Abstract
Melanoma differentiation associated gene-7 (mda-7)/interleukin-24 (IL-24), a member of the IL-10 cytokine gene family, preferentially induces cell death in neoplastic epithelial cells types while sparing their normal counterparts. The effects of mda-7/IL-24 in acute myeloid leukemia (AML) cells have not been extensively characterized. Treatment with recombinant GST-MDA-7/IL-24 potently induced apoptosis in diverse myeloid leukemia cell types including U937, HL60, MV4-11, EOL-1, and MLL/ENL cells. MDA-7/IL-24 also markedly induced apoptosis in and suppressed the colony-forming capacity of primary AML blasts but exerted minimal toxicity toward normal CD34+ hematopoietic progenitor cells. MDA-7/IL-24 lethality was associated with pronounced endoplasmic reticulum (ER) stress induction in leukemia cell lines and primary AML blasts, manifested by the accumulation of growth arrest and DNA damage-inducible protein 34 (GADD34), 78-kDa glucose-regulated protein (GRP78)/BiP, inositol-requiring enzyme 1α (IRE1α), and eukaryotic initiation factor 2α phosphorylation. It is noteworthy that short hairpin RNA (shRNA) knockdown of IRE1α, GADD34, or GRP78/BiP significantly enhanced MDA-7/IL-24-mediated apoptosis, indicating a protective role for these molecules against MDA-7/IL-24 lethality. MDA-7/IL-24 also down-regulated the antiapoptotic protein Mcl-1 and sharply increased expression of the proapoptotic proteins Bim and Noxa. Ectopic Mcl-1 expression or shRNA knockdown of Bim or Noxa significantly attenuated MDA-7/IL-24-mediated leukemia cell death. Finally, knockdown of Bax or Bak significantly reduced MDA-7/IL-24 lethality. Together, these findings indicate that MDA-7/IL-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by ER stress induction, Mcl-1 down-regulation, and Bim and Noxa up-regulation. They also suggest that MDA-7/IL-24 warrants further investigation in myeloid leukemia.
Introduction
Melanoma differentiation associated gene-7/interleukin-24 (MDA-7/IL-24), a cytokine classified as a member of the IL-10 family, was initially identified through a subtraction hybridization approach combined with a differentiation therapy model of human melanoma as a gene up-regulated during terminal differentiation (Jiang et al., 1995). MDA-7/IL-24 potently inhibits cell growth and induces apoptosis in various epithelial cancers in vitro and in vivo, including melanoma, glioma, breast, colorectal, lung, ovarian, and prostate cancers, among others (Fisher, 2005). In contrast to its effects on transformed cells, MDA-7/IL-24 has shown only minimal lethality toward normal cells (Gupta et al., 2006a). mda-7/IL-24 is currently undergoing clinical evaluation (phase I) in patients with melanoma and other advanced solid tumors and has shown significant preliminary evidence of activity.
The ability of MDA-7/IL-24 to inhibit cell growth of and to induce apoptosis in tumor cells has been attributed to multiple mechanisms including perturbations in signaling pathways (e.g., p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase), antiangiogenic actions, immune modulation, bystander antitumor activity, autocrine regulation, induction of oxidative damage, and modulation of the expression of pro- and antiapoptotic Bcl-2 family members (Fisher, 2005; Dash et al., 2010a). Recent attention has focused on the capacity of MDA-7/IL-24 to initiate endoplasmic reticulum stress (ER stress) in transformed cells. In response to diverse ER stress inducers, cells trigger a well orchestrated evolutionarily conserved response referred to as the unfolded protein response (UPR). This process involves inhibition of protein synthesis, induction of protein chaperones, and enhanced protein degradation (ER-assisted degradation). Initially, the UPR serves as an adaptive/cytoprotective response permitting cells to clear the mass of infolded or misfolded protein accumulated within the ER. However, if the stress within the ER becomes too severe, the UPR promotes cell death (Scorrano et al., 2003; Bernales et al., 2006; Ron and Walter, 2007). In this context, our group has recently reported that MDA-7/IL-24 localizes to the ER compartment, physically binds to the ER resident chaperone protein GRP78/BiP, and triggers ER stress, manifested by the induction of DNA damage-inducible genes such as GADD153, GADD45α, and GADD34, calcium release into the cytosol, PKR-like endoplasmic reticulum kinase activation, eIF2α phosphorylation, and protein translation inhibition (Sarkar et al., 2002; Gupta et al., 2006b; Park et al., 2008). In addition, MDA-7/IL-24 lethality is regulated by members of the Bcl-2 family. For example, the antiapoptotic protein Mcl-1, which is known to protect cells from ER stress-induced cell death (Fritsch et al., 2007; Rahmani et al., 2007a), markedly attenuated mda-7/IL-24 lethality in prostate cancer cells (Dash et al., 2010b). Together, these findings suggest a role for ER stress in the regulation of MDA-7/IL-24-mediated cytotoxicity toward transformed cells.
Although MDA-7/IL-24 has been extensively studied in epithelial tumors, the therapeutic potential of this molecule has been minimally explored in a limited number of hematologic malignancies, such as chronic lymphocytic leukemia (Sainz-Perez et al., 2006) or chronic myeloid leukemia (Dong et al., 2008). As a consequence, very little is known about the activity or mechanism of action of this molecule in acute myeloid leukemia (AML) cells. The results of this study indicate that MDA-7/IL-24 potently induces apoptosis in diverse human myeloid leukemia cell types, including primary leukemic blasts, but is relatively sparing toward normal CD34+ hematopoietic cells. Moreover, the present findings provide the first evidence of a functional role for components of the ER stress machinery in the regulation of MDA-7/IL-24 lethality toward AML cells.
Materials and Methods
Cells and Constructs.
