kim and Sheng, 2004), which in P2Y1R is DTSL, we mutated or deleted that motif. This abolished the coimmunoprecipitation of PSD-95 and P2Y1R, and their membrane colocalization.
Another mode of anchoring P2Y1Rs is known via a PDZ domain in NHERF-2 (Fam et al., 2005), and we have found that P2Y1R in cells can dimerize by binding thus to NHERF-2 (Choi et al., 2008). NHERF-2 is present postsynaptically in forebrain neurons in situ, as shown in the mouse cortex (Paquet et al., 2006), and we found P2Y1R also located there. NHERF-2 is presumed to contribute to the organization of P2Y1Rs at the membrane. However, contrary to the similar conclusion drawn here for PDS-95, Fam et al. (2005) observed no binding of P2Y1Rs to PSD-95. This was based on screening a proteomic array of PDZ domains against an overlay of a P2Y1R C-terminal 50-amino acid peptide, fused to glutathione transferase for detection. The PDZ-domain isolated from the PSD-95 sequence did not bind to that region of the P2Y1R in this system. Positive results from this screen have identified various PDZ-domain/receptor interactions, including NHERF-2/P2Y1R (Fam et al., 2005; Paquet et al., 2006). However, we considered whether another interaction might be made there for certain pairs such as PSD-95/P2Y1R. Thus, first, the full-length PSD-95 is known from crystallographic and other evidence to require a specific folding for a PDZ domain to bind a GPCR partner held within the PDS-95 tertiary structure (Kim and Sheng, 2004), and this may not always be attainable in an isolated PDZ domain/fusion protein screen. Second, in exceptional cases, PDZ complexing may require a nontail additional binding site in the GPCR, as with the mGluR1/tamalin scaffold PDZ-based pairing: there, such screening gave negative results (Paquet et al., 2006), but other methods (Hirose et al., 2004) show that a second binding site also acts, located between 89 and 179 amino acids from the mGluR1 C terminus (SSTL). The coimmunoprecipitation and DTSL-dependence tests that we applied (Figs. 2 and 3) to demonstrate this for P2Y1R/PSD-95 had likewise given positive results when used (Fam et al., 2005) in confirming the P2Y1R/NHERF-2 attachment in heterologous coexpression.
We also considered the possibility that the P2Y1R/PSD-95 interaction shown might be indirect through bridging by NHERF-2 binding to both using its two NHERF-2 PDZ-domains. Thus, some PDZ-domain-containing scaffolding proteins can indeed link to another family member to form heteromultimers (Lau and Hall, 2001); however, in that study (using coimmunoprecipitation of the full-length proteins), whereas NHERF-2 showed linkage to NHERF-1 or to itself, no NHERF-2 binding was found to PSD-95 or to any of several other PDZ proteins tested. Other potentially bridging neuronal scaffolds not tested here can also be disregarded, because we additionally showed that the P2Y1R/PSD-95 interaction is strong in transfected HEK cells. [For NHERF-2 itself, although also endogenous in our HEK cells, it is there only at a low level (Choi et al., 2008) and could not account for these effects (Figs. 2 and 3) on the much larger amounts of interacting P2Y1R and PSD-95 heterologously expressed there.] Hence, direct P2Y1R/PSD-95 interaction remains the most likely explanation of our findings.
Relationships of P2Y1 Receptors to Neuronal Functions.
Activation of forebrain P2Y1Rs is known electrophysiologically to modulate neuron excitability (Bowser and Khakh, 2004; Perea et al., 2009), and to inhibit the M current K+-channel to increase by 10-fold the firing rate of identified pyramidal neurons (Filippov et al., 2006). The modulation of excitability was proposed, in the studies cited, to arise from a relationship of synaptic P2Y1Rs to NMDA receptors. P2Y1Rs localized at a microdomain based on PSD-95 and NHERF-2, as deduced here, can provide the basis for this and for the P2Y1R signaling cascade to selective gene transcriptions.
Although most of the activating ATP/ADP derives from glia (see above), neuronal ATP/P2Y1R Ca2+-mobilization was also seen here with glia absent, plus longer-term gene-regulation of signaling components. We suggest that this behavior contributes to the finding that blocking P2Y1R by its antagonists or short hairpin RNA impairs the proliferation and migration of the intermediate neuronal progenitors in the developing mouse cortex (Liu et al., 2008). In addition, the cholinergic system has long been known to modulate other neurotransmission systems such as glutamatergic and GABAergic activity in the cerebral cortex (Pepeu and Blandina, 1998; Manns et al., 2001; McKay et al., 2007). Here, we showed that ATP/P2Y1R could be another such modulating pathway in the brain because it could induce the activity of cholinergic and glutamatergic genes. These conclusions, together with the other evidence presented here, suggest a functional significance for our findings, meriting further investigation.
Supplementary Material
Acknowledgments
We thank Drs. A. K. Filippov and J. Reilly (University College London) for hippocampal cultures. We thank Croucher Foundation for providing the scholarship to Nina L. Siow.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the University Grants Committee [Grant AoE/B-15/01]; Research Grants Council of the Hong Kong SAR China [Grants HKUST 660409; 6237/04M; 6404/05M; 6419/06M; and 3/03C]; and the Wellcome Trust [Grant 081706].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.066506.
