Abstract
Recent evidence suggests that passenger leukocytes migrate after organ transplantation and produce persistent chimerism, which is essential for sustained survival of the allografts. Here, Thomas Starzl and colleagues argure that this hematolymphopoietic chimerism provides an important framework for the interpretation of basic and therapeutically oriented transplantataion research.
Medawar’s characterization of rejection1 as a host-versus-graft (HVG) reaction (Fig. 1a) was the cornerstone of transplantation immunology. A decade later, this concept was transposed in the context of a graft-versus-host (GVH) reaction (Fig. 1b), in which histoincompatible hematolymphopoietic grafts rejected the immunologically defenseless recipients2,3. The resulting assumption that allograft acceptance or rejection could be understood by studying HVG or GVH immunologic responses in isolation led to prompt acceptance of the one-way in vitro tests of immune reactivity as ‘minitransplant’ surrogates. However, this assumption did not provide a blanket explanation for observations made in animal and human allograft recipients.
Fig 1.
(Upper panels) One-way paradigm in which transplantation is conceived as involving a unidirectional immune reaction: (a) host-versus-graft (HVG) with whole organs and (b) graft-versus-host (GVH) with bone marrow or other lymphopoietic transplants. (Lower panels) Two-way paradigm in which transplantation is seen as a bidirectional and mutually cancelling immune reaction that is (c) predominantly HVG with whole organ grafts, and (d) predominantly GVH with bone marrow grafts.
The one-way paradigm
Until 1959, preparatory donor leukocyte infusion into cytoablated organ recipients was an expected natural extension of the neonatal tolerance model of Billingham, Brent and Medawar4 and its adult cytoablation analogues3. However, when long-term survival of human kidney allografts was accomplished in a few sublethally irradiated recipients without donor leukocyte infusion, and then regularly without cytoreduction under continuous pharmacologic immunosuppression, the need either for chimerism or host preconditioning lost favor.
The identification of ‘passenger leukocytes’ as the primary antigenic component of organs6,7 led to the belief that their destruction by the host immune system was essential for organ engraftment. When these cells were found to be migratory8, including dendritic cells (DCs)9, their sensitization effects and presumed elimination at peripheral and intragraft sites was taken for granted.
Bone marrow transplantation
Major histocompatibility complex (MHC)-restricted models of acquired tolerance were widely considered to have validated Burnet’s prediction that developing lymphocytes could be purged of self-reactive cells before they achieved functional maturity, even following bone marrow transplantation. The alternative possibility that donor and recipient immune-cell populations coexisted in neonatally tolerant animals in a mutually nonreactive state while retaining the ability to function collaboratively (e.g. in a joint immune response to infection) was abandoned when no direct experimental support could be found10. However, it has since been learned that the outcome in the neonatal tolerance model is highly variable and that a state approaching permanent clonal deletion is uncommon11. Recently, it has been shown that the ability of donor-derived leukocyte subsets to proliferate in response to a skin graft challenge was a more critical determinant of neonatal tolerance outcome than the baseline level of chimerism12.
Organ transplantation
The conclusion that organ transplant acceptance was by different unidirectional mechanisms than those of bone marrow grafts was reinforced by the striking differences between the two varieties of procedures (Table 1). In addition, it was generally assumed that cytoablation (or cytoreduction) to ‘make microenvironmental space’ was a necessary condition for leukocyte engraftment and chimerism, in spite of early and recent evidence to the contrary (reviewed in Ref. 13).
Table 1.
Differences between conventional bone marrow and organ transplantation
| Bone Marrow | Feature | Organ |
|---|---|---|
| Yes | Recipient cytoablationa | No |
| Critical | MHC compatibility | Not critical |
| GVHD | Principal complication | Rejection |
| Common | Drug free state | Rare |
| Tolerance | Term for success | ‘Acceptance’b |
Abbreviations: GVHD, graft-versus-host disease; MHC, major histocompatibility complex.
All differences derive from this therapeutic step which in effect establishes an unopposed CVH reaction in the bone marrow recipient whose countervailing immune reaction is eliminated.
Or ‘operational tolerance’.