Human leukemia U937 (myelomonocytic), HL60 (promyelocytic), MV4-11, EOL1 (both FLT3-mutated), and MLL-ENL (leukemia stem cell-like) (Barabé et al., 2007) cells were cultured as reported previously (Rahmani et al., 2003). U937 cells overexpressing Mcl-1 were described previously. U937 cells in which GADD34 and GRP78/BiP were knocked down were obtained by infecting U937 cells with lentiviruses carrying specific short hairpin RNA (shRNA) constructs (Santa Cruz Biotechnology, Santa Cruz, CA). Stable clones from a single cell were selected in the presence of puromycin (1 μg/ml) and tested for the expression of the corresponding proteins. U937 cells stably expressing shRNA directed against Bax or Bak were described previously (Rahmani et al., 2009). Knockdown of Bim or Noxa was obtained by transfecting U937 cells with microRNA-adapted shRNA constructs specifically designed against human Bim or Noxa (Open Biosystems, Huntsville, AL). The sequences used were Bim, 5′-GCAGGCTGAACCTGCAGATAT-3′, and Noxa, 5′-ACACATACTTACATACTTATA-3′. U937 cells transfected with shRNA constructs against GFP were used as controls for various shRNA-expressing cells.
Isolation of Patient-Derived Leukemic Blasts.
Leukemic blasts were obtained with informed consent from the bone marrow of patients with relapsed AML, FAB subtype M2. These studies have been sanctioned by the Investigational Review Board of Virginia Commonwealth University/Medical College of Virginia, and all patients provided informed consent. In each case, the percentage of blasts in the peripheral blood was >70%. Bone marrow was collected into heparinized syringes, and mononuclear cells were isolated as described previously (Rahmani et al., 2005).
Isolation of CD34+ Cells.
Normal CD34+ cells were obtained with informed consent from the bone marrows of patients undergoing routine diagnostic procedures for nonmyeloid hematopoietic disorders (e.g., thrombocytopenia, iron-deficiency anemia). CD34+ cells were isolated from mononuclear cell preparations using an immunomagnetic bead separation technique (Miltenyi Biotec Inc., Auburn, CA) as described previously (Rahmani et al., 2007b). In some experiments, normal CD34+ cells were isolated from cord blood and processed as above. CD34+ cells were resuspended in RPMI medium containing 10% fetal calf serum and exposed to agents exactly as described above for human leukemia cells.
Reagents.
GST-MDA-7/IL-24 was generated as described in detail previously (Su et al., 2003).
Assessment of Apoptosis.
Apoptotic cells were routinely identified by Annexin V-fluorescein isothiocyanate staining as described previously (Rahmani et al., 2002).
Leukemic and Normal Hematopoietic Colony Formation Assays.
The colony-forming capacity of leukemic (L-CFU) and normal CD34+ cells (CFU-GM) was monitored by methylcellulose colony-forming assays as we have described recently in detail (Rahmani et al., 2009).
Western Blot Analysis.
Western blot analysis was performed using the whole-cell lysates as described previously in detail (Rahmani et al., 2002). The primary antibodies used in this study were caspase-3, Bax, Bcl-2, and Mcl-1 (BD Pharmingen, San Diego, CA); caspase-8 and Noxa (Alexis Corp, San Diego, CA); caspase-4 (Stressgene Bioreagents, Ann Arbor, MI); poly(ADP-ribose) polymerase (PARP) (BIOMOL Research Laboratories, Plymouth Meeting, PA); IRE1α, phospho-eIF2α, extracellular signal-regulated kinase 1/2, Bcl-xL, and cleaved caspase-3 (Cell Signaling Technology, Danvers, MA). GADD34, GRP78/BiP, AIF, cytochrome c (Santa Cruz Biotechnology, Santa Cruz, CA); and Bim, Bak, and α-tubulin (Calbiochem, San Diego, CA).
Subcellular Fractionation.
Leukemic cells (4 × 106) were lysed using digitonin buffer, after which cytosolic and membrane fractions were separated by centrifugation as described previously (Rahmani et al., 2005). The purity of cytosolic fraction was routinely confirmed by Western blot using antibodies against cytochrome c oxidase subunit IV, which resides strictly in the inner mitochondrial membrane.
Statistical Analysis.
The significance of differences between experimental conditions was determined using the Student's t test for unpaired observations.
Results
GST-MDA-7/IL-24 Potently Induces Apoptosis in Association with Profound Mitochondrial Injury and Caspase Activation in Various Human Myeloid Leukemia Cell Lines and in Primary AML Cells.
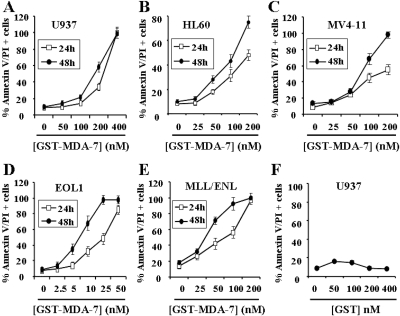
Previous studies in various epithelial tumors have demonstrated that GST-MDA-7/IL-24 potently induces apoptosis in transformed cells but exerts only minimal toxicity toward normal cells (Gupta et al., 2006a). To determine whether MDA-7/IL-24 triggered myeloid leukemia cell death, dose-response studies of GST-MDA-7/IL-24 were performed in various myeloid leukemia cell types, including U937 myelomonocytic, HL60 promyelocytic, MV4-11, and EOL1 FLT3-mutated AML and MLL-ENL-expressing cells. As shown in Fig. 1A, 24- to 48-h exposure to MDA-7/IL-24 induced pronounced cell death in U937 cells, reflected by Annexin V/PI positivity, when administered at concentrations ≥200 nM. It is noteworthy that MDA-7/IL-24 was even more potent in inducing apoptosis in HL60, MV4-11, and MLL/ENL cells, and particularly in EOL1 cells, in which only 5 to 10 nM concentrations were sufficient to induce extensive cell death after 48-h treatment (Fig. 1, B–E). It is noteworthy that GST protein alone had no effects on cell death in U937 cells (Fig. 1F) or the other cell lines (data not shown).
Fig. 1.