- CNS
- central nervous system
- A3P5P
- adenosine-3′,5′-bisphosphate
- AChE
- acetylcholinesterase
- Ara-C
- cytosine arabinoside
- ChAT
- choline acetyltransferase
- DIV
- days in vitro
- ERK
- extracellular signal-regulated kinase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GFAP
- glial fibrillary acidic protein
- GFP
- green fluorescent protein
- GPCR
- G protein-coupled receptor
- HA
- hemagglutinin
- HBSS
- Hanks’ balanced salt solution
- HEK
- human embryonic kidney
- Luc
- luciferase
- mAChR
- muscarinic acetylcholine receptor
- MAP-2
- microtubule-associated protein 2
- 2-MeSADP
- 2- (methylthio)adenosine 5′-diphosphate
- NHERF-2
- Na+/H+ exchanger regulatory factor type 2
- NR2
- N-methyl-d-aspartate receptor 2A
- PCR
- polymerase chain reaction
- PKC
- protein kinase C
- PRiMA
- proline-rich membrane anchor
- PSD-95
- postsynaptic density protein 95
- P2Y1R
- P2Y1 receptor
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- TTX
- tetrodotoxin
- NMDA
- N-methyl-d-aspartate
- bp
- base pair
- Ex
- excitation
- Em
- emission
- E
- embryonic day
- P
- postnatal day
- MRS2179
- 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium salt.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, et al. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Opazo C, Alarcón R, Garrido J, Inestrosa NC. (1997) Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J Mol Biol 272:348–361 [DOI] [PubMed] [Google Scholar]
- Ben Aziz-Aloya R, Seidman S, Timberg R, Sternfeld M, Zakut H, Soreq H. (1993) Expression of a human acetylcholinesterase promoter-reporter construct in developing neuromuscular junctions of Xenopus embryos. Proc Natl Acad Sci USA 90:2471–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GC, Ford AP, Smith JA, Emmett CJ, Webb TE, Boarder MR. (2003) P2Y receptor regulation of cultured rat cerebral cortical cells: calcium responses and mRNA expression in neurons and glia. Br J Pharmacol 139:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birikh KR, Sklan EH, Shoham S, Soreq H. (2003) Interaction of “readthrough” acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc Natl Acad Sci USA 100:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L. (2003) The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546:65–72 [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. (2004) ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci 24:8606–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. (1998) Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem 41:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp S, Zhang L, Marquez M, de la Torre B, Long JM, Bucht G, Taylor P. (2005) Acetylcholinesterase (AChE) gene modification in transgenic animals: functional consequences of selected exon and regulatory region deletion. Chem Biol Interact 157:79–86 [DOI] [PubMed] [Google Scholar]
- Choi RC, Man ML, Ling KK, Ip NY, Simon J, Barnard EA, Tsim KW. (2001a) Expression of the P2Y1 nucleotide receptor in chick muscle: its functional role in the regulation of acetylcholinesterase and acetylcholine receptor. J Neurosci 21:9224–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Siow NL, Cheng AW, Ling KK, Tung EK, Simon J, Barnard EA, Tsim KW. (2003) ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J Neurosci 23:4445–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Siow NL, Zhu SQ, Wan DC, Wong YH, Tsim KW. (2001b) The cyclic AMP-mediated expression of acetylcholinesterase in myotubes shows contrasting activation and repression between avian and mammalian enzymes. Mol Cell Neurosci 17:732–745 [DOI] [PubMed] [Google Scholar]
- Choi RC, Simon J, Tsim KW, Barnard EA. (2008) Constitutive and agonist-induced dimerizations of the P2Y1 receptor: relationship to internalization and scaffolding. J Biol Chem 283:11050–11063 [DOI] [PubMed] [Google Scholar]
- Delmas P, Crest M, Brown DA. (2004) Functional organization of PLC signaling microdomains in neurons. Trends Neurosci 27:41–47 [DOI] [PubMed] [Google Scholar]
- Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, Smith Y, Yun CC, Hall RA. (2005) P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA 102:8042–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Choi RC, Simon J, Barnard EA, Brown DA. (2006) Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons. J Neurosci 26:9340–9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. (2008) Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31:563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Zhang JY, Zhang XJ. (2009) Calcium signaling-induced Smad3 nuclear accumulation induces acetylcholinesterase transcription in apoptotic HeLa cells. Cell Mol Life Sci 66:2181–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SA, Zimmermann M, Bond CE. (2008) Non-hydrolytic functions of acetylcholinesterase. The significance of C-terminal peptides. FEBS J 275:604–611 [DOI] [PubMed] [Google Scholar]
- Hilgenberg LG, Smith MA. (2007) Preparation of dissociated mouse cortical neuron cultures. J Vis Exp 10:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Kitano J, Nakajima Y, Moriyoshi K, Yanagi S, Yamamura H, Muto T, Jingami H, Nakanishi S. (2004) Phosphorylation and recruitment of Syk by immunoreceptor tyrosine-based activation motif-based phosphorylation of tamalin. J Biol Chem 279:32308–32315 [DOI] [PubMed] [Google Scholar]