The two-way paradigm
A link between bone marrow and organ transplantation was provided when microchimerism was detected with sensitive immunocytochemical and polymerase chain reaction (PCR) techniques in the tissues or blood of all 30 human kidney or liver recipients studied from 2.5–30 years postoperatively14,15 (Fig. 1c). Many of the donor cells appeared to be DCs, potent antigen-presenting cells (APCs)16. Individual samples often do not contain the donor leukocytes, which wax and wane17. However, disseminated donor cells, including DCs, and/or donor DNA are consistently found if rodents bearing long-term grafts are thoroughly studied16–20.
Along with peripheral migration of the donor cells from a successfully transplanted graft, there is an influx of host leukocytes that do not cause graft damage (Fig. 1c)15 both the allograft and recipient become genetic composites. A mirror image condition exists after bone marrow transplantation21 (Fig. 1d), proved by demonstrating a trace residual population of host leukocytes in essentially all stable, human bone marrow recipients who previously were thought to have complete donor-cell chimerism22.
Cause or effect?
In the one-way paradigm, which excludes a role for lymphoid cell microchimerism, it has become axiomatic that antigens of the parenchymal (or vascular endothelial) cells of transplanted organs permit or induce allograft acceptance23 in various ways, e.g. via veto/suppressor cells, cytokine profile changes or enhancing antibodies. Furthermore, it has been argued that the microchimerism associated with successful transplantation, and conversely its disappearance with or just after irreversible rejection in experimental models18,20, is epiphenomenal24.
In a reassessment based on the discovery of microchimerism in organ recipients, we suggested that the donor leukocytes in organ recipients were components of antagonistic but reciprocally attenuated or abrogated HVG and GVH arms14,15,21. Deletion of the host arm by cytoablation prior to bone marrow but not organ transplantation altered the balance in this mutual antagonism and was thus responsible for the disparities in the two different kinds of transplantation (Table 1).
The microchimerism had consequences that could not be explained by the simple presence of antigen, as long as the balance was not disturbed and both cell populations were equally immunosuppressed. The dynamic ‘nullification’ effect of the two arms explained (1) the poor prognostic value of HLA matching for organ transplantation; (2) the rarity of GVH disease (GVHD) following the engraftment of immunologically active organs, such as the intestine and liver14,15,21, and (3) the characteristic cycle of immunologic crisis and resolution, first observed in kidney recipients25, that was most-practically monitored by serial changes in organ allograft function (Fig. 2).
Fig. 2.

Simultaneous host-versus-graft (HVG) and graft-versus-host (GVH) reactions in the two-way paradigm of transplantation immunology. Following the initial interaction, the evolution of nonreactivity of each leukocyte population to the other is seen as a predominantly low-grade stimulatory stale that may wax and wane, rather than a deletional one.
Finally, the discovery of chimerism cast new light on the B-cell lymphomas [post-transplant lymphoproliferative disorders (PTLDs)], that are usually of host origin in organ recipients and of donor origin after bone marrow transplantation. Except for their frequent Epstein-Barr virus association, these human malignancies are indistinguishable from those induced by Schwartz in a mouse chimerism model26 three years before the PTLD complication was first recognized clinically27 and explained by simple loss of surveillance28. By contrast, Schwartz ascribed the tumors to a lymphoproliferative response by the dominant immune apparatus to the persistent subclinical GVH counter-attack of the minority leukocyte population. The relevance of this conclusion, of ‘Schwartz’s rules’ of pathogenesis, and of their therapeutic implications could not be appreciated until three decades later in the context of the two-way paradigm29.
The role of immunosuppression
As in Schwartz’s ‘lymphoma-genic’ experiments, immunosuppression is a temporary requirement for reliable induction of tolerance in numerous rodent organ allograft models. The same is true, but unpredictably, after liver30 and, less commonly, kidney transplantation in outbred canines. Moreover, successful liver transplantation induces tolerance with no treatment at all in a significant percentage of outbred pigs as well as several rat20,31 and virtually all mouse strain combinations19. Mouse heart and kidney allografts are also accepted spontaneously in a much more limited number of MHC disparate conditions (reviewed in Ref. 19). When a thorough search is made for microchimerism in the rodent models, it can always be found19,20,32.
In all these species, the organs pass through an acute self-resolving rejection on the way to tolerance, which usually extends to subsequent transplantation of other donor-strain tissues and organs33. The tolerance is stable despite evidence from in vitro testing that anti-donor reactivity is retained (split tolerance)’9,20,31,34 or can be restored by the addition of appropriate cytokines.