GST-MDA-7/IL-24 potently induces apoptosis in various human myeloid leukemia cells. U937 (A), HL60 (B), MV4-11 (C), EOL1 (D), and MLL/ENL (E) cells were exposed for 24 h (□) or 48 h (●) to the designated concentration of GST-MDA-7/IL-24, after which the percentage of apoptotic cells was determined by Annexin V/PI analysis as described under Materials and Methods. F, U937 cells were exposed to GST protein alone for 48 h, after which the extent of cell death was determined as above. Values represent the means for three separate experiments ± S.D.
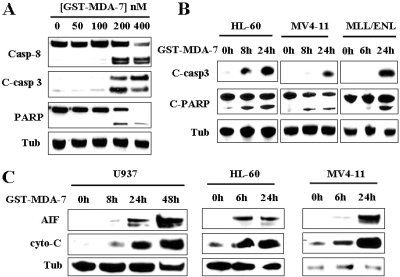
To confirm that MDA-7/IL-24 triggered apoptosis in AML cells, dose-response studies with GST-MDA-7/IL-24 were performed to monitor caspase activation and mitochondrial injury in U937, HL60, MV4-11, and MLL-ENL cells. It is noteworthy that concentrations of GST-MDA-7/IL-24 that effectively induced cell death in U937 cells (e.g., 200 nM; 48 h) resulted in a pronounced increase in caspase-3/-8 activation and PARP cleavage (Fig. 2A). Cleavage of caspase-3 and PARP were also observed in HL60, MV4-11, and MLL-ENL-expressing cells (Fig. 2B). Significantly, these effects were associated with a pronounced increase in cytosolic release of cytochrome c and AIF as shown in U937, HL60, and MV4-11 cells (Fig. 2C). Together, these findings indicate that GST-MDA-7/IL-24 effectively induces mitochondrial injury and apoptosis in diverse myeloid leukemia cell types.
Fig. 2.
GST-MDA-7/IL-24 induces profound mitochondrial injury and caspase activation in human myeloid leukemia cells. A, U937 cells were exposed to the designated concentration of GST-MDA-7/IL-24 for 48 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor caspase-3, caspase-8 cleavage/activation, and PARP degradation. B, HL60, MV4-11, and MLL/ENL cells were exposed to GST-MDA-7/IL-24 (100 nM for HL60 and MV4-11, and 50 nM for MLL/ENL) for the designated intervals, after which Western blot assays were performed as in A. C, U937, HL60, and MV4-11 cells were treated with GST-MDA-7/IL-24 (200 nM for U937 cells and 100 nM for HL60 and MV4-11 cells) for the designated intervals, after which mitochondria-free cytosolic fractions were obtained as described under Materials and Methods and subjected to Western blot analysis to monitor the release of cytochrome c and AIF into the cytosol. For this and all subsequent Western blot assays, lanes were loaded with 20 μg of protein; blots were subsequently reprobed with anti-tubulin (Tub) antibodies to document equivalent loading and transfer. The blots shown are representative of three or more independent experiments.
GST-MDA-7/IL-24 Induces Pronounced Caspase Activation and a Marked Reduction of the Clonogenic Capacity of Primary AML Blasts but Is Relatively Sparing toward Normal CD34+ Progenitor Cells.
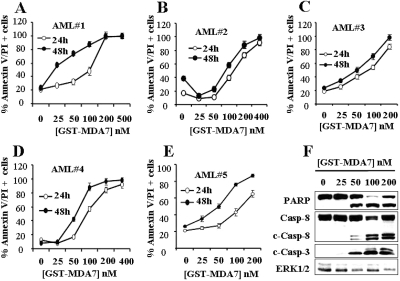
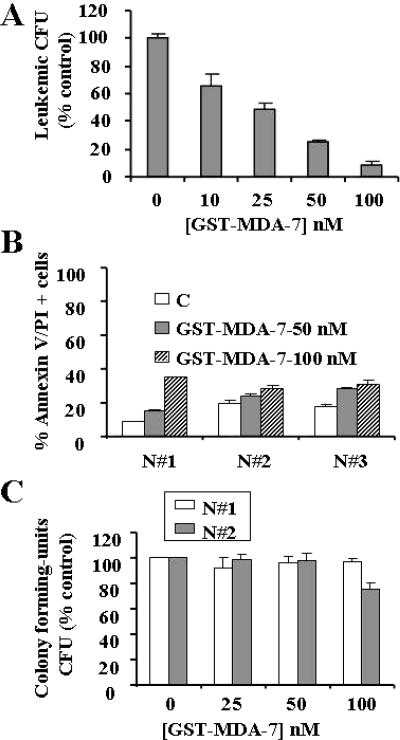
To determine whether MDA-7/IL-24-mediated apoptosis is restricted to AML cell lines, parallel dose-response studies with GST-MDA-7/IL-24 (24–48 h) were performed in five primary AML samples (all FAB M2). As shown in Fig. 3, A–E, patterns of cell death induction measured by Annexin V/PI positivity for all samples were virtually identical with those observed in continuously cultured myeloid leukemia cell lines. To establish whether GST-MDA-7/IL-24 triggers apoptosis in primary cells, primary AML blasts isolated from patient 3 were exposed to GST-MDA-7/IL-24 for 48 h, after which caspase activation and PARP cleavage were monitored. As shown in Fig. 3F, GST-MDA-7/IL-24 induced a marked increase in caspase-3 and caspase-8 cleavage as well as PARP degradation at concentrations effective in increasing Annexin V/PI positivity (≥ 50 nM). Similar results were observed in other primary AML cell specimens (data not shown). It is noteworthy that these effects were associated with a very pronounced reduction in L-CFU, which was essentially eliminated at 100 nM GST-MDA-7/IL-24 (Fig. 4A). In contrast, treatment with 100 nM GST-MDA-7/IL-24 for 48 h, which substantially induced cell death in AML blasts (e.g., ≥ 80%), exerted relatively modest toxicity toward normal CD34+ progenitor cells isolated from three normal subjects (Fig. 4B). Furthermore, the colony-forming capacity of normal CD34+ cells (CFU-GM) was reduced only minimally by an identical exposure (Fig. 4C). Together, these findings indicate that GST-MDA-7/IL-24 potently induces apoptosis in and markedly inhibits the colony-formation capacity of primary AML cells but exerts only modest toxicity toward normal hematopoietic cells.
