Abstract
Ingestion of aristolochic acid (AA) is associated with the development of aristolochic acid nephropathy, which is characterized by chronic renal failure, tubulointerstitial fibrosis and urothelial cancer. AA may also cause a similar type of kidney fibrosis with malignant transformation of the urothelium, the Balkan endemic nephropathy. Understanding which enzymes are involved in AA activation and/or detoxication is important in the assessment of a susceptibility to this carcinogen. The most important human enzymes activating AA by simple nitroreduction in vitro are hepatic and renal cytosolic NAD(P)H:quinone oxidoreductase, hepatic microsomal cytochrome P450 1A2 and renal microsomal NADPH:cytcohrome P450 reductase, besides cyclooxygenase, which is highly expressed in urothelial tissue. Despite extensive research, contribution of most of these enzymes to the development of these diseases is still unknown. Hepatic cytochromes P450 were found to detoxicate AA in mice, and thereby protect the kidney from injury. However, which of cytochromes P450 are the most important in this process both in animal models and in humans have not been entirely resolved as yet. In addition, the relative contribution of enzymes found to activate AA to species responsible for induction of urothelial cancer in humans remains still to be resolved.
Keywords: Aristolochic acid, metabolism, Aristolochic acid- and Balkan endemic-nephropathy, renal injury, tumor induction
Introduction
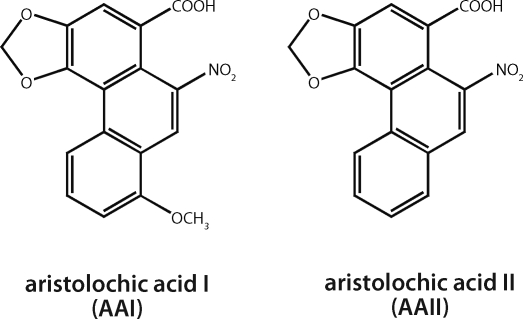
Aristolochic acid (AA), the plant extract of Aristolochia species, is a mixture of structurally related nitrophenanthrene carboxylic acids, with 8-methoxy-6-nitro-phenanthro-(3,4-d)-1,3-dioxolo-5-carboxylic acid (AAI) and 6-nitro-phenanthro-(3,4-d)-1,3-dioxolo-5-carboxylic acid (AAII), being the major components (Figure 1) (IARC, 2002). Recently AA was proven to be the cause of so-called Chinese herbs nephropathy (CHN), a unique type of rapidly progressive renal fibrosis associated with the prolonged intake of Chinese herbal remedies during a slimming regimen, observed for the first time in Belgium in 1991 (Vanherweghem et al., 1993; Vanhaelen et al., 1994; Schmeiser et al., 1996). About 100 CHN cases have been identified so far in Belgium, half of which needed renal replacement therapy, mostly including renal transplantation (Arlt et al., 2002b). The observed nephrotoxicity has been traced to the ingestion of herbal preparation Aristolochia fangchi containing nephrotoxic AA inadvertently included in slimming pills (Vanhaelen et al., 1994). CHN patients, who were exposed to Aristolochia species containing AA and had no relationship with the Belgian slimming clinic, have been identified in other European countries, in Asia and in the USA (about 170 cases) (Arlt et al., 2002b). Therefore, this disease is now called aristolochic acid nephropathy (AAN) (Gillerot et al., 2001; Arlt et al., 2002b; Cosyns, 2003). Recently, a high prevalence of urothelial cancer was found in the cohort of AAN patients in Belgium (Nortier et al., 2000) and cases of urothelial cancer have also been described in other countries (Arlt et al., 2004). These findings highlight the carcinogenic potential of AA to humans. Indeed, AA is among the most potent 2% of known carcinogens (Arlt et al., 2002b; IARC, 2002). As a consequence, herbal remedies containing species of the genus Aristolochia were recently classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (IARC) (IARC, 2002).
Figure 1.
Aristolochic acid I and II.