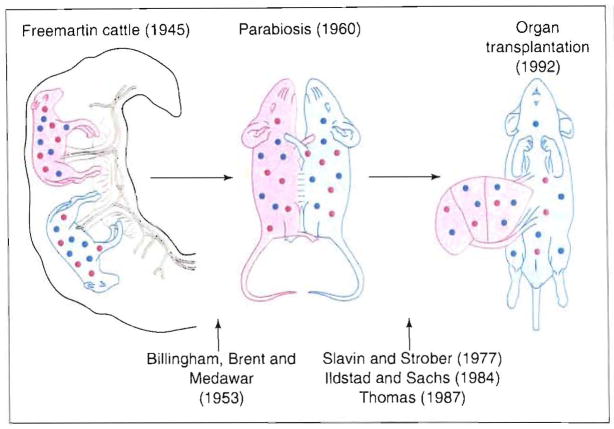
The cumulative weight of the above observations does not support the possibility that microchimerism is a passive consequence of organ transplantation. Instead, an active role of the organ-associated chimerism can be identified in a continuum of classical tolerance models beginning with the original observations by Owen in Freemartin cattle (Fig. 3).
Fig. 3.
The continuum of chimerism from observations of R. Owen in Freemartin cattle, which was rejected ns a mechanistic explanation of organ allograft acceptance from 1960 until the discovery in 1992 of microchimerism in organ recipients.
The stem cell question
The human chimerism studies suggested that hematopoietic stem and precursor cells were among the migratory cells from transplanted organs. In support of this contention, all lineages in supralethally irradiated mice can be reconstituted efficiently by the infusion of non-parenchymal cells with stem cell phenotype, isolated from syngeneic adult mouse livers35. In addition, irradiated rats can be reliably reconstituted with orthotopic liver transplantation rather than bone marrow36.
Importantly, heterotopic heart transplantation also results in permanent hematopoietic reconstitution in occasional irradiated rats36, a rescue that is increased to ≥70% by the post-cardiac transplant administration of lisofylline (N. Murase et al., unpublished). Lisofylline is a phosphatidic acid inhibitor that facilitates bone marrow engraftment by suppressing hematopoiesis-inhibiting cytokines (e.g. rumor necrosis factor α, transforming growth factor β, macrophage inhibitory protein 1α and platelet factor 4) that are typically released in response to activation stimuli in the post-transplant period, while not altering levels or activities of the myeloid, progenitor-cell-promoting cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF (Ref, 37).
Such experiments show that the chimerism produced with bone marrow infusion vs. conventional organ transplantation is the same, with apparent differences that are largely determined by the radically divergent treatment regimens. Consistent with this, the chimerism following transplantation of the bone-marrow-containing hind limb to non-cytoablated recipients is much the same as after engraftment of parenchymal organs36.
However, in practical terms the outcome (HVG, GVHD or both) is strongly influenced by the lineage profile of the mature immunocytes contained either in different vital organs (heart, kidney, liver and intestine) or in cell suspensions prepared from various primary or secondary lymphoid organs. Non-parenchymal cells of the liver (the most tolerogenic whole organ) resemble those of bone marrow (the lymphoid organ yielding the most tolerogenic cell suspension). Both include higher numbers of immature leukocytes and cells of myeloid origin than the lymphocyte-rich and GVHD-prone intestinal allograft and lymph node or spleen cell suspensions20.
Chimerism: level and duration
The implication of human and animal studies is that the threshold level of circulating donor leukocytes necessary for a tolerogenic effect has been set too high. Although treatment strategies that directly18,19 or indirectly augment chimerism37,39 in non-cytoablated experimental animals increase the reliability and completeness of tolerance, it is not at all clear that the process can be fundamentally hastened. One postulate is that the chimeric immune cells remain susceptible to further signals that reinforce specific nonreactivity in stages40. Rather than accelerating these steps, we have suggested that immunosuppressive agents, with diverse sites of action, merely permit them to develop (with variable success) by allowing the same underlying function of the immune system to be expressed as in models of spontaneous tolerance41 (see earlier).