Fig. 3.
GST-MDA-7/IL-24 potently induces apoptosis in primary AML cells. A to E, leukemic blasts were isolated as described under Materials and Methods from the bone marrow of five patients with AML (FAB classification M2) and exposed to the designated concentrations of GST-MDA-7/IL-24 for 24 or 48 h, after which the extent of apoptosis was determined using Annexin V/PI staining assay. On the other hand, protein lysates were prepared from patient 3 and subjected to Western blot analysis to monitor caspase-3 and caspase-8 cleavage/activation and PARP degradation.
Fig. 4.
GST-MDA-7/IL-24 induces a marked reduction in the clonogenic potential of primary AML blasts but is relatively sparing toward normal CD34+ progenitor cells. A, leukemic blasts isolated from patient 3 were plated in methylcellulose in the presence of the designated concentrations of GST-MDA-7/IL-24 for 14 days, after which L-CFUs were enumerated and expressed as a percentage relative to untreated cells. B, normal CD34+ cells isolated from three normal subjects (N#1–3) were exposed to GST-MDA-7/IL-24 (50 or 100 nM) for 48 h, after which cell death was determined by flow cytometry using the Annexin V staining assay. C, normal CD34+ cells (N#1–2) were plated in methylcellulose in the presence of the designated concentrations of GST-MDA-7/IL-24 for 8 days, after which CFU-GMs were enumerated and expressed as in A. Each sample was analyzed in triplicate; values represent the means ± S.D.
Evidence for ER Stress Induction by GST-MDA-7/IL-24 in Various Human Leukemia Cells Including Primary AML Blasts.
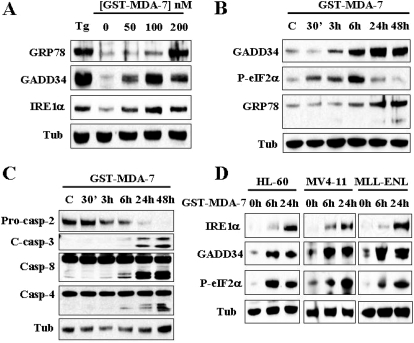
Previous studies from several groups, including our own, suggested that MDA-7/IL-24 induces ER stress in epithelial tumors (Gupta et al., 2006b; Park et al., 2008). To determine whether MDA-7/IL-24 induces ER stress in leukemia cells, U937 cells were exposed to GST-MDA-7/IL-24 (200 nM) for varying intervals, after which time-course analysis of various UPR components expression was performed. As shown in Fig. 5A, MDA-7/IL-24 triggered a dose-dependent induction of the classic UPR markers GRP78/BiP, GADD34, and IRE1α, comparable with the effects of the classic ER stress-inducer thapsigargin. In addition, time course analysis revealed that phosphorylation of eIF2α was first detected within 30 min of exposure to GST-MDA-7/IL-24 (200 nM), and substantially increased by 6 h. After 24 h of treatment, the extent of eIF2α phosphorylation returned to basal levels. Induction of GADD34 and GRP78/BiP was first noted at 3 and 6 h, respectively, and increased over the ensuing 48 h. In accord with these events, cleavage/activation of caspase-4, caspase-2, caspase-3, and caspase-8 were first noted within 6 h of exposure to GST-MDA-7/IL-24, and became more pronounced at later intervals (i.e., 24 or 48 h) (Fig. 5C). It is noteworthy that the observed induction of IRE1α, GADD34, and phosphorylation of eIF2α by GST-MDA-7/IL-24 was recapitulated in diverse leukemia cell lines including HL60-, MV4-11-, and MLL-ENL-expressing cells (Fig. 5D).
Fig. 5.
GST-MDA-7/IL-24 triggers UPR response in leukemia cells. U937 cells were exposed to the designated concentrations of GST-MDA-7/IL-24 for 48 h (A) or to 200 nM GST-MDA-7/IL-24 for the designated intervals (B and C), after which protein lysates were prepared and subjected to Western blot analysis using the indicated antibodies. D, HL60, MV4-11, and MLL-ENL were exposed to GST-MDA-7/IL-24 (100 nM for HL60 and MV4-11, and 50 nM for MLL/ENL) for the designated intervals, after which Western blot analysis was performed using indicated antibodies.
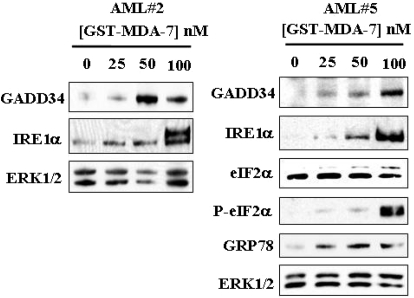
Parallel studies were performed in primary blasts isolated from two patients with AML. As shown in Fig. 6, GST-MDA-7/IL-24 significantly induced GADD34 and IRE1α expression in both specimens. Additional analysis of cells obtained from patient 2, for which sufficient material was available, revealed that GST-MDA-7/IL-24 also enhanced eIF2α phosphorylation and GRP78/BiP accumulation. Together, these findings indicate that GST-MDA-7/IL-24 induces various classic UPR-related proteins in human leukemia cells, including primary AML blasts, consistent with ER stress induction in these cells.
Fig. 6.
GST-MDA-7/IL-24 induces UPR response in primary AML cells. Leukemic blasts isolated from two patients (2 and 5) with AML were exposed to the designated concentrations of GST-MDA-7/IL-24 for 48 h, after which cells were lysed and subjected to Western blot analysis using the indicated antibodies.
IRE1α, GRP78/BiP, and GADD34 Play Functional Roles in Regulating GST-MDA-7/IL-24-Mediated Cell Death in Leukemia Cells.
