Abstract
Cytochrome b5 (cyt b5), a component of endoplasmic reticulum membrane, plays a role in modulation of enzymatic activity of some cytochrome P450 (CYP) enzymes. The effect of apo-cytochrome b5 on this enzymatic system has not been investigated in details, because preparation of cyt b5 as a pure protein failed in many laboratories. In order to prepare the native apo-cytochrome b5 in a large scale we utilized a protein with higher affinity toward the heme; the apo-myoglobin from the equine skeletal muscle. In the first step, we extracted heme moiety from the native myoglobin by butanone extraction. Than the effect of pH on spontaneous heme release from both proteins was investigated: purified rabbit cyt b5 as well as equine skeletal muscle myoglobin. The prepared apo-myoglobin was incubated with the cyt b5 and heme transfer was monitored as a shift of absorption maximum from 413 to 409 nm in pH varying between 3–6 (10 mM KH2PO4, pH 3–6). Here, we obtained 43 mg of the equine skeletal muscle apo-myoglobin (43% yield). The optimal pH range for heme transfer from cyt b5 into apo-myoglobin was between 4.2 and 5. Native apo-cytochrome b5 was successfully prepared using procedure described here.
Keywords: apo-myoglobin, cytochrome b5, butanon extraction, pH
Introduction
Cytochrome b5 (cyt b5), a component of endoplasmic reticulum membrane, is a heme protein with molecular weight of 16,800. It is composed of two functional domains, a soluble heme-containing core, and a short hydrophobic C-terminal tail, which anchors the protein into the microsomal membrane (Schenkman and Jansson, 2003). Cyt b5 has been shown to stimulate, inhibit or have no effect on cytochrome P450 (CYP)-mediated reactions. There are two major theories explaining this effect: (i) direct electron transfer from cyt b5 to CYP and (ii) conformational effects of cyt b5 on CYP without contribution of electron transfer (Yamazaki et al., 2001). Recently, we found that cyt b5 isolated from rabbit liver microsomes and reconstituted with CYP1A1 and NADPH:CYP reductase modulates the oxidation of carcinogenic azo dye Sudan I by this system (Stiborova et al., 2006).
To study the mechanism of this modulation, not only the effect of native cyt b5, but also that of the apo-cytochrome b5 (lacking the electron transferring cofactor – heme) on Sudan I oxidation is necessary to be evaluated.
The affinities of apohemoproteins for heme are very large, showing equilibrium dissociation constants in the 10–10–10–15 M region (Hargrove et al., 1996; Miksanova et al., 2006). In the holoproteins, the heme prosthetic group appears to be stabilized by a large number of hydrophobic (van der Waals) and electrostatic contacts. The vinyl groups are pointing toward the protein interior and surrounded by nonpolar aliphatic and aromatic side chains, whereas the propionates point toward the solvent and interact with a variety of charged or polar amino acids. These interactions and the extremely small dissociation constants imply a high degree of specificity in the binding process (Hargrove et al., 1994). However, a variety of experimental evidence suggests that the association of heme with apoglobin is little affected by globin structure (Hargrove et al., 1996).
In the present time there are just two methods for the apohemoproteins preparation in a large scale. (i) First it is a butanone extraction method (Rossi-Fanelli et al., 1959). (ii) Second it is a expression of recombinat hemoprotein in the system without heme precursor – δ-aminolevulic acid (Miksanova et al., 2006). However, the first method requires nonphysiological conditions such as pH 2.5 and the second method cannot guaranty that whole amount of the expressed hemoprotein would be present in its apoform. The E. coli used as an expression system contains its own heme, which can be incorporated into the produced hemoprotein. Therefore, here we present the new method for apohemoproteins preparation. Most important are the physiological conditions of the new method and the negligible amount of the residual holohemoprotein.
Materials and methods
Preparation of the apo-myoglobin from the equine skeletal muscle
In the first step, heme moiety was extracted from the native myoglobin by butanone extraction (Rossi-Fanelli et al., 1958). pH of the myoglobin solution (100 mg of the equine skeletal muscle myoglobin in 40 ml dist. H2O) adjusted to pH 2.5 by 1 M HCl. The solution was moved to the separation funnel and equal volume of 2-butanone was added. Mixture was slowly shaked 3 times for 5 minutes and than placed for 10 minutes into the cold room (8°C). Aqueous phase was dialyzed at 8°C against 2 l of water, than 2 days against 2 l of 10 mM Tris, pH 8.0. The solution was concentrated in an Amicon stirred cell using a PM-10 membrane. In order to prevent the precipitation of the apo-myoglobin, pH was adjusted to 5 using 2 M CH3COOH before it was stored at –80°C.
Protein analysis
Protein concentration was determined by the standard bicinchoninic acid protein assay (Wiechelman et al., 1988). Bovine serum albumin was used as a standard.