With liver transplantation in spontaneously tolerant and ‘immunosuppression-assisted’ rodent models, the cause (chimerism) and effect (tolerance) are induced almost simultaneously but these related events are usually separated by months or years in outbred animals and humans (Fig. 4). Many long-surviving human liver recipients have become immunosuppression-independent (most frequently because of treatment noncompliance) at highly variable postoperative times (Fig. 5). More-complete information was obtained in a prospective weaning trial of liver recipients who had at least five years of stable allograft function42. The majority of these patients were able to stop immunosuppression or are still in an uninterrupted weaning process43; 30% developed rejection, necessitating resumption of immunosuppression. No grafts were lost or had permanent impairment of function.
Fig. 4.

Time between cause (chimerism) and effect (donor specific tolerance) after liver allotransplantation in different species. Note that immunosuppression is not universally required in three of the five species shown,
Fig. 5.
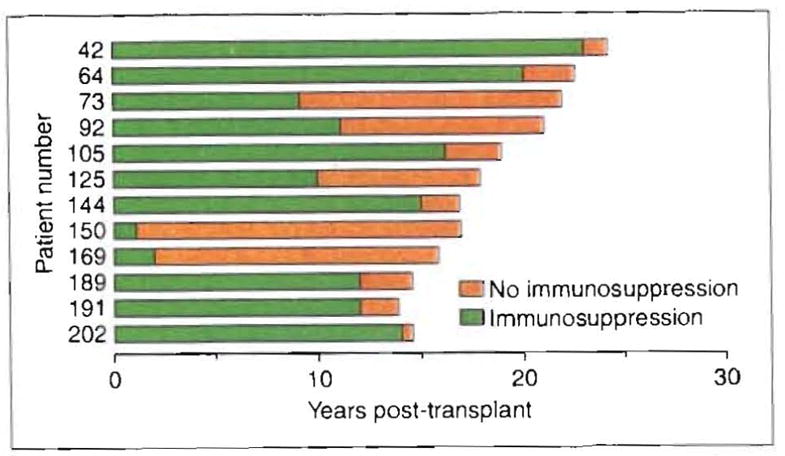
Time on (green) and off immunosuppression (orange) of 12 (28%) of our 42 longest-surviving liver recipients (15–26 years post-transplant) who are receiving no treatment as of December 1995. These drug-free patients remain well in September 1996.
The desired drug-free state might never be reached in a proportion of human liver recipients, but the disseminated donor-derived leukocytes (and their companion organ) apparently can be maintained for a lifetime under immunosuppression. The same principle has been demonstrated in rat cardiac and renal recipients in which continued immunosuppression prevented the slow disappearance of chimerism and the onset of indolent chronic rejection20.
As in animals, discontinuance of drug therapy in humans is thought to be more dangerous after transplantation of organs other than the liver. However, five of the ten longest-surviving patients bearing living-related kidney allografts have been completely off immunosuppression for between three and 30 years (Table 2). Patients 3 and 4, whose mixed lymphocyte response (MLR) tests to donor and third party targets were profoundly depressed prior to weaning44, had gradual restoration of MLR to both in the drug-free state, but with no evidence of rejection.
Table 2.
Discontinuance of immunosuppression in long-term living related kidney recipientsa
| Patient | Years post-transplantation | Haplotype mismatch | Indication for weaningb | Years off drugs |
|---|---|---|---|---|
| 1 | 33 | 0 | nc | 30 |
| 2c | 32 | 1 | comp | 15 |
| 3 | 32 | 0 | nc | 29 |
| 4c | 32 | 2 | comp | 0.5–3 |
| 5c | 33 | 1 | comp | 3 |
These are 5 of the 16 longest-functioning allografts in the world.
comp, complications: skin cancer, warts, infection, hypertension, obesity and orthopedic problems.
nc, non-compliant.
These were children at the time of transplantation.
There is no empirical method to determine the necessary duration of continued immunosuppression for maintenance of stable chimerism and allograft function in humans. Thus, quantitation of donor-derived leukocytes cannot be used to plan drug weaning protocols for patients. This must be done by cautious trial, with precautions to prevent irreversible error.