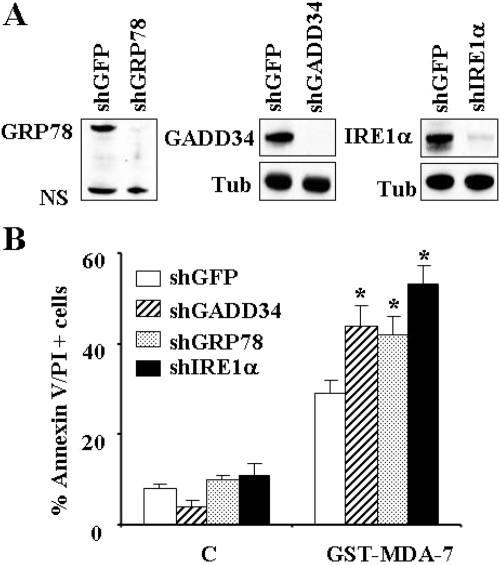
To assess the functional significance of up-regulation of IRE1α, GRP78/BiP, or GADD34 by GST-MDA-7/IL-24, U937 cell lines were generated in which these molecules were stably knocked down with specific shRNAs. As shown in Fig. 7A, cells transfected with shIRE1α, GRP78/BiP, or shGADD34 exhibited marked reductions in the expression of these proteins. It is noteworthy that each of these cells was significantly more sensitive to GST-MDA-7/IL-24-mediated cell death compared with control cells transfected with shRNA against GFP (P ≤ 0.05; Fig. 7B). These findings suggest that induction of the UPR-related proteins IRE1α, GRP78/BiP, and GADD34 by GST-MDA-7/IL-24 plays a significant functional role in regulating cell death and reinforce the notion that GST-MDA-7/IL-24 kills leukemia cells through an ER stress-regulated process.
Fig. 7.
Functional roles of IRE1α, GRP78/BiP, and GADD34 in regulating GST-MDA-7/IL-24-mediated cell death in leukemia cells. A, U937 cells were infected with lentiviruses carrying shRNA constructs against IRE1α, GRP78/BiP, GADD34, or their control GFP counterparts and selected with puromycin for 2 weeks, after which Western blot analysis was performed to monitor the level of knockdown of these proteins. B, these cells were treated with GST-MDA-7/IL-24 (150 nM) for 48 h, after which the extent of apoptosis was monitored using Annexin V/PI analysis assay. Values represent the means for three separate experiments ± S.D. *, significantly higher that values for shGFP-transfected cells (p < 0.05 in each case).
GST-MDA-7/IL-24-Mediated Lethality Involves Down-Regulation of Mcl-1 and Up-Regulation of Noxa and Bim in Human Leukemia Cells.
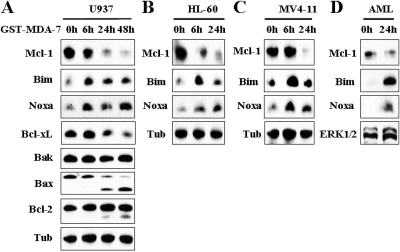
In view of the central role that Bcl-2 family members, particularly Mcl-1, Bim, and Noxa, play in survival/death decisions of malignant hematopoietic cells (Moulding et al., 2000; Derenne et al., 2002), expression and function of these proteins were examined in leukemic cells exposed to GST-MDA-7/IL-24. It is noteworthy that exposure of U937 cells to GST-MDA-7/IL-24 resulted in a down-regulation of the antiapoptotic protein Mcl-1 and significant up-regulation of the proapoptotic proteins Bim and Noxa (Fig. 8A). Similar results were observed in HL60 (Fig. 8B) and MV4-11 (Fig. 8C) cells and in primary blasts (Fig. 8D). Treatment with GST-MDA7-IL-24 also resulted in the time-dependent down-regulation of Bcl-xL and marked cleavage of Bax. In contrast, no major changes were noted in Bak protein levels, and only slight cleavage of Bcl-2 was observed at later intervals (i.e., 24–48 h).
Fig. 8.
GST-MDA-7/IL-24 down-regulates Mcl-1 and Bcl-xL and up-regulates Noxa and Bim in human leukemia cells. U937 (A), HL60 (B), MV4-11 (C) cells, or leukemic blasts (D) were exposed to GST-MDA-7/IL-24 for the designated intervals, after which Western blot analysis was performed. The GST-MDA7/IL-24 concentrations used were 200 nM for U937 cells and 100 nM for HL60, MV4-11, and leukemic blasts.
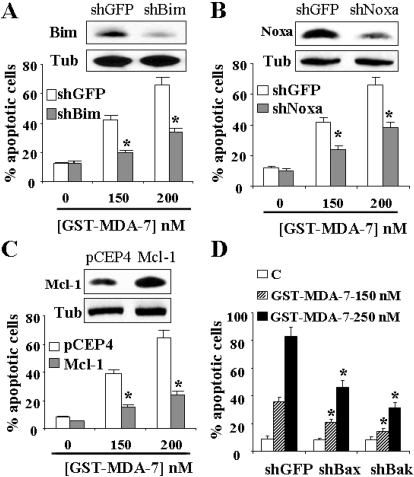
To determine whether up-regulation of Noxa and Bim contributed to MDA-7/IL-24 antileukemic activity, parallel studies were performed in U937 cells in which Bim or Noxa were knocked down by shRNA (Figs. 9, A and B, insets, respectively). It is noteworthy that these cells were significantly more resistant to MDA-7/IL-24-mediated apoptosis (P ≤ 0.05 in each case; Fig. 9, A and B). Parallel studies were performed in U937 cells ectopically expressing Mcl-1. As shown in Fig. 9C, these cells were substantially less susceptible to MDA-7/IL-24 lethality compared with their control counterparts (P ≤ 0.01). Together, these findings indicate that Mcl-1 down-regulation and Bim and Noxa up-regulation play significant functional roles in regulating the lethality of GST-MDA-7/IL-24.
Fig. 9.