It is also noteworthy that AA consumption may be a cause for the development of a similar type of kidney fibrosis with malignant transformation of the urothelium, the Balkan endemic nephropathy (BEN) (Ivic, 1969; Tatu et al., 1998; Arlt et al., 2002a; Stiborová et al., 2005b), which is widely found in certain areas of Romania, Croatia, Bosnia, Serbia and Bulgaria along the Danube river basin (Tatu et al., 1998; Stiborová et al., 2005b; Stefanovic et al., 2006). At least 25,000 individuals suffer from BEN or are suspected of having the disease, while the total number of people at risk in these countries may exceed 100,000. Although first described more than 50 years ago, the etiology of BEN remains unclear and is a matter of debate (Tatu et al., 1998; Stefanovic et al., 2006). For the last years evidence has accumulated that BEN is an environmental disease. Recent experimental data shows that AA might be one of the most important etiologic factors in BEN and associated urothelial cancer (Arlt et al., 2002a; Stefanovic et al., 2006; Grollman et al., 2007). AA exposure is associated with chronic dietary uptake of seeds of Aristolochia clematitis by the population living in BEN regions (Ivic, 1969; Arlt et al., 2002a; Hranjec et al., 2005).
Aristolochic Acid-Mediated Renal Injury and Carcinogenesis
The molecular mechanisms for AA-mediated renal injury, and if it is an early stage of the urothelial-specific tumor development, are still matter of debate and need further investigations. In this context, it is noteworthy that a case of AA-induced tumor development without renal injury (Nortier et al., 2003) suggests dissociation between AA-mediated nephrotoxicity and carcinogenicity. AA seems to directly cause renal injury through activating mitochondrial permeability transition, which was found recently in human renal tubular epithelial cells (Qi et al., 2007). This suggestion, however, needs to be confirmed by further studies. In contrast to suggestion that AA might be the direct cause of the instestitial nephropathy, metabolic activation of AA to species forming DNA adducts is an important step for AA-induced malignant transformation (Arlt et al., 2007; Stiborová et al., 2008b). Indeed, the molecular mechanism of AA-induced carcinogenesis demonstrates a strong association between DNA adduct formation, mutation pattern and tumour development (Arlt et al., 2007). The predominant AA-DNA adduct, 7-(deoxyadenosin-N6-yl)aristolactam I (dA-AAI), which is the most persistent of the adducts in the target tissue, is a mutagenic lesion leading to A→T transversions in the p53 gene in DNA from urothelial tumors of AAN and BEN patients (Lord et al., 2004; Arlt et al., 2007; Grollman et al., 2007).
Metabolism of Aristolochic Acid and Biotransformation Enzymes
One of the common features of AAN and BEN is that not all individuals exposed to AA suffer from nephropathy and tumor development. We have suggested earlier that one cause for these different responses may be individual differences in the activities of the enzymes catalyzing the biotransformation (detoxication and/or activation) of AA (for a summary, see Stiborová et al., 2008b) Many genes of enzymes metabolizing toxicants and carcinogens are known to exist in variant forms or show polymorphisms resulting in differing activities of the gene products. These genetic variations appear to be important determinants of cancer risk or other toxic effects of xenobiotics (Stiborová et al., 2008b).
