Abstract
The incidence of hepatocellular carcinoma (HCC) is significantly elevated in a Hispanic community in Bexar County, Texas. Chronic exposure to dietary aflatoxins (AFs) is a major risk factor for HCC; increased risk has been linked to polycyclic aromatic hydrocarbon (PAH) co-exposure and hepatitis virus infection. The aims of this study were to assess AF and PAH exposures, investigate dietary factors that may contribute to increased AF exposure, and determine the prevalence of hepatitis virus infection in Bexar Co. Blood and urine samples were collected from 184 volunteers for biomarker analyses and hepatitis screening. Serum AFB1-lysine adduct, urinary AFM1 and 1-hydroxypyrene (1-OHP) levels were measured using high-performance liquid chromatography. The average AFB1-lysine adduct level detected in 20.6% of serums was 3.84 ± 3.11 pg/mg albumin (range 1.01-16.57 pg/mg). AFM1 was detected in 11.7% of urines, averaging 223.85 ± 250.56 pg/mg creatinine (range 1.89-935.49 pg/mg). AFM1 detection was associated with increased consumption of corn tortillas (p = 0.009), nuts (p = 0.033) and rice (p = 0.037). A significant difference was observed between mean 1-OHP values of non-smokers (0.07 ± 0.13) and smokers (0.80 ± 0.68) μmol/mol creatinine (p < 0.01). A high hepatitis C virus positivity rate (7.1%) was observed. Findings suggest that the incidence and level of AF and PAH exposure was less than that observed in a high-risk population; however, participants consuming higher amounts of foods prone to AF contamination may be more vulnerable to exposure and interactions with other environmental/biological factors (i.e., HCV).
Keywords: aflatoxin, polycyclic aromatic hydrocarbons, hepatocellular carcinoma, hepatitis C virus, biomarkers of exposure, food safety, biomonitoring
1. Introduction
The incidence of hepatocellular carcinoma (HCC) in the United States has steadily increased over the recent decades (El-Serag and Mason, 1999). The state of Texas in particular has been shown to have the highest HCC mortality rate in the U.S. (Devesa et al., 1999). A Hispanic population residing within several zip codes in a community in Bexar County, TX has been disproportionately affected by a high incidence of HCC (ATSDR, 2001). Age-adjusted cancer incidence rates from the Texas Cancer Registry averaged from 2002-2006 show that Hispanics in Bexar Co. have an increased HCC incidence rate of 16.5 (95% CI = 15.0-18.0) compared to Hispanics in Texas, with an incidence rate of 10.9 (95% CI = 10.4-11.4). Incidence rates (per 100,000) are age-adjusted to the 2000 U.S. standard population, and confidence intervals (CI) are 95% for rates (Texas Cancer Registry, 2009). Notably, the HCC incidence rate for Hispanics living in Bexar Co. is considerably higher than all races in Bexar Co., with an incidence rate of 10.0 (95% CI = 9.2-10.8) and all races in Texas, with an incidence rate of 5.8 (95% CI = 5.7-6.0). Hispanic males in Bexar Co. had the highest incidence rate during this time period at 27.1 (95% CI = 24.2-30.2). Thus we were interested in exploring factors that may contribute to HCC in this community.