Genetic factors
Although the genetic basis for immune reactions is beyond question, the MHC effect is unambiguously evident only when the recipient is immunologically defenseless: i.e. in the neonatal tolerance model, recipient cytoablation in all species, or as the consequence of breeding (e.g. the F1 hybrid preparations). When the recipient immune system is competent, organ transplantation outcomes have defied detailed genetic analyses, even in congenic mouse19 and rat models45,46. A clear prognostic effect of MHC after organ transplantation in immunologically intact humans has been clearly identifiable only with a perfect or near perfect HLA match47. The lack of predictability can be explained by the interaction implicit with chimerism in which each population follows its own genetic program.
MHC did not evolve for immunologic segregation of transplant patients and their tissues but rather to meet the need of populations, not individuals, for immunologic flexibility: allograft rejection was an unforeseen byproduct of modern technology. Transplantation of surgically revascularized allografts was, in essence, no different than the induction and then the control of an organ-specific autoimmune disease. Thus, there were no hard genetic rules that prohibited chimerism or successful organ transplantation.
Cellular and molecular mechanisms
So-called ‘parking’ experiments, in which grafts are temporarily placed in a third-party recipient prior to retransplant into the intended host, have been put to good use in transplantation research7,48,49. However, we would argue that the presence of altered (nonreactive) leukocytes that repopulate an organ during residency in the intermediary allogeneic host make such retransplantation models inappropriate for the study of complex tolerance mechanisms. In addition, the leukocyte replacement during the parking period is incomplete. Even at one year of residence in a tolerant recipient, 10% of the non-parenchymal cells remain donor, a proportion that is essentially fixed from day 100 onward18. Not surprisingly, the results following retransplantation are hard to interpret50,51.
In simpler experiments involving only the depletion of organ leukocytes by donor irradiation or other means, both the tolerogenicity and antigenicity of heart39, liver52 and free pancreas islet allografts53 are abrogated or weakened. The tolerogenicity of liver can be restored by an infusion of donor-strain splenocytes into irradiated donors 24 hours before the organ is removed for transplantation54. The same is true of islets after adding back donor leukocytes.
In contrast to the interpretive artefacts introduced with the parking models, successful transplantation in the two-way paradigm is defined as persistent chimerism, whether or not it is immunosuppression-dependent. A failed transplantation connotes the therapeutically uncontrollable ascendency either of HVG or GVH (Refs 15, 41). Pathologic evidence of both processes is frequently found in failed cases, but the ultimate result is predominantly rejection or GVHD.
In this context, the vast literature addressing the basis of tolerance, and that preoccupied with rejection, can be brought to bear on problems of transplantation. Many experiments have been one-way paradigmatic, showing the effects of exogenous or transgenic antigen on T cells and other immune cell subpopulations. The interpretation of such data in transplantation must encompass the alterations in two cell populations, each of which can modulate the other. In addition to a mutual antigen stimulus, the two-way paradigm implies active protection of the coexisting arms (GVH or HVG), which is particularly important if one cell population is out-numbered or if there is severe MHC disparity. Such a reciprocal ‘defensive’ mechanism of graft enhancement has been the subject of investigation but only in connection with hematolymphopoietic reconstitution after recipient Cytoablation55–57.
Experimental manipulations under highly controlled conditions are usually directed at understanding T-cell tolerance. However, T cells are only one of a number of specialized immune regulatory leukocytes. For instance, Burlingham et al.58 have isolated a circulating donor leukocyte, resembling the veto cell of Miller59, in a tolerant human kidney recipient with such powerful function that a single cell could neutralize the in vitro activity of 10 000 recipientCTLs.
The possibility that transplantation tolerance is governed by APCs was raised by the invariably prominent presence of DCs in chimeric human14,15 and animal organ recipients18,19. Using culture techniques adapted from Inaba et al.60, donor-derived DC precursors have been propagated from disseminated locations in mouse recipients of spontaneously accepted liver allografts61: these are co-localized with recipient DCs that are undergoing the same changes61,62. These immature DCs, which are phagocytic63 and deficient in surface costimulatory molecule expression (B7 family)64, have been shown to induce T-cell anergy in vitro64 and to prolong organ allograft survival65.
Such clues are intriguing, but it is unlikely that allograft acceptance can be fully understood from the results of studies of individual leukocyte lineages. Overall, the mechanisms of transplantation tolerance suggests learning adaptive immune functions of the whole system involved in self-integrity (i.e. cytokines, immunoregulatory cells, antibodies and other factors).