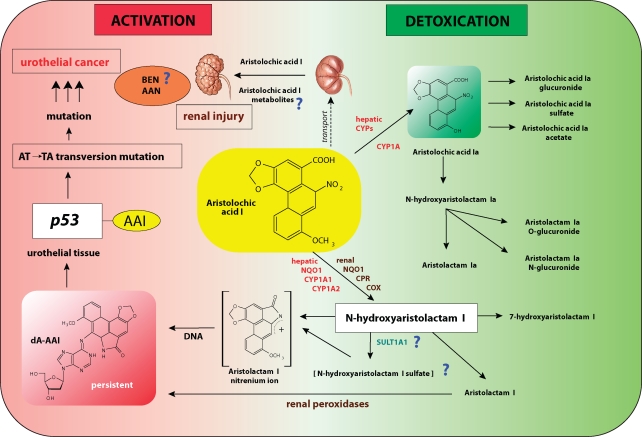
The proposed activation and detoxication pathways for the major component of AA, aristolochic acid I (AAI), are shown in the Figure 2. AAI is activated by simple nitroreduction to N-hydroxyaristolactam I that forms a cyclic N-acylnitrenium ion as the ultimate carcinogenic species binding to DNA to form 7-(deoxyadenosin-N6-yl)aristolactam I (dA-AAI) as the major persistent adduct, participating in initiation of carcinogenesis. The enzymes activating AA to species binding to DNA in vitro were studied in details (Stiborová et al., 2008b). Using in vitro studies we have found that the most important human and rat enzyme activating AAI in vitro in hepatic and renal cytosolic subcellular fractions is NAD(P)H:quinone oxidoreductase (NQO1)(Stiborová et al., 2002a; 2003; 2005a) followed by cytochrome P450 (CYP) 1A1/2 in liver microsomes (Stiborová et al., 2001c; 2005c) and NADPH:CYP reductase (POR) in kidney microsomes (Stiborová et al., 2001b; 2005a), besides prostaglandin H synthase (cyclooxygenase, COX) (Stiborová et al., 2001a), which is highly expressed in urothelial tissue. However, the confirmation that isolated (purified) human NQO1 is really capable of activating AAI remains still to be investigated. Such a proof is of great importance. It is namely noteworthy that NQO1-polymorphism (the genotype NQO1*2/*2) seems to predispose patients suffering from BEN to the development of urothelial malignancy of the upper urinary tract (OR=13.75, 95%CI 1.17-166.21) (Toncheva et al., 2004). Therefore, the targets of our future work are the confirmation of this finding (predisposition of patients to the development of cancer by NQO1-polymorphism), and the confirmation of the major role NQO1 in AAI activation. The results of such studies might answer the question why AAI-induced cancer is developed in only some of the AAN and BEN patients.
Figure 2.
Proposed pathway for metabolic activation and detoxication of aristolochic acid I (AAI), leading to renal injury and urothelial cancer. Aristolochic acid nephropathy (AAN); Balkan endemic nephropathy (BEN); 7-(deoxyadenosin-N6-yl)aristolactam I (dA-AAI); cytochrome P450 (CYP); NADPH:CYP reductase (CPR); cyclooxygenase (COX); sulfotransferase (SULT) (adapted from reference Stiborová et al., 2008c).
The competing conversion of N-hydroxyaristolactam I to the corresponding 7-hydroxyaristolactam or its further reduction to aristolactam I should be considered detoxication pathway; both these metabolites were found to be excreted. However, even though aristolactam I is not a direct DNA binding species, low amounts of the dA-AAI adduct, with the highest levels in one of the target tissues, the renal pelvis, were generated in rats treated with aristolactam I (Dong et al., 2006). This result is consistent with finding that formation of the dA-AAI adduct by aristolactam I was observed after its in vitro activation with different peroxidases of which several, such as COX-1 and/or COX-2, are expressed at high levels in renal tissue (Stiborová et al., 1999). It is still questionable if enzymes capable of conjugating the proximate carcinogenic metabolite of AAI, N-hydroxyaristolactam I, are involved in AAI activation. Meinl et al. (2006) demonstrated that expression of some human sulfotransferases (SULTs), particularly SULT1A1, in bacterial and mammalian target cells enhances the mutagenic activity of AAI. However, our preliminary experiments did not bring unambiguous results. On one hand, we found that an increase in AAI-induced mutagenicity was correlated with higher AAI-DNA adduct levels in V79 cells transfected with human SULT1A1 (Glatt et al., unpublished results). However, our further results suggest that SULTs in human hepatic and renal cytosols do not participate in an increase in AAI-DNA adduct formation in these subcellular systems. Thus, the exact role of conjugation enzymes in AAI activation awaits further investigation and is another aim of our additional studies.
While most of the enzymes catalyzing the reductive activation of AAI in vitro have already been identified, the question, which of them actually participates in this process in vivo, remains to be answered. Additional factors such as route of administration, absorption, renal clearance and tissue-specific enzyme expression make it difficult to extrapolate from data found in vitro (Stiborová et al., 2008b) to the in vivo situation. Such a study is, therefore, the next target of our future investigation.