Multiple factors including diet, environment, lifestyle, health status, gender and genetic susceptibility play a role in the etiology of HCC. Chronic dietary exposure to aflatoxins (AFs), fungal contaminants commonly detected in grain and nut crops (i.e., corn and peanuts), has been established as a major risk factor for HCC development (CAST, 2003; Wogan, 1992). AFB1, the most prevalent of the AFs, is a potent hepatocarcinogen in animals and humans (IARC, 2002). Biomarkers of AF exposure, e.g., AFB1-lysine albumin adduct and AFM1 metabolite, are reliable indicators of chronic and acute exposure, respectively, that have been correlated with elevated HCC risk in several human populations (Groopman et al., 2005). Hoque et al. (1999) previously demonstrated the presence of AFB1-lysine adducts in a small number of HCC patients (5/5 sera samples) registered at the University of Texas (U.T.) M.D. Anderson Cancer Center, prompting the question ‘does AFB1 play a role in the etiology of HCC in the U.S.?’ Populations may be at increased risk for HCC due to additional biological factors, namely hepatitis virus, and/or environmental carcinogen exposures. Wu et al. (2007) documented an elevated HCC risk associated with exposure to a class of environmental contaminants known as polycyclic aromatic hydrocarbons (PAHs). In that report, the greatest risk was found among participants concurrently exposed to high levels of AFs and chronically infected with hepatitis B virus (HBV). While a variety of biological indicators of PAH exposure exist, the urinary biomarker 1-hydroxypyrene (1-OHP) has been validated in many human populations and is widely accepted due to the presence of pyrene in most PAH mixtures (Bouchard and Viau, 1999). In recent work, we reported that a population in Ghana (highly exposed to AFs) was co-exposed to PAHs based on the presence of 1-OHP in the majority of urines collected (Johnson et al., 2009). Cancer mortality patterns in Ghana show liver cancer is the leading cause of cancer mortality in men and the third highest in women (Wiredu and Armah, 2006). In areas of high HCC incidence, such as sub-Saharan Africa, China, and Southeast Asia, HCC occurrence is closely related to HBV infection. Ross et al. (1992) formerly demonstrated a synergistic interaction between HBV and AFB1 in the development of liver cancer. Subsequently, Sun et al. (1999) followed a cohort of Chinese men with chronic HBV for 10 years and found the relative risk of HCC was significantly increased in subjects with detectable AFM1 levels. In addition, co-infection with hepatitis C virus (HCV) further increased HCC risk, indicating HBV and HCV interact as risk factors.
While HBV is endemic to parts of the world with high HCC cases, the frequency of HBV infection in the U.S. is far lower. Conversely, an association between HCV infection and HCC incidence has been demonstrated in the U.S., particularly in Texas (Davila et al., 2004). Records from U.T.M.D. Anderson Cancer Center have shown that more than 50% of HCC cases observed in Texas could be attributed to HCV infection (Hassan et al., 2002). In recent work, Chen et al. (2007) showed AF biomarkers of exposure were associated with advanced liver disease in HCV patients in an endemic area in Taiwan. While it is well-established that a viral-chemical interaction exists between the hepatitis virus and AFs, the possible contribution of AFs in the human diet has not yet been assessed in Bexar Co. Due to the disproportionate occurrence of HCC observed in a minority community in Bexar Co., an environmental health study was conducted as a preliminary survey to 1) assess AF and PAH exposures; 2) investigate dietary factors that may contribute to increased AF exposure, and 3) determine the prevalence of HBV and HCV infection.
2. Materials and Methods
2.1. Participant recruitment and sample collection
Study participants were recruited from three zip codes (where the incidence of liver cancer is significantly elevated) located within the San Antonio metropolitan area of Bexar Co. This area encompasses nearly 11% of Bexar Co.'s population, and residents are predominantly Hispanic (90.2%). Like other southern regions of Texas, corn and corn-based products represent a staple food source; hence, we were interested in assessing AF exposure in this community. A total of 186 participants were recruited at the San Antonio Metropolitan Health District (SAMHD) from October 2007 to May 2008. Volunteers (males and females) who qualified as study participants met the following criteria: 1) at least 18 years of age and 2) a minimum of two years residency (within the last 12 months) in one of the three specified study zip codes. The study protocol was approved by the Institutional Review Board at Texas A&M University, and all participants were provided written informed consent, as well as an oral explanation of the study protocol prior to beginning the study. Upon enrollment, SAMHD public health officials administered an environmental and personal health questionnaire (in English or Spanish) and collected demographic information through in-person interviews. Biological samples, including venous blood and urine, were collected and stored frozen (-20°C) until transport to Texas A&M University and the University of Georgia. Following sample collection, it was noted that two participants did not meet the eligibility criteria concerning residency, and data collected from these subjects were not included. Thus, 184 participants comprised our study population.
