Abstract
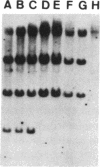
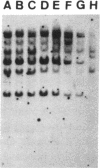
The P element is a type of transposable element in Drosophila melanogaster. Characteristics of the syndrome of “hybrid dysgenesis” are due to transposition of P elements, and the molecular mechanism for regulation of this transposition has been unknown. In this study a Q strain (which carries only defective P elements in its genome but still is able to repress the transposition of complete P elements although defective in transposase activity) was used to determine the structure of the P element with this repressor (or P cytotype-determining) domain. Examination of the cytotype and structure of the P elements of particular strains with reduced copy number of P elements showed that the P element with a repressor domain was defective, being deleted between bases 1991 and 2448. This region corresponds to most of the third intron [between open reading frame (ORF) 2 and ORF 3] as well as half the ORF 3 of an intact P element. Therefore ORF 3 was deemed to be unnecessary for repressor production.
Keywords: P repressor, hybrid dysgenesis, MR element
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Engels W. R. A trans-acting product needed for P factor transposition in Drosophila. Science. 1984 Dec 7;226(4679):1194–1196. doi: 10.1126/science.6095450. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Hybrid dysgenesis in Drosophila and the Stochastic loss hypothesis. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):561–565. doi: 10.1101/sqb.1981.045.01.072. [DOI] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Hybrid dysgenesis in Drosophila melanogaster: the biology of female and male sterility. Genetics. 1979 May;92(1):161–174. doi: 10.1093/genetics/92.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Green M. M. Controlling element mediated transpositions of the white gene in Drosophila melanogaster. Genetics. 1969 Feb;61(2):429–441. doi: 10.1093/genetics/61.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. Genetic instability in Drosophila melanogaster: De novo induction of putative insertion mutations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3490–3493. doi: 10.1073/pnas.74.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. Genetic instability in Drosophila melanogaster: the genetics of an MR element that makes complete P insertion mutations. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1036–1040. doi: 10.1073/pnas.83.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. The genetics of a mutable gene at the white locus of Drosophila melanogaster. Genetics. 1967 Jul;56(3):467–482. doi: 10.1093/genetics/56.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraizumi Y. Spontaneous recombination in Drosophila melanogaster males. Proc Natl Acad Sci U S A. 1971 Feb;68(2):268–270. doi: 10.1073/pnas.68.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986 Jan 17;44(1):7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Laski F. A., Rubin G. M. Identification and immunochemical analysis of biologically active Drosophila P element transposase. Cell. 1986 Jan 17;44(1):21–32. doi: 10.1016/0092-8674(86)90481-2. [DOI] [PubMed] [Google Scholar]
- Sakoyama Y., Todo T., Ishiwa-Chigusa S., Honjo T., Kondo S. Structures of defective P transposable elements prevalent in natural Q and Q-derived M strains of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6236–6239. doi: 10.1073/pnas.82.18.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Simmons M. J., Bucholz L. M. Transposase titration in Drosophila melanogaster: a model of cytotype in the P-M system of hybrid dysgenesis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8119–8123. doi: 10.1073/pnas.82.23.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]