We recently reported that intestinal transplantation from Lewis (LEW) to Brown Norway (BN) was intractable to FK 506 therapy because of a high incidence of a graft-versus-host disease (GVHD) syndrome1 similar to that in the parent to F1 hybrid offspring experiments of Monchik and Russell.2 However, transplantation in the opposite direction (BN to LEW) was highly successful without either GVHD or difficult rejection reactions. Genetic analysis did not provide an explanation for the directional difference, in spite of the fact that genetic factors are known to control alloreactions.3,4 Consequently, we undertook the experiments herein reported to determine the influence on intestinal transplant outcome of 12 donor-recipient combinations of 4 fully allogeneic rat strains, with or without a 2-week induction course of FK 506. The outcome indices of rejection and GVHD proved to be influenced overwhelmingly by the choice of the recipient strain, with the choice of the donor being a far less consequential factor.
MATERIALS AND METHODS
Intestinal Transplantation
Animals
Inbred male LEW (RT1l), BN (RT1n). ACI (RT1a), and PVG (RT1c) rats weighing 200 to 300 g were purchased from Harlan Sprague Dawley Inc (Indianapolis, Ind) and used as donors or recipients. All animals were kept in conventional facilities. Water and commercial rat chow were provided ad libitum.
Intestinal Transplant Operation
Under methoxyflurane anesthesia, orthotopic small bowel transplantation (SBTx) was performed as described before.5 For convenience, venous drainage was into the recipient inferior vena cava, a procedure that has been shown in our laboratories6 and elsewhere7 not to affect the immune reactions. The entire small intestine from the ligament of Trietz to the ileocecal valve was harvested on a vascular pedicle, consisting of the superior mesenteric artery with a piece of aorta and portal vein. End-to-side vascular anastomoses between the graft aorta and recipient infrarenal aorta, and between the graft portal vein and recipient vena cava, were hand sewn with 10-0 Novafil suture. The entire recipient intestine was removed and enteric continuity was restored via proximal and distal end-to-end intestinal anastomoses.
Immunosuppression
FK 506 was dissolved in HCO-60 and D-mannitol carrier solvent and was given intramuscularly at a dose of 0.64 mg/kg/d for 14 days starting on the day of intestinal transplantation. Body weight, activity, skin color, and defecation patterns were monitored daily for the first 14 days, and at least twice a week thereafter for 150 days. Clinical signs of rejection were defined as continuous or intermittent diarrhea and weight loss. GVHD was clinically defined by cutaneous erythema, and by hair and weight loss.
Tissue Collection
A complete necropsy was performed on the animals that died before 150 days. Pieces of tissue from graft intestine, including Peyer’s patches, graft and recipient mesenteric lymph nodes, spleen, and skin were submitted for pathological evaluation. Animals surviving for more than 150 days were used either for skin graft experiments or killed to obtain lymphoid tissues for in vitro immunologic testing and for histopathologic examination.
Immunologic Testing of Long-Term Survivors
Skill Grafting
In a subgroup of long-surviving animals, full-thickness tail skin grafts (1 × 1 cm) were taken from syngeneic bowel donor and syngeneic recipient strains and third party rats. These were placed on 4 PVG and 4 ACI recipients who had lived for 152 to 217 days after small intestinal replacement, having received no immunosuppression since the 14th postoperative day. The skin grafts were shielded with a plaster cast for 7 days, and inspected daily thereafter. Rejection was considered complete when no part of the graft remained viable.
Mixed Lymphocyte Reactions
Cervical lymph node cells collected from 3 ACI and 2 PVG intestinal recipients after 190 to 291 days were used as responders in one-way mixed lymphocyte reactions (MLR). Irradiated (2000 rads) lymphocytes were obtained from the cervical lymph nodes of naive rats of the intestinal donor strain, recipient strain, and third parties. Triplicate cultures with 1.75 × 105 responder and 3.0 × 105 stimulator cells were prepared in 96-well round-bottomed microculture plates at a final volume of 0.2 mL RPMI-1640 (GIBCO Inc, Grand Island, NY). The medium was supplemented with 25 mmol/L HEPES buffer, 5 × 10−5 mol/L 2-mercaptoethanol, 50 U/mL penicillin, 50 µg/mL streptomycin, 2 mmol/L L-glutamine, and 10% normal rat serum. The cultures were incubated in a humidified atmosphere of 5% CO2 for 4 days at 37°C. One µCi of 3H-thymidine was added to the cultures 16 hours prior to read-out with a multiple sample harvester (Skatron Inc, Steerling, Va). 3H-thymidine uptake was determined by liquid scintillation spectrometry.