The oxidation of AAI to aristolochic acid Ia (AAIa) has been suggested to be a detoxication pathway of AAI (Arlt et al., 2002b; Stiborová et al., 2008b). Namely, AAIa or its conjugates, the O-glucuronide, the O-acetate and the O–sulfate esters, were excreted in urine (Chan et al., 2006; 2007). AAIa is also reduced to N-hydroxyaristolactam Ia forming aristolactam Ia, which together with its conjugates, the N- and O-glucuronides, is excreted (Chan et al., 2006; 2007). Enzymatic reactions leading to aristolactam Ia and its metabolites seem to be purely detoxication pathway, because DNA adducts containing aristolactam Ia structure have as yet never been found. In contrast to the enzymes activating AAI in vitro, those participating in AAI oxidation to AAIa both in vitro and in vivo have not been extensively studied so far. Our preliminary studies indicated that CYP enzymes can generate this oxidative metabolite (Stiborová et al., 2008b). However, the question which of the CYP enzymes are responsible for formation of AAIa remains still to be investigated. In this context it is noteworthy that a large-scale investigation in BEN patients on the role of genetic polymorphisms in genes of some phase I detoxication CYP enzymes revealed a possible risk for BEN (OR 2.41) in individuals carrying CYP3A5*1 allele G6989 (Toncheva et al., 2004; Toncheva 2006). Although we found that this CYP did not activate AAI to dA-AAI adduct (Stiborová, unpublished results), we do not know, if this CYP species is involved in AA detoxication. Furthermore, as mentioned above, we also do not know if other CYPs, and which of them, are capable of detoxicating AAI. Therefore, the evaluation of the oxidative detoxication of AAI by individual CYP enzymes is the target of studies in several laboratories. Indeed, very recently, Xiao et al. (2008) showed novel data concerning the enzymes detoxicating AAI. The HRN (Hepatic Cytochrome P450 Reductase Null) mice, which we had shown previously to be a suitable model to determine hepatic xenobiotic metabolism in vivo (Arlt et al., 2005; 2006; 2008; Stiborová et al., 2008a) and suggested to use to elucidate AA metabolism (Stiborová et al., 2008b), were successfully used in their study (Xiao et al., 2008). The authors' results indicate that hepatic CYPs detoxify AAI by its demethylation to aristolochic acid Ia (AAIa), and thereby protect the kidney from AAI-induced injury. The observations of Xiao et al. (2008) combined with results found previously, support strongly the former hypothesis (Stiborová et al., 2008b) that a key point determining the carcinogenic and nephrotoxic effects of AAI lies in the balance of activities of reductases such as NQO1, catalyzing the AAI-DNA adduct formation, and enzymes such as CYPs, which detoxicate AAI to AAIa.
The question which of the CYP enzymes are responsible for formation of AAIa remains still to be investigated. The in vitro experiments of Xiao et al. (2008) indicate that CYP1A generate AAIa. However, the model used to evaluate CYP1A participation in formation of AAIa in vivo, mice treated with an inducer of CYP1A 3-methycholanthrene (MC), did not bring unambiguous results. Namely, MC also induces other enzymes beside CYP1A. Although treatment of mice with MC leads to decrease in AAI concentrations in the liver and kidney, no increase in AAIa concentrations was found in the liver, only in the kidney of mice treated with the higher dose of AAI (20 mg/kg). An increase in AAIa excretion due to its conjugation with glucuronide, caused by induction of UDP-glucuronosyltransferase with MC, could occur. Nevertheless, because CYP1A enzymes also activate AAI to species forming DNA adducts (Stiborová et al., 2008b), the decrease of AAI in liver and kidney might also result from this reaction. Moreover, NQO1, which is also efficiently induced by MC, could contribute to decreased AAI levels in MC-treated mice.
Taking into account all data known at the present time, we propose that the pathways of AAI metabolism are dictated by the binding affinity of AAI to CYP1A or NQO1, and their enzymatic turnover as well as by the balance between the efficiency of CYP1A to oxidize and reduce AAI. In order to confirm this assumption and to complement our former studies (Stiborová et al., 2008b; c), and the work of Xiao et al. (2008), we started a study investigating formation of AAI-DNA adducts in the HRN mouse model, and in models, in which CYP1A genes are deleted.