2.2. Chemicals and laboratory analysis
Authentic AFB1, AFM1 and 1-OHP standards were purchased from Sigma Chemical Co. (St. Louis, MO). Blood specimens were analyzed for complete blood count, HBV surface antigen (HBsAg) and anti-HCV antibodies according to standard laboratory operating procedures at the SAMHD.
2.3. Serum aflatoxin B1-lysine adduct analysis
Serum AFB1-lysine adduct levels were measured by a modified high-performance liquid chromatography fluorescence (HPLC-f) method (Qian et al., 2009). In brief, serum samples (150 μl) were digested by Pronase (Calbiochem, San Diego, CA) and loaded onto a Waters Oasis Max cartridge (Milford, MA). Cartridges were sequentially washed and eluted with 2% formic acid in methanol. The eluents were evaporated to dryness and reconstituted in 150 μl of 10% methanol prior to HPLC injection. Analysis was carried out on an 1100 liquid chromatography system (Agilent Technologies, Wilmington, DE), and chromatographic separation was performed on a 250 × 4.6 mm Agilent C18 column, particle size 5 μm. The mobile phase consisted of 20 mM ammonium phosphate monobasic (pH 7.2) and methanol in a linear gradient profile. The concentration of AFB1-lysine adducts was monitored at 405 nm (excitation) and 470 nm (emission). Peaks for authentic AFB1-lysine adduct standard and samples were co-eluted at retention times averaging 12.7 min. The detection limit of this method was 10 pg/ml. Results were adjusted for serum albumin levels.
2.4. Urinary aflatoxin M1 and 1-hydroxypyrene analyses
Urinary AFM1 levels were analyzed using immunoaffinity column purification followed by HPLC-f using methods previously described by Wang et al. (2008). Urinary 1-OHP levels were also measured with an HPLC-f method based on a procedure developed by Gardiner et al. (1992), as previously described by Johnson et al. (2009). Quantification of AFM1 and 1-OHP were based on peak area and retention times as compared to external standards run daily. The limit of detection for urinary AFM1 and 1-OHP using these methods was 0.5 pg/ml and 0.25 nmol/L of urine, respectively. Creatinine concentrations were measured at St. Joseph's Regional Health Center Laboratory in order to correct for variations in urine dilution.
2.5. Statistical analyses
Median, mean, standard deviation (SD) and detectable range were calculated for concentrations of all biomarkers measured. Statistical analyses were done using SPSS software version 15.0 (Chicago, IL). For comparisons, t-tests or Wilcoxon tests were used as appropriate to examine differences between biomarker data. Chi-square tests were performed to examine demographic data and variables assessed by the questionnaire. Crude odds ratio estimates for the relationship between various dietary factors and aflatoxin biomarkers were determined by generating 2 × 2 contingency tables. A p-value ≤ 0.05 (two-tailed) was considered significant.
3. Results
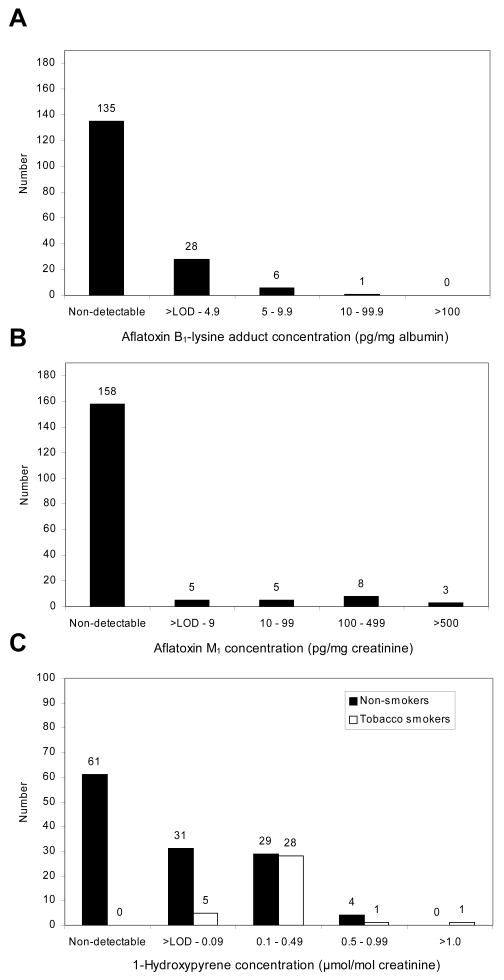