Cell-Mediated Lympholysis
The cytotoxic activity of 4 × 106 splenocytes from 2 ACI and 3 PVG intestinal recipients surviving for 218 to 421 days was assessed with a standard 51Cr-release assays.8 The effector splenocytes were depleted of B and accessory cells by incubation with a nylon wool column for 45 minutes at 37°C. The effectors were cultured for 5 days in 2.0 mL Dulbecco’s Modified Eagle Medium (GIBCO Inc) with 4 × 106 irradiated (2000 rads) cervical lymph node cells obtained from naive rats of the bowel donor strain or third party rats. The medium was supplemented with 1 mmol/L sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin, 10 mmol/L HEPES buffer, 13.6 µmol/L folic acid. 0.3 mmol/L L-asparagine, 2 mmol/L L-glutamine, 5 × 10−5 mol/L 2-mercaptoethanol, 10% fetal calf serum, and 0.5 mmol/L NG monomethyl-L-arginine.8
Targets were prepared by culturing the cervical lymph node cells of naive rats of the bowel donor strain or of third party rats with 2 µg/mL phytohemagglutinin (DIFCO, Detroit, Mich) for 2 days, followed by labeling with 51Cr (New England Nuclear, Boston, Mass). The target cells (4 × 103) were mixed with effector cells in V-bottomed 96-well microtiter wells at ratios of 3:1, 10:1, 30:1, and 100:1 and incubated for 6 hours at 37°C. One hundred microliters of the supernatant were then removed and 51Cr release measured by a gamma radiation counter (Beckman). Cytotoxicity was calculated according to the following formula: (experimental [cpm] – background cpm/maximum cpm – background cpm) × 100. Radioactivity spontaneously released from the target cells in the absence of effector cells was defined as the background cpm, and maximum cpm was obtained by directly counting 4 × 103 detergent-lysed target cells.
Pathologic Studies
Routine
Tissues obtained at necropsy from the intestinal graft and mesenteric lymph nodes, recipient spleen, cervical and mesenteric lymph nodes, thymus, skin, and liver were fixed in neutral buffered formalin, embedded in paraffin, and routinely stained with hematoxylin-eosin for blinded histologic examination, without knowledge of the experimental group.
Immunopathologic
An indirect immunoperoxidase technique was used to identify positively staining donor cells in ACI recipients of PVG grafts (n = 3) and PVG recipients of ACI grafts (n = 2) who survived more than 150 days. A separate piece of tissue from each site was frozen in OCT compound for immunohistochemical studies. The monoclonal antibodies OX27 and MN4-91-6 (Serotec Ltd, Bicester, England), which react with a polymorphic determinant of major histocompatibility complex (MHC) class I on PVG (RT1 Ac) and ACI (RT1 Aa), respectively, were used to identify cells of donor origin within host tissues, and the presence of host cells in the small bowel graft.9,10 An immunoglobulin class-matched irrelevant antibody was substituted for the primary antibody as a negative staining control. The immunostained slides were examined without knowledge of the experimental group or primary immunoreactant (test antibody or control).
Statistical Analyses
The significance of differences in intestinal recipient and skin graft survival were analyzed with the Log rank test. MLR and cell-mediated lympholysis results were analyzed with Student’s t test. A P value < .01 was considered significant.
RESULTS
Survival and Course
Untreated Rats
Survival in different strain combinations ranged from 5 to 14 days (Table 1), the shortest being with ACI to LEW. Although survival was not significantly different in most strain pairs with transplantation in either direction, a trend toward unbalanced results with a median disparity of at least 3 days was present in half of the pairings (Table 1). All of the BN recipients of LEW or 4 out of 5 PVG grafts developed signs of transient skin GVHD between days 7 and 9 before dying of graft rejection. In other strain combinations, the transient dermal erythema was seen only in one PVG recipient of a BN intestine.