Conclusions
Although hepatic CYP enzymes were found to detoxicate AAI in mice, thus decreasing its renal toxicity (Xiao et al., 2008), individual enzymes, which might metabolize (activate and/or detoxicate) AAI in vivo, and their impact on AAI-mediated nephrotoxicity and carcinogenicity, have not been fully resolved as yet. Therefore, such a subject remains to be investigated. Namely, the evaluation of inter-individual variations in the human enzymes playing a major role in AAI activation and detoxication, including their genetic polymorphisms, remain a major challenge to explain an individual's susceptibility to AAI, and to predict cancer risk among the AAN and BEN patients. Therefore, the study we started in our laboratory addresses still unsettled question whether the metabolism of AAI, and if so, which enzymes participating in this process, determine pathophysiological effects of this compound in development of AAN and BEN diseases.
Acknowledgement
The work is supported by the Grant Agency of the Czech Republic (grant 303/06/0329), the Ministry of Education of the Czech Republic (grant MSM0021620808) and German Cancer Research Center.
REFERENCES
- Arlt VM, Ferluga D, Stiborova M, Pfohl-Leszkowicz A, Vukelic M, Vdovic S, Schmeiser HH, Cosyns JP. Is aristolochic acid a risk factor for Balkan endemic nephropathy-associated urothelial cancer? Int J Cancer. 2002a;101:500–502. doi: 10.1002/ijc.10602. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Henderson CJ, Wolf CR, Schmeiser HH, Phillips DH, Stiborova M. Bioactivation of 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone: evidence for DNA adduct formation mediated by cytochrome P450 enzymes and peroxidases. Cancer Lett. 2006;234:220–231. doi: 10.1016/j.canlet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborová M, Henderson CJ, Thiemann M, Frei E, Aimová D, Singhs R, da Costa GG, Schmitz OJ, Farmer PB, Wolf CR, Phillips DH. Metabolic activation of benzo[a]pyrene in vitro by hepatic cytochrome P450 contrasts with detoxification in vivo: experiments with hepatic cytochrome P450 reductase null mice. Carcinogenesis. 2008;29:656–665. doi: 10.1093/carcin/bgn002. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 2002b;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Alunni-Perret M, Quatrehomme G, Ohayon P, Albano L, Gaid H, Michiels JF, Meyrier A, Cassuto E, Wiessler M, Schmeiser HH, Cosyns J-P. Aristolochic acid (AA)-DNA adduct as marker of AA exposure and risk factor for AA nephropathy-associated cancer. Int J Cancer. 2004;111:977–980. doi: 10.1002/ijc.20316. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborová M, vom Brocke J, Simoes ML, Lord GM, Nortier JL, Hollstein M, Phillips DH, Schmeiser HH. Aristolochic acid mutagenesis: molecular clues to the etiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogensis. 2007;28:2253–2261. doi: 10.1093/carcin/bgm082. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborova M, Henderson CJ, Osborne MR, Bieler CA, Frei E, Martinek V, Sopko B, Wolf CR, Schmeiser HH, Phillips DH. Environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols. Cancer Res. 2005;65:2644–2652. doi: 10.1158/0008-5472.CAN-04-3544. [DOI] [PubMed] [Google Scholar]
- Chan W, Cu L, Xu G, Cai Z. Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry, Rapid Commun. Mass Spectrom. 2006;20:1755–1760. doi: 10.1002/rcm.2513. [DOI] [PubMed] [Google Scholar]
- Chan W, Luo H-B, Zheng Y, Cheng Y-K, Cai Z. Investigation of the metabolism and reductive activation of carcinogenic aristolochic acid in rats. Drug Metab Dispos. 2007;35:866–874. doi: 10.1124/dmd.106.013979. [DOI] [PubMed] [Google Scholar]