Knockdown of Bim or Noxa or enforced the expression of Mcl-1 renders cells less susceptible to GST-MDA-7/IL-24 lethality. U937 cells in which Bim (A, inset) or Noxa (B, inset) was stably knocked down with shRNA, and their control counterpart shGFP-transfected cells were lysed, and protein lysates were subjected to Western blot analysis to monitor down-regulation of Bim and Noxa. Cells were then exposed to GST-MDA-7/IL-24 (150 or 200 nM) for 48 h, after which the extent of cell death was determined using the Annexin V/PI staining assay (A and B). C, U937 cells ectopically expressing Mcl-1 or the empty vector (pCEP4) were exposed to GST-MDA-7/IL-24 for 48 h, after which the extent of cell death was monitored as above. Protein lysates were also prepared from cells before treatment and Mcl-1 protein levels were monitored by Western blot analysis (C, inset). *, significantly less than values obtained for pCEP4 cells (P < 0.01). D, U937 cells in which Bak or Bax was stably knocked down with shRNA or their control counterpart cells transfected with GFP-shRNA (shGFP) were exposed to GST-MDA-7/IL-24 for 48 h, after which the extent of cell death was monitored using the Annexin V/PI staining assay. For all studies, values represent the means for three separate experiments ± S.D. For A, B, and D, *, significantly lower than values obtained for shGFP-transfected cells (P < 0.05).
In view of the critical role that Bim plays in Bax/Bak activation (Kuwana et al., 2005), the functional role of Bax and Bak in GST-MDA-7/IL-24-mediated apoptosis was investigated. To this end, U937 cells, in which Bax or Bak were stably knocked down with specific shRNA (Rahmani et al., 2009), were used. As shown in Fig. 9D, Bax or Bak knockdown cells were significantly more resistant to GST-MDA-7/IL-24-mediated apoptosis than control counterparts (P ≤ 0.05 in each case). Together these findings indicate that Bax and Bak play significant functional roles in leukemia cell death induced by GST-MDA-7/IL-24.
Discussion
Previous studies from several laboratories, including our own, have shown that MDA-7/IL-24 inhibits the growth of and induces apoptosis in cancer cells through multiple mechanisms, including perturbations in signaling pathways (e.g., p38 mitogen-activated protein kinase, c-Jun NH2-terminal kinase, and signal transducer and activator of transcription 3) antiangiogenic actions, immune modulation, bystander antitumor activity, induction of reactive oxygen species, and modulation of the expression of pro- and antiapoptotic Bcl-2 family members (Fisher, 2005; Dash et al., 2010a). We and others have reported that MDA-7/IL-24 initiates ER stress in epithelial tumor cells (Gupta et al., 2006b; Park et al., 2008). However, the precise contribution of ER stress to MDA-7/IL-24-induced cell death remains to be defined. In this context, our group has shown that in HeLa cells, MDA-7/IL-24 binds to the ER-resident chaperone GRP78/BiP in association with several DNA damage-related genes (Sarkar et al., 2002; Gupta et al., 2006b), raising the possibility that this phenomenon may contribute to lethality. In contrast to the case of epithelial malignancies, the effects of MDA-7/IL-24 have been minimally explored in acute myeloid leukemia cells, and the functional role that ER stress plays in MDA-7/IL-24-mediated antileukemic actions has not been investigated previously.
The results of the present study indicate that GST-MDA-7/IL-24 strikingly induces cell death in diverse human leukemia cell types and in primary AML blasts. It is noteworthy that the concentrations of MDA-7/IL-24 used in the present study were comparable with those shown previously to induce cell death in solid tumor cells (Sauane et al., 2004). It is noteworthy that at concentrations that induced cell death in ≥80% of cultured and primary AML cells, MDA-7/IL-24 exerted relatively minimal toxicity toward normal hematopoietic cells (CD34+). Such findings are consistent with previous reports demonstrating that MDA-7/IL-24 preferentially targets transformed versus normal cells (Sarkar et al., 2002). The present findings stand in contrast to previous results suggesting that MDA-7/IL-24 promotes the survival of chronic lymphocytic leukemia cells (Sainz-Perez et al., 2006), although this phenomenon was subsequently found to be proliferation-dependent (Sainz-Perez et al., 2008). Such a discrepancy could reflect cell type-specific effects of MDA-7/IL-24. In this context, Qian et al. (2008) described the induction of apoptosis in B lymphoblastic leukemia cells by a conditionally replicating mda-7/IL-24 adenovirus. On the other hand, stable transfection of chronic myeloid leukemia cells (K562) or Nawalma lymphoma cells with MDA-7/IL-24 inhibited cell growth without inducing apoptosis (Dong et al., 2008). In this context, recent studies suggest that the ability of enforced expression of adenoviral mda-7/IL-24 to induce apoptosis in leukemia cells is attenuated by autophagy (Yang et al., 2010). Regardless of the mechanisms responsible for these discrepancies, our findings indicate that diverse human myeloid leukemia cells are highly sensitive to GST-MDA-7/IL-24-mediated apoptosis.
The present results also demonstrate that MDA-7/IL-24 triggers a response characteristically associated with ER stress induction, including up-regulation of GRP78/BiP, IRE1α, and GADD34 expression, as well as eIF2α phosphorylation, in various myeloid cells, including primary blasts. These findings suggest that, as observed in epithelial tumor cells (Gupta et al., 2006b; Park et al., 2008) and in a myelodysplastic syndrome cell line (Mutz-1) stably transfected with MDA-7/IL-24 (Qian et al., 2008), GST-MDA-7/IL-24 potently induces ER stress in myeloid leukemia cells. However, to date, the functional role of ER stress in MDA-7/IL-24 lethality has not been investigated in myeloid leukemia or in hematopoietic malignancies in general. It is noteworthy that functional studies using shRNA directed against GRP78/BiP revealed that induction of this molecule significantly protected myeloid leukemia cells from MDA-7/IL-24 lethality. GRP78/BiP is a major ER-resident chaperone and sensor that is induced by various physiological and nonphysiological ER stresses and that signals downstream to the three major arms of the UPR (PKR-like endoplasmic reticulum kinase, IRE1, and ATF6) (Bertolotti et al., 2000). GRP78/BiP is known to protect cells against various stimuli, particularly those triggering ER stress, in diverse cell types (Shu et al., 2008). It has also been reported to play a protective role against MDA-7/IL-24 lethality in GBM cells (Park et al., 2008). The observation that MDA-7/IL-24 induced GRP78/BiP and that knockdown of this molecule significantly sensitized AML cells to GST-MDA-7/IL-24 lethality is consistent with its established role as a cytoprotective factor in the face of ER stress inducers. In addition, we have reported previously that GST-MDA-7/IL-24 localizes to the ER compartment and physically binds to GRP78/BiP (Gupta et al., 2006b), raising the possibility that MDA-7/IL-24 may disrupt GRP78/BiP function, leading to apoptosis initiation. In this setting, down-regulation of GRP78/BiP by shRNA may lower the threshold for MDA-7/IL-24-mediated interruption of GRP78/BiP cytoprotective functions. Further studies will be necessary to confirm this hypothesis.