Table 1.
Survival Without Immunosuppression in 6 Rat Strains in Which Intestinal Transplantation Was Performed in Both Directions (12 Combinations)
| Median | Survival* | No. | No. | Survival* | Median | |
|---|---|---|---|---|---|---|
| 14 | 10, 13, 15, 144 | 4 | ← ACI to BN → | 4 | 7, 8, 9, 88 | 8.5 |
| 12.5 | 12, 12, 13, 13 | 4 | ← BN to LEW → | 6 | 9, 9, 10, 11, 12, 14 | 10.5 |
| 13 | 13, 13, 13 | 3 | ← LEW to PVG → | 5 | 5, 7, 14, 19, 25 | 14 |
| 8 | 6, 8, 10 | 3 | ← PVG to ACI → | 5 | 10, 10, 11, 11, 13 | 11 |
| 8.5 | 7, 8, 9, 12 | 4 | ← ACI to LEW → | 4 | 5, 5, 5, 6 | 5 |
| 12 | 8, 9, 12, 12, 13 | 5 | ← BN to PVG → | 3 | 11, 11, 13 | 11 |
Underlined numbers indicate temporary GVHD.
One ACI recipient of a BN intestine lived for 144 days, and in the other direction of transfer, a BN recipient of an ACI intestine survived for 88 days (Table 1). Aside from these 2 exceptions, the clinical course was similar in all of the other 48 experiments. After an uneventful recovery from operation, the animals had progressive diarrhea, body weight loss, and death from rejection.
Treated Rats
The 2-week course of FK 506 improved survival in all strain combinations tested when compared with the same strain combinations in untreated animals (P < .01 overall). However, the magnitude of the survival effect shown in Table 2 for individual animals, as well as the maintenance of body weight, were profoundly influenced by the direction of the graft transfer. For example, BN intestines were accepted and supported nutrition well in virtually all PVG and ACI recipients; in LEW recipients survival beyond 150 days also was the rule although only 30% of the animals could maintain their weight (Table 2).
Table 2.
Survival Using FK 506 Induction in 6 Strain Combinations With Intestinal Transplantation in One Strain Direction or the Other
| Weight* Loss |
Median | Survival† | No. | No. | Survival† | Median | Weight* Loss |
|
|---|---|---|---|---|---|---|---|---|
| 0/4 | > 150 | 24‡, > 150 × 3 | 4 | ← ACI to BN → | 5 | 9‡, 87, 91, 104, > 150 | 91 | 4/5 |
| 5/5 | 32 | 31, 32, 32, 32, 34 | 5 | ← BN to LEW → | 10 | 42, 105, 107, 112, > 150 × 6 | > 150 | 7/10 |
| 2/4 | > 150 | 5‡, > 150 × 3 | 4 | ← LEW to PVG → | 4 | 10‡, > 150 × 3 | > 150 | 0/4 |
| 0/3 | > 150 | > 150 × 3 | 3 | ← PVG to ACI → | 4 | > 150 × 4 | > 150 | 0/4 |
| 0/5 | > 150 | 11‡, > 150 × 4 | 5 | ← ACI to LEW → | 3 | 85, 133, 143 | 133 | 3/3 |
| 7/7 | 42 | 38, 42, 42, 42, 43, 44, 63 | 7 | ← BN to PVG → | 7 | > 150 × 7 | > 150 | 0/7 |
Number of animals with weight loss > 10% body weight after completion of 0.64 mg/kg FK 506 for 14 days.
Underlined numbers Indicate animals that died of GVHD.
Died of intestinal obstruction without evidence of rejection or GVHD.
In contrast, transplantation from any of these strains to the BN recipient led to death after a median time of 32 to 91 days. However, the cause for the poor outcome with BN recipients varied. Those given ACI intestines died of rejection with a median survival of 91 days, but when PVG or LEW donors were used, the invariable cause of death after 31 to 63 days was GVHD. In contrast, none of the ACI, PVG, or LEW recipients of intestines from any of the test strains had skin changes.