Transplant tolerance: central or peripheral
The role of the thymic vs. peripheral mechanisms in graft acceptance under both experimental and clinical circumstances has been controversial66–68. The prompt appearance of donor-derived leukocytes in the recipient thymus following organ transplantation18 was of particular interest because of the strikingly tolerogenic effect in rodents of intrathymic inoculation of donor leukocytes53. However, thymectomy in adult rats does not influence either the chimerism or spontaneous tolerance induced by liver transplantation69. Dejbakhsh-Jones et al.70 have shown that, after thymectomy and lethal irradiation, adult mice reconstituted with purified hematolymphopoietic stem cells developed similar levels of αβ T cells to those seen in control animals except for a reduced proportion in the spleen.
Between 1962 and 1965, 32 patients, including 24 who were part of a controlled randomized trial, underwent transthoracic thymectomy from 8 to 112 days (average 22) before renal transplantation either from living related or unrelated donors. Between 3.5 and 7 years later, no clinical differences were apparent between the thymectomized and control recipients, although there was a trend towards better histopathology in the thymectomized group71. In 1992, comprehensive in vitro immunologic studies of many of the remaining recipients and their donors did not reveal any distinguishing features of one cohort vs. the other (G. Shearer and A. Zeevi, unpublished). After 25 to 30 years, the thymectomized patients had no clinical advantage or disadvantage.
Therapeutic implications
In the context of the two-way paradigm, early efforts to improve transplantation results with donor-specific blood transfusion72 and the donor bone marrow augmentation of organ recipients73,74 were based on sound therapeutic principles involving the unrecognized augmentation of chimerism. Also in retrospect, it is obvious why whole organs are inherently tolerogenic as first convincingly demonstrated by Calne et al.32.
Understanding the concept of a donor-recipient leukocyte dialogue should help predetermine what can (and cannot) be accomplished with various tolerance-inducing strategies, all of which are attempts to influence this interaction. Our first clinical premise was that the spontaneous microchimerism of organ transplantation could be greatly augmented by the co-administration of unmodified donor bone marrow cells without a significant risk of GVHD, providing the two immunocyte populations were initially competent and that immunosuppression was delivered to both equally. It was also predicted that the timing, severity and frequency of acute rejection would be approximately the same as in non-bone-marrow-augmented control patients14,41,75.
These expectations have been fulfilled in 150 human organ recipients treated at the University of Pittsburgh75,76. The presence of donor DNA in the myeloid and erythroid colonies generated from recipient’s peripheral blood mononuclear cells (PBMCs) as measured in standard76 or innovative clonal hematopoietic progenitor cell assays77 has provided unequivocal evidence of augmented stem cell chimerism. There were no examples of significant GVHD.
The hypotheses of therapeutic efficacy being tested were that the threat of delayed (acute or chronic) rejection could be reduced and that the frequency of ultimate drug independence would be increased by the higher persistent level of chimerism. An efficacy evaluation is expected to take 5–10 years41, roughly the same time frame mapped out by clinical experience with MHC-incompatible liver and bone marrow transplantation (Figs 4 and 5).
Other chimerism-enhancing strategies (e.g. G-CSF, GM-CSF or lisofylline) should follow the same safety/efficacy rules. By contrast, procedures that alter only one of the interacting arms must be approached with caution, as exemplified by the historical experience with GVHD following cytoablation and bone marrow transplantation. When the converse tactic of leukocyte or T-cell-specific depletion of intestinal allografts was attempted as GVHD-prophylaxis in the 1980s, virtually every bowel recipient who survived the perioperative period developed lethal Epstein-Barr-virus-associated B-cell lymphomas78.
In an experimental example of unbalance which has potential clinical relevance, prior induction of tolerance with bone marrow in briefly immunosuppressed rats followed by delayed liver transplantation resulted in GVHD (Ref. 19), a complication not seen after either bone marrow or liver transplantation, or both simultaneously. The results of the second stage transplantation resembled those in the parent to defenseless offspring F1 models.
Conclusion
The assumption that stem cell driven hematolymphopoietic chimerism was irrelevant to successful whole organ transplantation, as currently practiced, has led to inadequate explanations of organ allograft acceptance and clouded the meaning of successful bone marrow transplantation, thus precluding the development of a central principle of transplantation. Incorporation of the chimerism factor into a two-way paradigm has allowed previous enigmas of organ and bone marrow engraftment to be explained and should allow key advances in basic immunology to be more meaningfully exploited in transplantation.