Table 1 provides the descriptive characteristics and HBV and HCV status in our study population. Slightly more than one fourth (26.6%) of the participants were male and 73.4% were female. The average participant age was 48 (median: 49; range: 18-83 years). The majority of participants (97.3%) were of Hispanic ethnicity; the remaining percentage of the study population (1.1 and 1.6%) was Native American and African American, respectively. Serum analysis at SAMHD included screening for HBsAg and anti-HCV antibodies. None of the participants were HBsAg+, while 7.1% (13/184) of the study population was anti-HCV+. Fig. 1A and 1B show the distribution of AF biomarkers of exposure in the study population. Of the total serums analyzed for AFB1-lysine adduct (n = 170), 20.6% had detectable levels with the average level at 3.84 ± 3.11 pg/mg albumin (median: 2.96; detectable range: 1.01-16.57 pg/mg albumin). Urinary AFM1 was detectable in 11.7% of samples analyzed (n = 179) with the average level at 223.85 ± 250.56 pg/mg creatinine (median: 141.53; detectable range: 1.89-935.49 pg/mg creatinine). Characteristics described above did not differ significantly among the participants in the AF-detectable and non-detectable groups.Fig. 1 (C) illustrates the distribution of 1-OHP in study participants stratified by smoking status. Of the samples available for 1-OHP analysis, 51.2% of 125 non-smokers and 100% of 35 tobacco-smokers had detectable levels of 1-OHP with median excretion values of 0.01 and 0.17 μmol/mol creatinine, respectively. Moreover, there was a significant difference between mean 1-OHP levels measured in non-smokers (0.07 ± 0.13 μmol/mol creatinine) and smokers (0.26 ± 0.33 μmol/mol creatinine) (p < 0.01). While a slight lack of concordance between the total number of study participants and amount of samples analyzed for AF and PAH biomarkers arose due to unforeseeable events during sample collection and transfer, adequate amounts of samples for each biomarker were analyzed to validate statistical analysis. A primary aim of the environmental and personal health questionnaire administered at SAMHD was to investigate dietary factors that may contribute to increased AF exposure in the study population. Results from questions on food consumption showed that > 98% of participants reported that they ate commodities prone to AF-contamination (e.g., corn, nuts, rice and a variety of corn/peanut-based foods) at varying frequencies. For instance, a large percentage of the population consumed corn tortillas (44.8%) and rice (30.1%) frequently (3-14 times per week); the vast majority ate ≥ 1 tortilla (97.6%) or ≥ ½ cup of rice (82.7%) at each time of consumption. When food consumption was examined according to AF biomarker detection, the amount of corn tortillas (p = 0.009), rice (p = 0.037), and nuts (p = 0.033) consumed was found to be significantly associated with urinary AFM1 detection (Table 2). No association was found between food consumption and detectable AFB1-lysine adduct, except a marginally significant association with rice consumption (p = 0.053).
Table 1. Descriptive characteristics and HBV/HCV serology in Bexar County study participants (n = 184).
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 49 (26.6) |
| Female | 135 (73.4) |
| Ethnic Group | |
| Hispanic | 179 (97.3) |
| African American | 2 (1.1) |
| Native American | 3 (1.6) |
| Age (years) | |
| 18-29 | 24 (13.0) |
| 30-39 | 37 (20.1) |
| 40-49 | 34 (18.5) |
| 50-59 | 34 (18.5) |
| 60-69 | 36 (19.6) |
| > 70 | 19 (10.3) |
| Smoking Status | |
| Smokera | 31 (16.8) |
| Non-smoker | 153 (83.2) |
| Hepatitis Status | |
| HBsAg+b | 0 (0.0) |
| Anti-HCV+c | 13 (7.1) |
Tobacco smoking status based on participant questionnaire.