An influence of the direction of the transplantation also could be seen with the ACI-LEW strain combination, but not to the extent as when BN was involved and never with the complication of GVHD. In contrast, the LEW-PVG and PVG-ACI combinations were “balanced” in that the survival outcome and ability of the grafts to maintain nutrition was the same no matter which strain of these pairs served as donor or recipient (Table 2).
These data are categorized in Table 3 in terms of the donor and recipient characteristics of each of the 4 strains in relation to the other 3. With the group of 4, BN was a universal donor whereas PVG and ACI were universal recipients. All PVG and ACI recipients survived for more than 150 days regardless of the donor strain, except for 3 rats who died early because of an obstructive ileus. These animals were free from any clinical signs of rejection, and steadily gained weight throughout the entire observation period.
Table 3.
Summary of Donor and Recipient Characteristics of 4 Rat Strains for Intestinal Transplantation
| As Donor | As Recipient | |
|---|---|---|
| 1 BN | *Accepted by PVG (150) and ACI (150); less well by LEW (150) | Rejects ACI (91); fatal GVHD caused by PVG and LEW |
| 2 LEW | Causes GVHD in BN (32); accepted by PVG (150) and ACI (150) | Rejects ACI; (133) accepts BN (150) and PVG (150) imperfectly |
| 3 PVG | Causes GVHD in BN (42); accepted by ACI (150) and less well by LEW (150) | Accepts all† |
| 4 ACI | Rejected by LEW (133) and BN (91); accepted by PVG (150) | Accepts all† |
Universal donor.
Universal recipient.
Parentheses indicate days median graft survival.
Immunologic Testing
Late Skin Graft Challenge
Eight of the “universal recipients” (4 PVG and 4 ACI) of well-functioning intestinal allografts from BN, PVG, or ACI donors were given skin grafts more than 150 days later from the recipient, intestinal donor strain and third party donors (Table 4). Skin grafts from third party donors were rejected promptly. Skin grafts from the original intestinal donor strain had more than doubling of survival (10.5 to 23.5 days) in the 4 PVG recipients, and survived permanently in the 4 ACI recipients (Table 4).
Table 4.
Skin Graft Survival After Acceptance of Small Bowel Allografts in PVG and ACI Recipients
| Recipient | Skin Graft Donor | Skin Graft Survival (d) | Median (d) |
|---|---|---|---|
| PVG after SBT× (n = 4)* | PVG | > 100 × 4 | > 100.0 |
| Bowel donor strain | 20, 22, 25, 30 | 23.5† | |
| Third party strains | 8, 10 × 3, 11 × 3, 13 | 10.5 | |
| ACI after SBT× (n = 4)‡ | ACI | > 100 × 4 | > 100.0 |
| Bowel donor strain | > 100 × 4 | > 100.0† | |
| Third party strains | 9, 10 × 4, 11 × 3 | 10.0 |
ACI (n = 1) and BN (n = 3) donor.
Skin graft survival prolonged (P < .01). Normal PVG rats rejected LEW, BN, and ACI skin grafts with median survival of 11.5, 10.0, and 10.0 days, respectively (n = 4 each group). Median LEW, BN, and PVG skin graft survival in normal ACI recipients was 10.0 days (n = 4 each group).
PVG (n = 2) and BN (n = 2) donor.