The induction of ER stress response is a dynamic process that can serve either protective functions (generally at early intervals) or that can contribute to cell death, often at later intervals after prolonged stress. The temporal relationship governing the relative abundance of pro- versus antiapoptotic components of the UPR can therefore determine whether a cell lives or dies in response to ER stress (Lin et al., 2007). To add to the complexity, components of the UPR can exert either cytoprotective or prodeath effects, depending on the cell context and stimulus. For example, GADD34 (MyD116), a protein initially described as regulator of myeloid differentiation (Lord et al., 1990), has been shown to play a proapoptotic role in some contexts (i.e., SW480 cells exposed to ionizing radiation) (Adler et al., 1999). On the other hand, GADD34 has been shown to protect cells against apoptosis triggered by energy depletion, possibly a consequence of inhibitory effects on mammalian target of rapamycin activity (Watanabe et al., 2007). In addition, pharmacological inhibition of GADD34 by salubrinal has been shown to promote cell death in multiple myeloma cells (Schewe and Aguirre-Ghiso, 2009) and pancreatic β-cells (Cnop et al., 2007). In the present study, the finding that GADD34 shRNA knockdown significantly enhanced apoptosis suggests that GADD34 plays a protective role against MDA-7/IL-24 lethality in myeloid leukemia cells. Although exceptions exist, IRE1α is also believed to play primarily a cytoprotective role against ER stress-mediated cell death (Lin et al., 2007; Rahmani et al., 2007a). Consistent with these findings, the present results indicate that IRE1α induction protects leukemia cells from MDA-7/IL-24 lethality.
A question remaining to be answered is why GST-MDA-7/IL- 24 preferentially targets leukemic blasts compared with normal CD34+ progenitor cells. There is evidence, albeit indirect, to suggest that this effect is unlikely to be explained by differential expression of MDA-7/IL-24 receptors (e.g., IL-20R1/IL20R2 or IL-22R1/IL-20R2) on the surface of leukemia blasts versus normal CD34+ progenitor cells. First, GST-MDA-7/IL-24 does not seem to require classic MDA-7/IL-24 receptors to kill cells as much as cells lacking functional MDA-7/IL-24 receptors, such as A549 cells, remain fully sensitive to GST-MDA-7/IL-24 (Dent et al., 2005). Second, endogenous expression of MDA-7/IL-24 via adenoviral delivery also exhibits selectivity toward transformed versus normal cells (Sarkar et al., 2002). An alternative explanation for MDA-7/IL-24 selectivity is that the high levels of ER stress characteristically displayed by transformed cells may lower the apoptotic threshold for an ER stress inducer such as MDA-7/IL-24.
The down-regulation of Mcl-1 by GST-MDA-7/IL-24 observed in various AML cell types, including primary blasts, as well as evidence of a requirement for this process in GST-MDA-7/IL-24 lethality, are consistent with our recent findings in prostate cancer cells (Dash et al., 2010b). In addition, previous studies from our group and others have shown that Mcl-1 plays a critical role in protecting cells against ER stress induced by diverse agents, including sorafenib, thapsigargin, and tunicamycin (Fritsch et al., 2007; Rahmani et al., 2007a). Furthermore, induction of ER stress can contribute to Mcl-1 down-regulation by inhibiting its translation (Rahmani et al., 2005, 2007a). In this context, the present findings involving Mcl-1 further implicate ER stress in MDA-7/IL-24 lethality in myeloid leukemia. This notion is reinforced by the observation that MDA-7/IL-24 induced up-regulation of Noxa and Bim, and that these proapoptotic proteins played important functional roles in MDA-7/IL-24 lethality. Both Noxa and Bim have been shown to be involved in ER stress-mediated cell death (Puthalakath et al., 2007; Wang et al., 2009). Finally, the observation that both Bax and Bak contributed functionally to MDA-7/IL-24 lethality implicate activation of the mitochondrial apoptotic pathway in myeloid leukemia cell death. However, the specific molecular link between ER stress and mitochondrial dysfunction remains to be determined.
In summary, the present findings demonstrate that MDA-7/IL-24 strikingly induces apoptosis in both cultured and primary human myeloid leukemia cells and provide, for the first time, evidence of a functional contribution of ER stress induction in the regulation of MDA-7/IL-24-mediated leukemia cell death. The observation that equivalent MDA-7/IL-24 exposures exerted relatively little toxicity toward normal CD34+ hematopoietic cells suggests a possible role for this molecule in future antileukemic strategies. Finally, the present findings raise the possibility that agents capable of interfering with cytoprotective UPR-related proteins such as GRP78/BiP, IRE1α, or GADD34 might effectively enhance the antileukemic activity of MDA-7/IL-24. Studies designed to test this hypothesis are currently underway.
This work was supported by the National Institutes of Health National Cancer Institute [Grants P01-CA104177, R01-CA93738-05]; and awards from the V Foundation and the Leukemia and Lymphoma Society of America. D.S. is a Harrison Scholar and P.B.F. holds the Thelma Newmeyer Chair in Cancer Research.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.068007.