Hepatitis B virus surface antigen.
Antibodies to hepatitis C virus.
Fig. 1.
Distribution of aflatoxin B1-lysine adducts in serum (A), aflatoxin M1 metabolite in urine (B), and 1-hydroxypyrene in urine of smokers and non-tobacco smokers (C) from Bexar Co., Texas.
Table 2. Food consumption in study population by distribution of aflatoxin M1 biomarker in urine.
| Amount of Food Consumeda | Aflatoxin M1[n (%)]b | ||
|---|---|---|---|
| Detectable | Non-detectable | ||
| Corn | < ½ cup (1 ear) | 2 (10.5) | 28 (18.9) |
| ≥ ½ cup (1 ear) | 17 (89.5) | 120 (81.1) | |
| Corn tortillas | < 1 tortilla | 2 (11.8) | 2 (1.4) |
| ≥ 1 tortilla | 15 (88.2)* | 142 (98.6) | |
| Corn bread/muffins | < 1 piece (muffin) | 1 (12.5) | 12 (13.0) |
| ≥ 1 piece (muffin) | 7 (87.5) | 80 (87.0) | |
| Corn chips | < 1 cup (10 chips) | 5 (26.3) | 44 (30.3) |
| ≥1 cup (10 chips) | 14 (73.7) | 101 (69.7) | |
| Rice | < ½ cup | 0 (0.0) | 31 (19.9) |
| ≥ ½ cup | 18 (100.0)* | 125 (80.1) | |
| Peanut butter | < 1 tablespoon | 4 (25.0) | 17 (19.3) |
| ≥ 1 tablespoon | 12 (75.0) | 71 (80.7) | |
| Nuts | < ¼ cup | 2 (12.5) | 54 (39.7) |
| ≥ ¼ cup | 14 (87.5)* | 82 (60.3) | |
Amount of food consumed at each time of consumption.
Numbers within subgroups differ slightly from the total number of samples analyzed for AFM1 due to missing responses.
p ≤ 0.05 in comparison of distribution in AFM1-detectable and AFM1-non-detectable groups in Fisher exact test.
4. Discussion
Interaction between dietary AFs and hepatitis virus infection increases the risk for HCC development. Areas located between latitudes 40° N and S (which includes Texas) encompass populations at risk for chronic AF exposure based on suitable temperature, humidity, and vulnerability of staple commodities for mycotoxin contamination (Williams et al., 2004). Since contamination has been reported in foods from Texas and surrounding areas (Torres et al., 1995; Wood, 1992), particularly after periods of drought, our primary objective was to assess AF exposure in a predominantly Hispanic population in Bexar Co. with an increased incidence of HCC. McCoy et al. (2008) compared three procedures (ELISA, HPLC-f and HPLC with isotope dilution mass spectrometry) for measuring human AF-albumin adduct levels and found a good correlation between the three independent methods. In our study, serum AFB1-lysine adduct levels, quantified by HPLC-f, revealed that the majority of participants had a non-detectable AF exposure, which was confirmed by urinary AFM1 data. While the correlation between serum and urine AF biomarkers was not significant, results indicate that some individuals showed low levels of chronic exposure whereas others exhibited low to moderate to high short-term AF exposure. This may be due to the differences in half-life of the two biomarkers. The AFB1-lysine adduct has a longer in vivo half-life reflecting integrated exposures over weeks to months compared to AFM1 excretion representing recent exposure (i.e., 24 to 48 hours) (Wang et al., 1996).