- Hussl S, Boehm S. (2006) Functions of neuronal P2Y receptors. Pflugers Arch 452:538–551 [DOI] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. (2003) Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278:23331–23342 [DOI] [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F. (2004) Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci 24:10835–10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5:771–781 [DOI] [PubMed] [Google Scholar]
- Lau AG, Hall RA. (2001) Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry 40:8572–8580 [DOI] [PubMed] [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. (2007) Astrocytic control of synaptic NMDA receptors. J Physiol 581:1057–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KK, Siow NL, Choi RC, Tsim KW. (2005) ATP potentiates the formation of AChR aggregate in the co-culture of NG108–15 cells with C2C12 myotubes. FEBS Lett 579:2469–2474 [DOI] [PubMed] [Google Scholar]
- Liu X, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P. (2008) The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci USA 105:11802–11807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. (2001) Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience 107:249–263 [DOI] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. (2007) Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 74:1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. (2001) Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521:107–119 [DOI] [PubMed] [Google Scholar]
- Neary JT, Zimmermann H. (2009) Trophic functions of nucleotides in the central nervous system. Trends Neurosci 32:189–198 [DOI] [PubMed] [Google Scholar]
- Paquet M, Asay MJ, Fam SR, Inuzuka H, Castleberry AM, Oller H, Smith Y, Yun CC, Traynelis SF, Hall RA. (2006) The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. J Biol Chem 281:29949–29961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraoanu LE, Layer PG. (2004) Mouse acetylcholinesterase interacts in yeast with the extracellular matrix component laminin-1beta. FEBS Lett 576:161–164 [DOI] [PubMed] [Google Scholar]
- Pepeu G, Blandina P. (1998) The acetylcholine, GABA, glutamate triangle in the rat forebrain. J Physiol Paris 92:351–355 [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431 [DOI] [PubMed] [Google Scholar]
- Perrier AL, Massoulié J, Krejci E. (2002) PRiMA: the membrane anchor of acetylcholinesterase in the brain. Neuron 33:275–285 [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 25:6286–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo RL. (2003) Expression and localization of acetylcholinesterase at the neuromuscular junction. J Neurocytol 32:743–766 [DOI] [PubMed] [Google Scholar]
- Schaeffer L, de Kerchove d'Exaerde A, Changeux JP. (2001) Targeting transcription to the neuromuscular synapse. Neuron 31:15–22 [DOI] [PubMed] [Google Scholar]
- Schönwasser DC, Marais RM, Marshall CJ, Parker PJ. (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18:790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. (2001) Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem 276:16379–16390 [DOI] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. (2009) MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31:965–973 [DOI] [PubMed] [Google Scholar]
- Simon J, Vigne P, Eklund KM, Michel AD, Carruthers AM, Humphrey PP, Frelin C, Barnard EA. (2001) Activity of adenosine diphosphates and triphosphates on a P2Y(T) -type receptor in brain capillary endothelial cells. Br J Pharmacol 132:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow NL, Choi RC, Cheng AW, Jiang JX, Wan DC, Zhu SQ, Tsim KW. (2002) A cyclic AMP-dependent pathway regulates the expression of acetylcholinesterase during myogenic differentiation of C2C12 cells. J Biol Chem 277:36129–36136 [DOI] [PubMed] [Google Scholar]
- Tonazzini I, Trincavelli ML, Storm-Mathisen J, Martini C, Bergersen LH. (2007) Co-localization and functional cross-talk between A1 and P2Y1 purine receptors in rat hippocampus. Eur J Neurosci 26:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung EK, Choi RC, Siow NL, Jiang JX, Ling KK, Simon J, Barnard EA, Tsim KW. (2004) P2Y2 receptor activation regulates the expression of acetylcholinesterase and acetylcholine receptor genes at vertebrate neuromuscular junctions. Mol Pharmacol 66:794–806 [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Barnard EA. (1998) Regional distribution of [35S]2′-deoxy 5′-O-(1-thio) ATP binding sites and the P2Y1 messenger RNA within the chick brain. Neuroscience 84:825–837 [DOI] [PubMed] [Google Scholar]
- Xie HQ, Choi RC, Leung KW, Chen VP, Chu GK, Tsim KW. (2009) Transcriptional regulation of proline-rich membrane anchor (PRiMA) of globular form acetylcholinesterase in neuron: an inductive effect of neuron differentiation. Brain Res 1265:13–23 [DOI] [PubMed] [Google Scholar]
- Xie HQ, Choi RC, Leung KW, Siow NL, Kong LW, Lau FT, Peng HB, Tsim KW. (2007) Regulation of a transcript encoding the proline-rich membrane anchor of globular muscle acetylcholinesterase. The suppressive roles of myogenesis and innervating nerves. J Biol Chem 282:11765–11775 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. (2003) Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci USA 100:15194–15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40:971–982 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kimelberg HK. (2001) Developmental expression of metabotropic P2Y(1) and P2Y(2) receptors in freshly isolated astrocytes from rat hippocampus. J Neurochem 77:530–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.