MLR
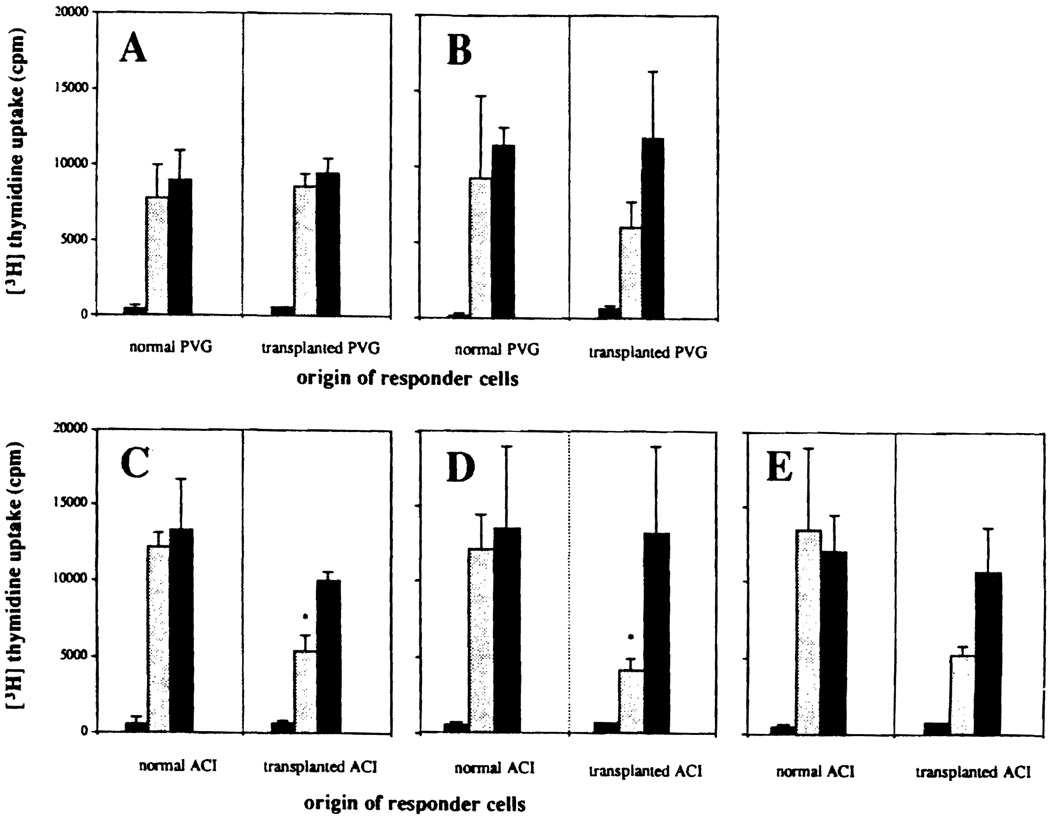
All 5 long-surviving recipients were nonresponsive to their naive syngeneic lymphocytes but responded normally to third party stimulation (Fig 1). The PVG recipients also responded vigorously to donor strain lymphocytes, but all of the ACI intestinal recipients had reduced reactivity to donor strain stimulator cells (Fig 1).
Fig 1.
One-way MLR, using cervical lymph node lymphocytes of naive and chronically surviving PVG or ACI intestinal recipients as responding cells with stimulation by irradiated cervical lymphocytes from unaltered recipient (syngeneic) strain ( , first bar), intestinal donor strain (
, first bar), intestinal donor strain ( , middle bar), and third party strain (■, third bar). In each double panel (A–E), the activation of the responding naive recipient strain lymphocytes is on the left, and that of the intestinal recipient’s lymphocytes is on the right. Mean cpm ± SD of triplicate cultures. (A) PVG recipient of BN bowel. (B) PVG recipient of LEW bowel. (C) ACI recipient of BN bowel. (D) ACI recipient of LEW bowel. (E) ACI recipient of PVG bowel. *3H-thymidine uptake significantly lower (P < .01) than with lymphocytes of normal animal to the same stimulator cells.
, middle bar), and third party strain (■, third bar). In each double panel (A–E), the activation of the responding naive recipient strain lymphocytes is on the left, and that of the intestinal recipient’s lymphocytes is on the right. Mean cpm ± SD of triplicate cultures. (A) PVG recipient of BN bowel. (B) PVG recipient of LEW bowel. (C) ACI recipient of BN bowel. (D) ACI recipient of LEW bowel. (E) ACI recipient of PVG bowel. *3H-thymidine uptake significantly lower (P < .01) than with lymphocytes of normal animal to the same stimulator cells.