Though there was a measurable AF exposure in Bexar Co., the percentage and levels were lower than those we previously observed in the Ashanti Region of Ghana, which represents a population at high risk for aflatoxicosis due to frequent and high level consumption of contaminated foods (Jolly et al., 2006; Wang et al., 2008). For instance, in two surveys, 91.2% and 88.1% of 91 and 159 participants, respectively, had detectable AFM1 levels ranging from 0.66 to 13,297.67 pg/mg creatinine. Findings from our environmental health survey in Bexar Co. suggest that participants overall had a lower exposure to AFs than participants from a developing country (i.e., Ghana). Proper production, storage and processing of foods and effective enforcement of regulations all contribute to reduced AF exposures in developed countries. Further work delineating the source of food (e.g., store bought, home-grown crops, human food grade quality, etc.) may be of importance since low socioeconomic conditions in rural and metropolitan communities in developed countries may necessitate the use of lower quality foodstuffs. Importantly, no tolerable daily intake has been set for AFB1, as determined for other (less carcinogenic) mycotoxins. The U.S. Food and Drug Administration has set an action level of 20 ppb in foods intended for human consumption, which corresponds to ∼30 μg AFs/day, assuming that an average adult consumes approximately 1500 g of food/day. Using a urinary excretion rate of 2 - 5% (Cheng et al., 1997) and a metabolic excretion rate of 1500 ml urine/day, it can be estimated that the mean AFM1 excretion in Bexar Co. corresponds to an average daily AFB1 consumption ranging from 9.8 - 24.6 μg/day. Although the estimated average daily AFB1 intake is below 30 μg, individuals in the 75th and 95th percentiles may have estimated daily AFB1 consumptions ranging from 15.3 - 38.2 and 49.2 - 122.9 μg/day, respectively. Thus, human health hazards associated with such AF exposure over time cannot be ruled out.
Of additional concern in Bexar Co. was assessing co-exposure to PAHs, which may increase the risk for HCC in the presence of AFs and hepatitis virus infection (Wu et al. 2007). Findings from this portion of our study illustrated that all study participants classified as tobacco-smokers had detectable levels of urinary 1-OHP, whereas approximately half of non-smoking participants did not show a measurable exposure to PAHs. Data further demonstrated a significant difference in mean 1-OHP concentrations when participants were stratified by smoking status. This is in agreement with previous work showing statistically significant increases in 1-OHP excretion in tobacco-smokers exposed to background levels of environmental PAHs (Levin et al., 1995; Viau et al., 1995). Conversely, in our population in Ghana smoking failed to produce any differences in urinary 1-OHP levels, compared to not smoking, indicating a predominant environmental PAH exposure (Johnson et al., 2009). Moreover, 1-OHP levels measured in Bexar Co. were considerably lower than those previously recorded in Ghana. Findings in this U.S. population were comparable or lower than those previously recorded for non-smoking individuals in numerous developed countries (Levin, 1995). Overall, results suggest that non-tobacco smokers in Bexar Co. are not at high risk for PAH exposure, based on this short-term biomarker.
An additional objective of our environmental health study in Bexar Co. was to determine the prevalence of HBV and HCV since hepatitis virus infection clearly contributes to the overall burden of HCC. Previous findings from a study in a Texas male prison population indicated that inmates who were older, Hispanic, and infected with HCV or HBV had elevated rates of both HCC prevalence and mortality (Baillargeon et al., 2009). While no participants in our study population were HBsAg+, 7.1% were positive for HCV. HBV infection is closely linked to HCC in developing countries; however, its impact may be far less in areas of the U.S. where HBV vaccination is common. In contrast, the prevalence of HCV is of significant importance, especially since no vaccination is currently available. Data from this Bexar Co. community was higher than the overall prevalence in Texas, reported to be 1.79% (varying from 1.25-2.63% across Texas counties) (Yalamanchili et al., 2005). Thus, the implementation of biomonitoring and intervention strategies, particularly in vulnerable individuals may play an important role in reducing the overall negative public health impact of dietary AF exposure. This is especially relevant in individuals at high risk for HCV-induced HCC, such as women considered to be at risk in their child bearing years and infants that acquire the virus early on as a result of perinatal infection.
A limitation of our study was the uneven recruitment of females due to the lack of male participation in the recruitment process. In future studies in this area, partnerships with local non-profit groups have been established and will serve to recruit participants with emphasis on equal gender participation. These mutually beneficial partnerships and collaborations with researchers at Texas A&M University and the San Antonio Metropolitan Health District will facilitate future work in this area.