- MDA-7/IL-24
- melanoma differentiation associated gene-7/interleukin-24
- GST
- glutathione transferase
- L-CFU
- leukemic colony-forming units
- GADD
- growth arrest and DNA damage-inducible protein
- IRE1α
- inositol-requiring enzyme 1α
- eIF2α
- eukaryotic initiation factor 2α
- PARP
- poly(ADP-ribose) polymerase
- UPR
- unfolded protein response
- MLL-ENL
- eleven-nineteen-leukemia
- GRP78
- 78-kDa glucose-regulated protein
- AML
- acute myeloid leukemia
- ER
- endoplasmic reticulum
- shRNA
- short hairpin RNA
- AIF
- apoptosis-inducing factor
- CFU-GM
- myeloid colony-forming units
- GFP
- green fluorescent protein
- PI
- propidium iodide
- FAB
- French-American-British classification.
References
- Adler HT, Chinery R, Wu DY, Kussick SJ, Payne JM, Fornace AJ, Jr, Tkachuk DC. (1999) Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol 19:7050–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabé F, Kennedy JA, Hope KJ, Dick JE. (2007) Modeling the initiation and progression of human acute leukemia in mice. Science 316:600–604 [DOI] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P. (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22:487–508 [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332 [DOI] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. (2007) Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem 282:3989–3997 [DOI] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su Z-z, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee S-G, Park MA, et al. (2010a) mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-specific toxicity. Cytokine Growth Factor Rev doi:10.1016/j.cytogfr.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, et al. (2010b) Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res 70:5034–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Grant S, Curiel DT, Fisher PB. (2005) MDA-7/IL-24 regulates proliferation, invasion and tumor cell radiosensitivity: a new cancer therapy? J Cell Biochem 95:712–719 [DOI] [PubMed] [Google Scholar]
- Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, Bataille R, Amiot M. (2002) Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 100:194–199 [DOI] [PubMed] [Google Scholar]
- Dong CY, Zhang F, Duan YJ, Yang BX, Lin YM, Ma XT. (2008) mda-7/IL-24 inhibits the proliferation of hematopoietic malignancies in vitro and in vivo. Exp Hematol 36:938–946 [DOI] [PubMed] [Google Scholar]
- Fisher PB. (2005) Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res 65:10128–10138 [DOI] [PubMed] [Google Scholar]
- Fritsch RM, Schneider G, Saur D, Scheibel M, Schmid RM. (2007) Translational repression of MCL-1 couples stress-induced eIF2 alpha phosphorylation to mitochondrial apoptosis initiation. J Biol Chem 282:22551–22562 [DOI] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, et al. (2006a) mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther 111:596–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, Valerie K, Sarkar D, Fisher PB. (2006b) BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res 66:8182–8191 [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. (1995) Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11:2477–2486 [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525–535 [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord KA, Hoffman-Liebermann B, Liebermann DA. (1990) Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res 18:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. (2000) Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood 96:1756–1763 [PubMed] [Google Scholar]
- Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, et al. (2008) PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy 4:513–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349 [DOI] [PubMed] [Google Scholar]
- Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, Liu X. (2008) Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia 22:361–369 [DOI] [PubMed] [Google Scholar]
- Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, Ferreira-Gonzalez A, Dent P, Grant S. (2009) The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood 114:4507–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Dai Y, Grant S. (2002) The histone deacetylase inhibitor sodium butyrate interacts synergistically with phorbol myristate acetate (PMA) to induce mitochondrial damage and apoptosis in human myeloid leukemia cells through a tumor necrosis factor-alpha-mediated process. Exp Cell Res 277:31–47 [DOI] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Bauer C, Dent P, Grant S. (2005) Apoptosis induced by the kinase inhibitor BAY 43–9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem 280:35217–35227 [DOI] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S. (2007a) The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol 27:5499–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Nguyen TK, Dent P, Grant S. (2007b) The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol Pharmacol 72:788–795 [DOI] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, Grant S. (2003) Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene 22:6231–6242 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529 [DOI] [PubMed] [Google Scholar]
- Sainz-Perez A, Gary-Gouy H, Gaudin F, Maarof G, Marfaing-Koka A, de Revel T, Dalloul A. (2008) IL-24 induces apoptosis of chronic lymphocytic leukemia B cells engaged into the cell cycle through dephosphorylation of STAT3 and stabilization of p53 expression. J Immunol 181:6051–6060 [DOI] [PubMed] [Google Scholar]
- Sainz-Perez A, Gary-Gouy H, Portier A, Davi F, Merle-Beral H, Galanaud P, Dalloul A. (2006) High Mda-7 expression promotes malignant cell survival and p38 MAP kinase activation in chronic lymphocytic leukemia. Leukemia 20:498–504 [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. (2002) mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA 99:10054–10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, Dent P, Fisher PB. (2004) Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene 23:7679–7690 [DOI] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA. (2009) Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res 69:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139 [DOI] [PubMed] [Google Scholar]
- Shu CW, Sun FC, Cho JH, Lin CC, Liu PF, Chen PY, Chang MD, Fu HW, Lai YK. (2008) GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J Cell Physiol 215:627–635 [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher PB. (2003) Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene 22:1164–1180 [DOI] [PubMed] [Google Scholar]
- Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, et al. (2009) ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA 106:2200–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Tambe Y, Inoue H, Isono T, Haneda M, Isobe K, Kobayashi T, Hino O, Okabe H, Chano T. (2007) GADD34 inhibits mammalian target of rapamycin signaling via tuberous sclerosis complex and controls cell survival under bioenergetic stress. Int J Mol Med 19:475–483 [PubMed] [Google Scholar]
- Yang C, Tong Y, Ni W, Liu J, Xu W, Li L, Liu X, Meng H, Qian W. (2010) Inhibition of autophagy induced by overexpression of mda-7/interleukin-24 strongly augments the antileukemia activity in vitro and in vivo. Cancer Gene Ther 17:109–119 [DOI] [PubMed] [Google Scholar]