- Cosyns JP. Aristolochic acid and “Chinese herbs nephropathy”: a review of the evidence to date. Drug Safety. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- Dong H, Suzuki N, Torres MC, Bonala RR, Johnson F, Grollman AP, Shibutan S. Quantitative determination of aristolochic acid-derived DNA adducts in rats using 32P-postlabeling/polyacrylamide gel electrophoresis analysis. Drug Metab Dispos. 2006;34:1122–1127. doi: 10.1124/dmd.105.008706. [DOI] [PubMed] [Google Scholar]
- Gillerot G, Jadoul M, Arlt VM, van Ypersele de Strihou C, Schmeiser HH, But PHH, Bieler CA, Cosyns J-P. Aristolochic acid nephropathy in a Chinese patient: time to abandon the term “Chinese herbs nephropathy”? Am J Kidney Dis. 2001;38:E26. doi: 10.1053/ajkd.2001.28624. [DOI] [PubMed] [Google Scholar]
- Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky R, Goodenough AK, Rieger R, Vukelic M, Jelakovic B. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sc USA. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranjec T, Kovac A, Kos J, Mao W, Chen JJ, Grollman AP, Jelakovic B. Endemic nephropathy: the case for chronic poisoning by aristolochia. Croat Med J. 2005;46:116–125. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum. 2002;82 [PMC free article] [PubMed] [Google Scholar]
- Ivic M. Etiology of endemic nephropathy. Lijec Vjesn. 1969;91:1273–1281. [PubMed] [Google Scholar]
- Liu Z, Hergenhahn M, Schmeiser HH, Wogan GN, Hong A, Hollstein M. Human tumor p53 mutations are selected for in mouse embryonic fibroblasts harboring a humanized p53 gene. Proc Nat Acad Sc USA. 2004;101:2963–2968. doi: 10.1073/pnas.0308607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Hollstein M, Arlt VM, Roufosse C, Pusey CD, Cook T, Schmeiser HH. DNA adducts and p53 mutations in a patient with aristolochic acid-associated nephropathy. Am J Kidney Dis. 2004;43:e11–17. doi: 10.1053/j.ajkd.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Meinl W, Pabel U, Osterloh-Quitroz H, Hengstler JG, Glatt H. Human sulphotransferases are involved in the activation of aristolochic acids and are expressed in renal target tissue. Int J Cancer. 2006;118:1090–1097. doi: 10.1002/ijc.21480. [DOI] [PubMed] [Google Scholar]
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petřin M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- Nortier JL, Schmeiser HH, Martinez MCM, Arlt VM, Vervaet C, Garbar CH, Daelemans P, Vanherweghem JL. Invasive urothelial carcinoma after exposure to Chinese herbal medicine containing aristolochic acid may occur with-out severe renal failure. Nephrol Dial Transplant. 2003;18:426–428. doi: 10.1093/ndt/18.2.426. [DOI] [PubMed] [Google Scholar]
- Qi X, Cai Y, Gong L, Liu L, Chen F, Xiao Y, Wu X, Li Y, Xue X, Ren J. Role of mitochondrial permeability transition in human renal tubuar epithelial cell death induced by aristolochic acid. Toxicol Appl Pharmacol. 2007;222:105–110. doi: 10.1016/j.taap.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Schmeiser HH, Bieler CA, Wiessler M, van Ypersele de Strihou C, Cosyns JP. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res. 1996;56:2025–2028. [PubMed] [Google Scholar]
- Stefanovic V, Toncheva D, Atanasova S, Polenakovic M. Etiology of Balkan endemic nephropathy and associated urothelial cancer. Am J Nephrol. 2006;26:1–11. doi: 10.1159/000090705. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Arlt VM, Henderson CJ, Wolf CR, Kotrbová V, Moserová M, Hudeček J, Phillips DH, Frei E. Role of hepatic cytochromes P450 in bioactivation of the anticancer drug ellipticine: studies with the hepatic NADPH:cytochrome P450 reductase null mouse. Toxicol Appl Pharmacol. 2008a;226:318–327. doi: 10.1016/j.taap.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Arlt VM, Schmeiser HH. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res. 2008b;658:55–67. doi: 10.1016/j.mrrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Breuer A, Bieler C A, Schmeiser HH. Aristolactam I a metabolite of aristolochic acid I upon activation forms an adduct found in DNA of patients with Chinese herbs nephropathy. Exp Toxic Pathol. 