Spectrophotometric measurements
Spectrophotometric measurements were performed on a Hewlett Packard 8453 UV spectrophotometer with 1 ml samples. 10 µl of purified rabbit cyt b5 or equine skeletal muscle myoglobin was diluted to 990 µl of 10 mM KH2PO4, pH 3–5. Afterwards the cyte b5 was incubated with the apo-myoglobin in the molar ratio 1:1.5. The heme transfer in various pH was monitored as a shift of absorption maximum of heme from 413 to 409 nm. The exact pH values of individual incubation mixtures were measured after the spectrophotometric measurement.
Results
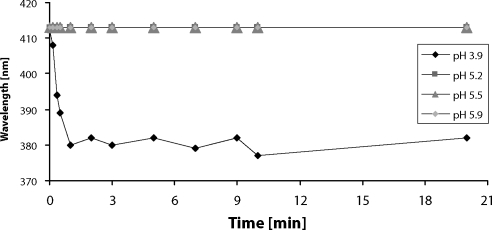
The effect of pH on spontaneous heme release from rabbit cyt b5 and equine skeletal muscle myoglobin was investigated. Figure 1 shows the low pH induced dissociation of heme from cyt b5. The absorption maximum of heme shifts, during incubation at pH 3.9, from 413 nm to 382 nm indicating release of the free heme cofactor. This process was fast, cyt b5 lost its heme completely during the first minute of incubation. On the contrary, the heme bound in molecule of cyt b5 is stable at pH between 5.2–5.9 (absorption maximum stays at 413 nm).
Figure 1.
The effect of pH on spontaneous heme release from purified rabbit cytochrome b5.
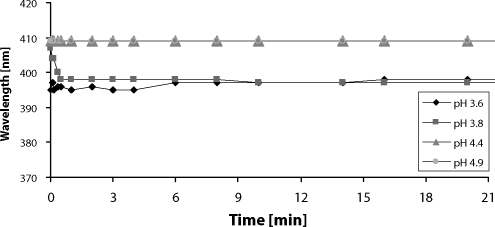
Under the more acidic conditions (pH 3.6–3.8) heme cofactor readily dissociated from the molecule of myoglobin. This process was observed as a shift of heme absorption maximum from 409 to 397 nm within 30 s of the incubation (Figure 2). No changes in absorption maximum of myoglobin were detected for pH 4.4–4.9 (Figure 2).
Figure 2.
The effect of pH on heme dissociation of equine skeletal muscle myoglobin.
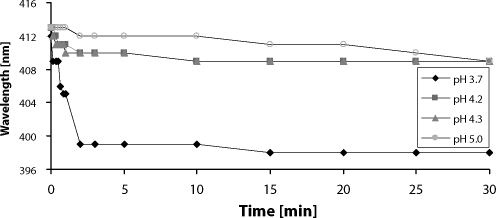
Significant changes in UV/VIS spectra were observed during incubation of cyt b5 with apo-myoglobin in various pH. The initial spectrum eliciting maximum at 413 nm corresponds to pure cyt b5. Both proteins are loosing heme spontaneously at pH 3.7, therefore the heme transfer at this pH was not observed (Figure 3), and the resulting spectrum resembles spectrum of the free hemin.
Figure 3.
The effect of pH on heme transfer or release from cytochrome b5 to apo-myoglobin. Cytochrome b5 (2.1 mg/ml) was mixed with apo-myoglobin (3.6 mg/ml) to molar ratio 1:1.5 in phosphate buffer (10 mM KH2PO4) pH 3-6.
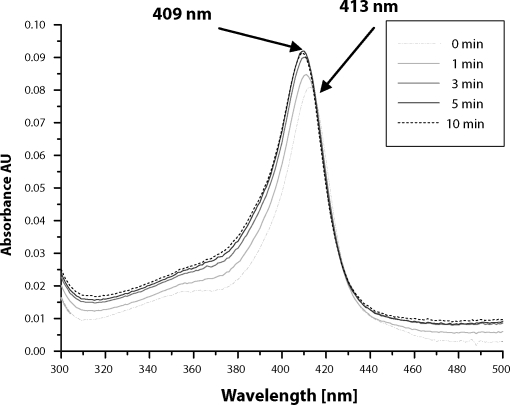
The absorption maximum of heme chromophore in the incubation mixtures (pH 4.2, 4.3 and 5) was shifted from 413 nm to 409 nm (Figure 3). This finding indicates that heme transfer from cyt b5 to apo-myoglobin occurs. Detail spectral changes, observed in incubation mixture pH 4.2, are shown in Figure 4. The resulting spectrum resembles the holo-myoglobin spectrum (maximum 409 nm) (Figure 5), this is indicating that equilibrium state was achieved. Although, there was only slight excess of myoglobin over cyt b5 (molar ratio 1:1.5), the heme transfer should be nearly complete ~99%, due to a significantly higher affinity of myoglobin to heme cofactor, ~2 orders of magnitude more than cyt b5.
Figure 4.
Spectral changes observed during heme transfer from cytochrome b5 to apo-myoglobin at pH 4.2.