Acknowledgments
We would like to thank G. Nossal, R. Good, R. Steinman and L. Brent for their contributions to the two-way paradigm. This work was aided by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD, USA.
References
- 1.Medawar PB. J Auat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- 2.Billingham R, Brent L. Trans Bull. 1957;4:67–71. [PubMed] [Google Scholar]
- 3.Simonsen M. Acta Path Microbiol Scand. 1957;40:480–500. [PubMed] [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Main JM, Prehn RT. J Natl Cancer Inst. 1955;15:1023–1029. [PubMed] [Google Scholar]
- 6.Snell GD. Annu Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 7.Lechler RI, Batchelor JR. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemlander A, Soots A, Willebrand EV, Husberg B, Hayry P. J Exp Med. 1982;156:1087–1100. doi: 10.1084/jem.156.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen CP, Steinman R, Witmer-Pack M, Morris PJ, Austyn JM. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonsen M. Prog Allergy. 1962;6:349–461. [PubMed] [Google Scholar]
- 11.Streilein JW. Transplantation. 1991;52:1–10. doi: 10.1097/00007890-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Alard P, Matriano JA, Socarras S, Ortega MA, Streilein JW. Transplantation. 1995;60:1125–1130. doi: 10.1097/00007890-199511270-00012. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DE. Blood. 1993;81:2473–2474. [PubMed] [Google Scholar]
- 14.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Trucco M, et al. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman RM, Cohn ZA. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlitt HJ, Hundrieser J, Hisanaga M, et al. Lancet. 1994;343:1469–1471. doi: 10.1016/s0140-6736(94)92584-4. [DOI] [PubMed] [Google Scholar]
- 18.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Transplant Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 19.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murase N, Starzl TE, Tanabe M, et al. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Demetris AJ. J Am Med Assoc. 1995;273:876–879. doi: 10.1001/jama.273.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, Thomas ED, Durham DM, Fisher L. Am J Clin Pathol. 1991;95:201–206. doi: 10.1093/ajcp/95.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Morecki S, Leshem B, Eid A, Slavin S. J Exp Med. 1987;165:1468–1480. doi: 10.1084/jem.165.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushell A, Pearson TC, Morris PJ, Wood KJ. Transplantation. 1995;59:1367–1371. doi: 10.1097/00007890-199505270-00001. [DOI] [PubMed] [Google Scholar]
- 25.Starzl TE, Marchioro TL, Waddell WR. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz R, Andre-Schwartz J. Ann New York Acad Sci. 1966;129:804–821. [Google Scholar]
- 27.Penn I, Hammond W, Brettschneider L, Starzl TE. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Penn I, Putnam CW, Groth CG, Halgrimson CG. Transplant Rev. 1971;7:112–145. doi: 10.1111/j.1600-065x.1971.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todo S, Reyes J, Furukawa H, et al. Ann Surg. 1995;222:270–282. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Marchioro TL, Porter KA, et al. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 31.Kamada N. Experimental Liver Transplantation. CRC Press; 1988. pp. 67–80. [Google Scholar]
- 32.Murase N, Demetris AJ, Tsamandas AC, Ye Q, Starzl TE. Transplantation. 1996;61:1126–1131. doi: 10.1097/00007890-199604150-00027. [DOI] [PubMed] [Google Scholar]
- 33.Calne RY, Sells RA, Pena JR, et al. Nature. 1969;223:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 34.Dahmen U, Qian S, Rao AS, et al. Transplantation. 1994;58:1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 36.Murase N, Starzl TE, Ye Q, et al. Transplantation. 1996;61:1–3. doi: 10.1097/00007890-199601150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer JW, Bursten SL, Rice GC, Gordon WP, Bianco JA. Exp Opin Invest Drugs. 1994;3:631–643. [Google Scholar]