5. Conclusions
The primary goal of this pilot study was to gain insight into the current public health of this vulnerable community and gather information to support further exploration of potential factors that can contribute HCC risk. Biomarkers measured in this study reflect current exposures to AFs and PAHs. Results from our environmental health study showed a significant association of increased consumption of certain foods and the excretion of AFM1 in a minority population in Texas. In addition, the HCV positivity rate is considerably high in this community and warrants attention.
Acknowledgments
Special thanks to all volunteers and study participants whose participation made this study possible. The authors declare they have no competing financial interests. This work was supported by the National Institute of Environmental Health Sciences (NIEHS; Grant No. ES09106).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry Health Consultation 2001. ATSDR; Kelly Air Force Base, San Antonio, Bexar County, Texas: [11 June 2010]. available: http://www.atsdr.cdc.gov/HAC/pha/PHA.asp?docid=100&pg=0. [Google Scholar]
- Baillargeon J, Snyder N, Soloway RD, Paar D, Baillargeon G, Spaulding AC, et al. Hepatocellular carcinoma prevalence and mortality in a male state prison population. Public Health Rep. 2009;124:120–6. doi: 10.1177/003335490912400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Viau C. Urinary 1-hydroxypyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: biological monitoring strategies and methodology for determining biological exposure indices for various work environments. Biomarkers. 1999;4:159–87. doi: 10.1080/135475099230859. [DOI] [PubMed] [Google Scholar]
- CAST. Mycotoxins: risks in plant, animal, and human systems. Council for Agricultural Science and Technology Task Force Report. 2003;39:13–85. [Google Scholar]
- Chen CH, Wang MH, Wang JH, Hung CH, Hu TH, Lee SC, et al. Aflatoxin exposure and hepatitis C virus in advanced liver disease in a hepatitis C virus endemic area in Taiwan. Chen Am J Trop Med Hyg. 2007;77:747–52. [PubMed] [Google Scholar]
- Cheng Z, Root M, Pan W, Chen J, Campbell TC. Use of an improved method for analysis of urinary aflatoxin M1 in a survey of mainland China and Taiwan. Cancer Epidemiol Biomarkers Prev. 1997;6:523–29. [PubMed] [Google Scholar]
- Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–80. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Devesa SS, Grauman DG, Blot WJ, Pennello G, Hoover RN, Fraumeni JF., Jr . Atlas of cancer mortality in the United States, 1950-94. Washington, DC: US Govt Print Off; 1999. NIH Publ No. (NIH) 99–4564. [Google Scholar]
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Gardiner K, Hale KA, Calvert IA, Rice C, Harrington JM. The suitability of the urinary metabolite 1-hydroxypyrene as an index of poly nuclear aromatic hydrocarbon bioavailability from workers exposed to carbon black. Ann Occup Hyg. 1992;36:681–8. doi: 10.1093/annhyg/36.6.681. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Johnson D, Kensler TW. Aflatoxin and hepatitis B virus biomarkers: a paradigm for complex environmental exposures and cancer risk. Cancer Biomark. 2005;1:5–14. doi: 10.3233/cbm-2005-1103. [DOI] [PubMed] [Google Scholar]
- Hassan MN, Frome PA, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–69. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Hoque A, Patt YZ, Yoffe B, Groopman JD, Greenblatt MS, Zhang YJ, Santella RM. Does aflatoxin B1 play a role in the etiology of hepatocellular carcinoma in the United States? Nutr Cancer. 1999;35:27–33. doi: 10.1207/S1532791427-33. [DOI] [PubMed] [Google Scholar]
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. International Agency for Research on Cancer Monogr Eval Carcinog Risk Hum. 2002;82:171–274. [PMC free article] [PubMed] [Google Scholar]