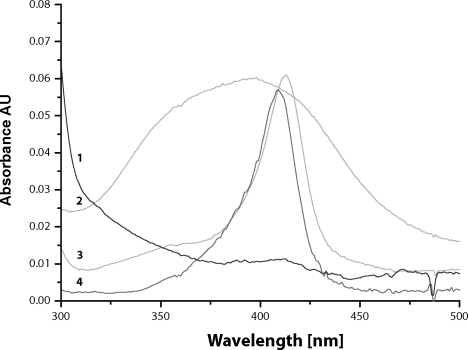
Figure 5.
UV/VIS spectra of individual species. (1) spectrum of the equine skeletal muscle apo-myoglobin, (2) spectrum of hemin with maximum absorbance at ~385 nm, (3) spectrum of purified rabbit cytochrome b5 with maximum absorbance at 413 nm, (4) spectrum of the equine skeletal muscle myoglobin with maximum absorbance at 409 nm.
The heme transfer from cyt b5 to apo-myoglobin is much slower compared to release of free hemin observed in more acidic conditions (pH 3.9 and 3.8, for cyt b5 and myoglobin, respectively). This heme transfer is also extremely sensitive to pH. It tooks 10 minutes at pH 4.2 and 4.3, but at slightly less acidic conditions (pH 5), the transfer was completed in 30 min (Figure 3). Besides, heme transfer from cyt b5 to apo-myoglobin at pH 5.25 is extremely slow and required 6 hours to complete (data not shown).
Discussion
In this study we utilized the principle of the assay for hemin dissociation rate constant (Hargrove et al., 1994, 1996) for the preparation of the apohemoprotein in a large scale. The method employs the fact that apomyoglobin has a very high affinity for heme when compared to others hemoproteins (Hargrove et al., 1996; Miksanova et al., 2006). For example, heme dissociation rate constant for myoglobin is 8.4 × 10–7/s (Hargrove et al., 1996), however for the bovine cyt b5, which is highly homologous (83.5% identity) to rabbit cyt b5 used in this study, is 7.7 × 10–5/s (Altuve et al., 2004). Considering the fact that association of heme with apoglobin is little affected by globin structure (Hargrove et al., 1996), the heme equilibrium dissociation constant for myoglobin is at least two orders of magnitude smaller when compared to cyt b5, showing much higher affinity to heme.
In order to prepare the apo-cytochrome b5 in a large scale we utilized a protein with high affinity toward the heme; the apo-myoglobin from the equine skeletal muscle. We obtained 43 mg of the equine skeletal muscle apo-myoglobin (43% yield). We investigated the effect of pH on spontaneous heme release from both proteins: purified rabbit cyt b5 as well as equine skeletal muscle myoglobin. These realize shows that the optimal pH for heme transfer from cyt b5 into apo-myoglobin is 4.2–5.0.
Acknowledgement
The work is supported by the Grant Agency of the Czech Republic (grants 203/06/0329 and 303/06/0928) and the Ministry of Education of the Czech Republic (grants MSM0021620808 and 1M0505).
REFERENCES
- Altuve A, Lijun Wang L, Benson DR, Rivera M. Mammalian mitochondrial and microsomal cytochromes b5 exhibit divergent structural and biophysical characteristics. Biochem Biophys Res Comm. 2004;314:602–609. doi: 10.1016/j.bbrc.2003.12.138. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Barrick D, Olson JS. The Association Rate Constant for Heme Binding to Globin Is Independent of Protein Structure. Biochemistry. 1996;35:11293–11299. doi: 10.1021/bi960371l. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Singleton EW, Quillin ML, Ortiz LA, Phillips GN, Jr, Olson JS, Mathews AJ. His64- (E7)-Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J Bio Chem. 1994;269:4207–4214. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- Miksanova M, Igarashi J, Minami M, Sagami I, Yamauchi S, Kurokawa H, Shimizu T. Characterization of heme-regulated eIF2alpha kinase: roles of the N-terminal domain in the oligomeric state, heme binding, catalysis, and inhibition. Biochemistry. 2006;45:9894–905. doi: 10.1021/bi060556k. [DOI] [PubMed] [Google Scholar]
- Rossi-Fanelli A, Antonini E, Caputo A. Studies on the structure of hemoglobin. II. Properties of reconstituted protohemoglobin and protoporphyringlobin. Biochim Biophys Acta. 1959;35:93–101. doi: 10.1016/0006-3002(59)90338-5. [DOI] [PubMed] [Google Scholar]
- Shenkman JB, Jansson I. The many roles of cytochrome b5. Pharm Therap. 2003;97:139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Martínek V, Schmeiser HH, Frei E. Modulation of CYP1A1-mediated oxidation of carcinogenic azo dye Sudan I and its binding to DNA by cytochrome b5. Neuro Endocrinol Let. 2006;27(2):35–39. [PubMed] [Google Scholar]
- Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem. 1988;175:231–237. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shimada T, Martin MV, Guengerich FP. Stimulation of cytochrome P450 reactions by apo-cytochrome b5: evidence against transfer of heme from cytochrome P450 3A4 to apo-cytochrome b5 or heme oxygenase. J Biol Chem. 2001;276:30885–30891. doi: 10.1074/jbc.M105011200. [DOI] [PubMed] [Google Scholar]