1999;51:421–427. doi: 10.1016/S0940-2993(99)80033-5. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Breuer A, Wiessler M, Schmeiser HH. Evidence for reductive activation of carcinogenic aristolochic acids by prostaglandin H synthase – 32P-postlabeling analysis of DNA adduct formation. Mutat Res. 2001a;493:149–160. doi: 10.1016/s1383-5718(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Wiessler M, Schmeiser HH. Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: evidence for reductive activation by cytochrome P450 1A1 and 1A2. Chem Res Toxicol. 2001c;14:1128–1137. doi: 10.1021/tx010059z. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Schmeiser HH. Biotransformation enzymes in development of renal injury and urothelial cancer cause by aristolochic acid. Kidney Int. 2008c;73:1209–1211. doi: 10.1038/ki.2008.125. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Sopko B, Wiessler M, Schmeiser HH. Carcinogenic aristolochic acids upon activation by DT-diaphorase form adducts found in DNA of patients with Chinese herbs nephropathy. Carcinogenesis. 2002a;23:617–625. doi: 10.1093/carcin/23.4.617. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Sopko B, Sopková K, Marková V, Laňková M, Kumstýřová T, Wiessler M, Schmeiser HH. Human cytosolic enzymes involved in the metabolic activation of carcinogenic aristolochic acid: evidence for reductive activation by human NAD(P)H:quinone oxidoreductase. Carcinogenesis. 2003;24:1695–1703. doi: 10.1093/carcin/bgg119. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Hodek P, Wiessler M, Schmeiser HH. Human hepatic and renal microsomes, cytochromes P450 1A1/2, NADPH:cytochrome P450 reductase and prostaglandin H synthase mediate the formation of aristolochic acid-DNA adducts found in patients with urothelial cancer. Int J Cancer. 2005a;113:189–197. doi: 10.1002/ijc.20564. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Hájek M, Frei E, Schmeiser HH. Carcinogenic and nephrotoxic alkaloids aristolochic acids upon activation by NADPH:cytochrome P450 reductase form adducts found in DNA of patients with Chinese herbs nephropathy. Gen Physiol Biophys. 2001b;20:375–392. [PubMed] [Google Scholar]
- Stiborová M, Patočka J, Frei E, Schmeiser HH. Biochemistry and toxicological aspects of etiology of Balkan endemic nephropathy. Chem Listy. 2005b;99:782–788. [Google Scholar]
- Stiborová M, Sopko B, Hodek P, Frei E, Schmeiser HH, Hudeček J. The binding of aristolochic acid I to the active site of human cytochromes P450 1A1 and 1A2 explains their potential to reductively activate this human carcinogen. Cancer Lett. 2005c;229:193–204. doi: 10.1016/j.canlet.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Tatu CA, Oren WH, Finkelman RB, Feder GL. The etiology of Balkan endemic nephropathy: still more questions than answers. Environ Health Perspect. 1998;106:689–700. doi: 10.1289/ehp.106-1533478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toncheva DI, von Ahsen N, Atanasova SY, Dimitrov TG, Armstrong VM, Oellerich M. Identification of NQO1 and GSTs genotype frequencies in Bulgarian patients with Balkan endemic nephropathy. J Nephrol. 2004;17:384–389. [PubMed] [Google Scholar]
- Toncheva D. Genetic studies in BEN and associated urothelial cancers. Coll. Antropol. 2006;30(1):34. [Google Scholar]
- Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Valdervelde D, Verbeelen D, Vanhaelen-Fastre B, Vanhaelen M. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL. Identification of aristolochic acid in Chinese herbs. Lancet. 1994;343:174. doi: 10.1016/s0140-6736(94)90964-4. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Ge M, Xue X, Wang H, Wu X, Li L, Liu L, Qi X, Zhang Y, Li Y, Xie T, Gu J, Ren J. Detoxication role of hepatic cytochrome P450s in the kidney toxicity induced by aristolochic acid. Kidney Int. 2008;73:1231–1239. doi: 10.1038/ki.2008.103. [DOI] [PubMed] [Google Scholar]