- Johnson NM, Afriyie-Gyawu E, Huebner HJ, Marroquin-Cardona A, Robinson A, Tang L, et al. PAH Exposure in a Ghanaian population at high risk for aflatoxicosis. Sci Total Environ. 2009;407:1886–91. doi: 10.1016/j.scitotenv.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Jolly PE, Jiang Y, Ellis W, Awuah R, Nnedu O, Phillips TD, et al. Determinants of aflatoxin levels in Ghanaians: sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Health. 2006;209:345–58. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Levin JO. First international workshop on hydroxypyrene as a biomarker for PAH exposure in man–summary and conclusions. Sci Total Environ. 1995;163:165–8. [PubMed] [Google Scholar]
- Levin JO, Rhén M, Sikström E. Occupational PAH exposure: urinary 1-hyroxypyrene levels of coke oven workers, aluminum smelter pot-room workers, road pavers, and occupationally non-exposed persons in Sweden. Sci Total Environ. 1995;163:169–77. doi: 10.1016/0048-9697(95)04488-m. [DOI] [PubMed] [Google Scholar]
- McCoy LF, Scholl PF, Sutcliffe AE, Kieszak SM, Powers CD, Rogers HS, et al. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17:1653–7. doi: 10.1158/1055-9965.EPI-07-2780. [DOI] [PubMed] [Google Scholar]
- Qian G, Tang L, Xu L, Johnson NM, Tietze D, Rodriguez M, et al. Serum levels of aflatoxin B1-lysine adduct in a U.S. population compared to a high risk population in China. 48th Annual Society of Toxicology Meeting; 2009 Mar 15-19; Baltimore, MD. Abstract. [Google Scholar]
- Ross R, Yuan JM, Yu M, Wogan GN, Qian GS, Tu JT, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–6. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Sun Z, Lu P, Gail MH, Pee D, Zhang Q, Ming L, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology. 1999;30:379–83. doi: 10.1002/hep.510300204. [DOI] [PubMed] [Google Scholar]
- Texas Department of State Health Services. Texas Cancer Registry, Incidence 1995-2006. Aug 17, 2009. Cancer Epidemiology and Surveillance Branch. NPCR-CSS Sub 11-26-2008, SEER Pop-Adj, SEER*Prep 2.4.0. Data request #09293. [Google Scholar]
- Torres EE, Acuña AK, Naccha Torres LR, Montoya OR, Castrellón Santa Anna JP. Quantification of aflatoxins in corn distributed in the city of Monterrey, Mexico. Food Addit Contam. 1995;12:383–6. doi: 10.1080/02652039509374319. [DOI] [PubMed] [Google Scholar]
- Viau C, Vyskocil A, Martel L. Background urinary 1-hydroxypyrene in non-occupationally exposed individuals in the Province of Québec, Canada, and comparison with its excretion in workers exposed to PAH mixtures. Sci Total Environ. 1995;163:191–4. doi: 10.1016/0048-9697(95)04496-n. [DOI] [PubMed] [Google Scholar]
- Wang JS, Qian GS, Zarba A, He X, Zhu YR, Zhang BC, et al. Temporal patterns of aflatoxin-albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong County, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1996;5:253–61. [PubMed] [Google Scholar]
- Wang P, Afriyie-Gyawu E, Tang Y, Johnson NM, Xu L, Tang L, et al. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Addit Contam. 2008;25:622–34. doi: 10.1080/02652030701598694. [DOI] [PubMed] [Google Scholar]
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- Wiredu EK, Armah HB. Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality. BMC Public Health. 2006;6:159–65. doi: 10.1186/1471-2458-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992;52:2114s–8s. [PubMed] [Google Scholar]
- Wood GE. Mycotoxins in foods and feeds in the United States. J Anim Sci. 1992;70:3941–9. doi: 10.2527/1992.70123941x. [DOI] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Wang LW, Yang HI, Ahsan H, Tsai WY, et al. Polycyclic aromatic hydrocarbon- and aflatoxin-albumin adducts, hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Cancer Lett. 2007;252:104–14. doi: 10.1016/j.canlet.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili K, Saadeh S, Lepe R, Davis GL. The prevalence of hepatitis C virus infection in Texas: implications for future health care. BUMC Proceedings. 2005;18:3–6. doi: 10.1080/08998280.2005.11928024. [DOI] [PMC free article] [PubMed] [Google Scholar]