Cytotoxic T-Lymphocyte Response
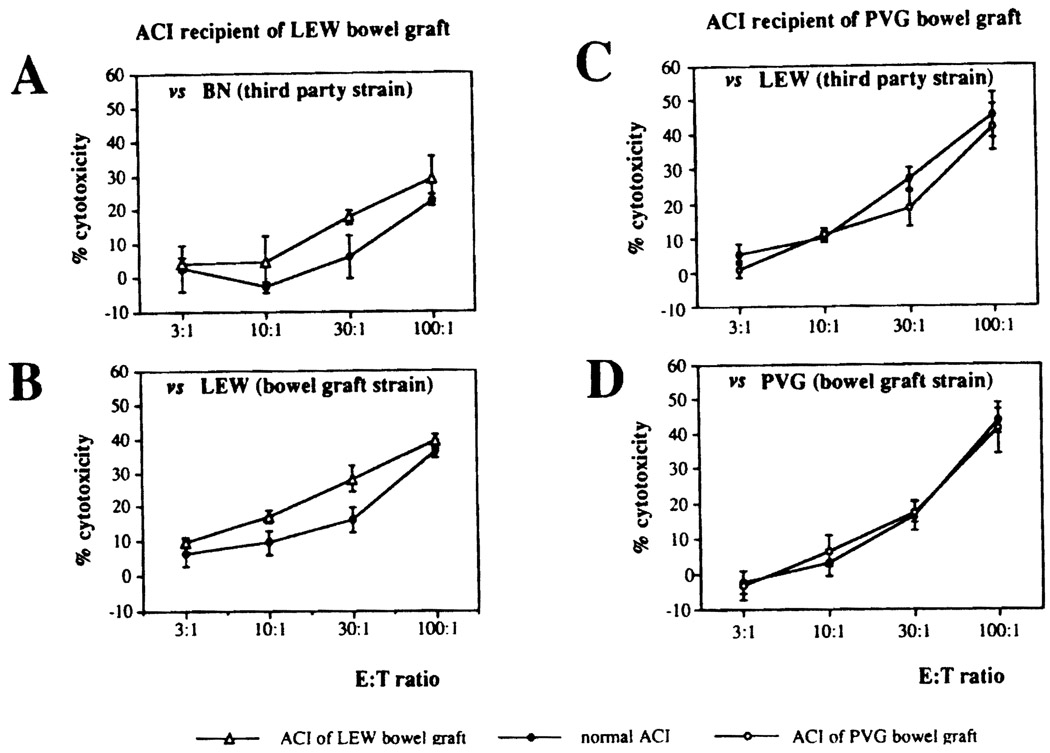
The B-cell-depleted splenocytes of long-surviving PVG recipients did not develop a vigorous cytotoxic T-lymphocyte (CTL) response either to third party lymphocytes or to lymphocytes of the intestinal donor strain. The CTL responses to donor strain and third party stimulation shown in Fig 2 from 2 ACI intestinal recipients (experiments) was essentially the same using responding lymphocytes from the chronically surviving intestinal recipients and from naive recipient strain recipients.
Fig 2.
Cytolytic activity of the splenocytes from long-surviving ACI recipient of (A, B) LEW (open triangles) or (C, D) PVG (open circles) bowel graft. Isolated splenocytes were cultured with irradiated donor or third party strain lymphocytes for 5 days. Cytolytic function against the (B, D) bowel graft and (A, C) third party strain target cells was assessed in a standard 51Cr-release assay. Bars and circles/triangles represent mean ± SD of triplicate cultures. The closed circles represent the percent cytotoxicity of normal ACI rat splenocytes.
Standard Pathological Studies
Untreated Rats
The gross and microscopic changes were essentially the same as we described before,5 with purulent ascites, interintestinal adhesions, patchy mural or transmural necrosis of the bowel allograft, and enlarged mesenteric lymph nodes.
In the 2 exceptional animals with survival of 88 and 144 days, there was clear histological evidence of chronic rejection11 with obliterative arteritis, villous atrophy, and depletion and scarring of the graft Peyer’s patches and mesenteric lymph.