- 38.Talmor M, Steinman RM, Codner MA, Chen M, Harper AD. Immunology. 1995;86:448–455. [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson TC, Alexander DZ, Hendrix R, et al. Transplantation. 1996;61:991–1004. doi: 10.1097/00007890-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 40.Arnold B, Schönrich G, Hämmerling GJ. Immunol Today. 1993;14:12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 41.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Immunol Today. 1993;14:326–332. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos HC, Reyes J, Abu-Elmagd K, et al. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazariegos GV, Reyes J, Marino IR, et al. Transplantation. (in press) [Google Scholar]
- 44.Starzl TE, Demetris AJ, Trucco M, et al. Transplantation. 1993;55:1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murase N, Demetris AJ, Woo J, et al. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanabe M, Murase N, Demetris AJ, et al. Transplant Proc. 1994;26:3733–3740. [PMC free article] [PubMed] [Google Scholar]
- 47.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. New Engl J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 48.Steinmuller D. Science. 1967;158:127–129. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- 49.Hart DNJ, Winearls CG, Fabre JW. Transplantation. 1980;30:73–80. [PubMed] [Google Scholar]
- 50.Sriwatanawongsa V, Davies HS, Calne RY. Nat Med. 1995;1:428–432. doi: 10.1038/nm0595-428. [DOI] [PubMed] [Google Scholar]
- 51.Thai NL, Qian TS, Fu F, et al. Transplant Proc. 1995;27:509–510. [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, McCaughan GW, Gallagher ND, Sheil AGR, Bishop GA. Transplantation. 1995;60:233–236. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Campos L, Posselt AM, Deli BC, et al. Transplantation. 1994;57:950–953. doi: 10.1097/00007890-199403270-00030. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu Y, Goto S, Lord R, et al. Transplant Int. 1996;9:593–595. doi: 10.1007/BF00335561. [DOI] [PubMed] [Google Scholar]
- 55.Vriesendorp HM. In: Bone Marrow Transplantation. van Bekkum DW, Lowenberg B, editors. Marcel Dekker; 1985. pp. 73–145. [Google Scholar]
- 56.Gale RP, Reisner Y. Lancet i. 1986:1468–1470. doi: 10.1016/s0140-6736(86)91503-5. [DOI] [PubMed] [Google Scholar]
- 57.Plotnicky H, Touraine JL. Bone Marrow Transplant. 1993;12:307–314. [PubMed] [Google Scholar]
- 58.Burlingham WJ, Grailer AP, Fechner JH, et al. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 59.Miller RG. Nature. 1980;287:544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- 60.Inaba K, Steinman RM, Pack MW, et al. J Exp Med. 1992;175:1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Rudert WA, Qian S, et al. J Exp Med. 1995;182:379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomson AW, Lu L, Wan Y, Qian S, Larsen CP, Starzl TE. Transplantation. 1995;60:1555–1559. doi: 10.1097/00007890-199560120-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu L, Woo J, Rao AS, et al. J Exp Med. 1994;179:1823–1834. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu L, McCnslin D, Starzl TE, Thomson AW. Transplantation. 1995;60:1539–1545. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu F, Li Y, Qian S, et al. Transplantation. 1996;62:659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nossal GJV, Pike BL. Proc Natl Acad Sci U S A. 1981;78:3844–3847. doi: 10.1073/pnas.78.6.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nossal GJV. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 68.Miller JF, Morahan G. Annu Rev Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi E, Kamada N, Delriviere L, et al. Immunology. 1995;84:333–336. [PMC free article] [PubMed] [Google Scholar]
- 70.Dejbakhsh-Jones S, Jerabek L, Weissman IL, Strober S. Immunology. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 71.Starzl TE, Porter KA, Andres G, et al. Clin Exp Immunol. 1970;6:803–814. [PMC free article] [PubMed] [Google Scholar]
- 72.Salvatierra O, Jr, Vincenti F, Amend WJ, et al. Ann Surg. 1980;192:543–552. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monaco AP, Clark AW, Wood ML, Sahyoun AI, Codish SD, Brown RW. Surgery. 1976;79:384–392. [PubMed] [Google Scholar]
- 74.Barber WH, Mankin JA, Laskow DA, et al. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Fontes P, Rao A, Demetris AJ, et al. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao AS, Fontes P, Dodson F, et al. Transplant Proc. (in press) [Google Scholar]
- 77.Garcia-Morales R, Esquenazi V, Zucker K, et al. Transplantation. (in press) [Google Scholar]
- 78.Starzl TE, Todo S, Tzakis A, et al. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]