Treated Rats
Tissue examination was congruent with the clinical impressions in the different strain combinations (Table 5). There were changes typical of acute GVHD in the skin of the BN recipients of LEW or PVG bowel grafts, with a superficial dermal and perivascular mononuclear infiltrate. The picture was completed by epidermal exocytosis, satellitosis, keratinocyte necrosis, and spongiosis. The bowel allografts were normal in these animals in contrast to the BN recipients of ACI intestines in which the findings of chronic rejection were unequivocal (Table 5).
Table 5.
Pathological Findings* in the Small Bowel Grafts Following a 2-Week Course of FK 506
| Strain Combination | Pathological Findings of the Graft | ||||
|---|---|---|---|---|---|
| Recipient | Donor | Mucosa† | Peyer’s Patches‡ |
Mesenteric Lymph Nodes‡ |
Obliterative Arteriopathy |
| BN | LEW | 0 | 0 | 0 | 0 |
| BN | PVG | 0 | 0 | 0 | 0 |
| BN | ACI | 1 | 2 | 1–2 | 0–2 |
| LEW | ACI | 1 | 2 | 2 | 0–1 |
| LEW | BN | 1 | 1 | 2 | 0–1 |
| LEW | PVG | 0 | 1–2 | 1 | 0–2 |
| PVG | LEW | 0 | 0 | 0–1 | 0 |
| PVG | ACI | 0 | 0 | 0–1 | 0 |
| PVG | BN | 0 | 0 | 0–1 | 0 |
| ACI | LEW | 0 | 0 | 0 | 0 |
| ACI | BN | 0 | 0 | 0 | 0 |
| ACI | PVG | 0 | 0 | 0 | 0 |
Tissues from animals who died before 150 days or those surviving 150 to 506 days after small bowel transplantation.
Blunting of villi, cryptitis, and lymphoid depleted lamina propria.
Lymphoid depletion and fibrosis.
Diagnosis was classified histologically as 0, nothing ~ rare; 1, present; 2, severe. Two to four animals were examined in each donor-recipient combination.
Although a far better survival record was compiled clinically with the LEW (9 of 17 > 150 days) than the BN recipients (1 of 17 > 150 days), the late pathology was the same. Obliterative arteriopathy and the other stigmas of chronic rejection were found in the intestines of the LEW recipients, regardless of the donor strain.
ACI and PVG recipients of all strains of intestines appeared clinically equivalent in terms of long survival and weight gain. However, pathologic examination of their allografts after 150 days revealed a difference between these 2 cohorts of apparently healthy intestinal recipients. Graft lymphoid tissues were mildly atrophic or scarred in PVG recipients, in contrast to minimal alteration in ACI recipients. Obliterative arteriopathy was rare or absent in both ACI and PVG recipients, no matter what the donor strain. The pathologic findings suggested that the PVG recipients were slowly rejecting their grafts, whereas the ACI recipients appeared to be completely spared from this process.
Immunohistochemical Studies
ACI Recipients
Tissues from the three ACI recipients of PVG grafts were stained for cells bearing the PVG phenotype (OX27) at 343, 421, and 506 days after transplantation. The endothelial cells, epithelial cells, and rare lymphoid cells within the Peyer’s patches within the graft remained donor phenotype, while the vast majority of cells in the Peyer’s patches were OX27-negative. When the immunostained slides of recipient lymphoid tissues were reviewed without knowledge of the primary immunoreactant, the presence of positively staining cells correlated with the use of OX27 as the primary immunoreactant in more than 75% of tissue samples, supporting the diagnosis of microchimerism.
PVC Recipients
Tissues from 2 PVG recipients of ACI grafts were stained for cells bearing the ACI phenotype (MN4-91-6) at 153 and 517 days posttransplantation. In the animal killed at 153 days, the graft mesenteric lymph nodes were enlarged because of scarring, but endothelial (epithelial) and stromal cells remained donor origin. The rat killed at 517 days did not show scarring of the graft mesenteric lymph nodes or Peyer’s patches. MN4-91-6+ (donor) cells were identified more often in the tissue sections stained with MN4-91-6 than in the appropriate negative controls. The findings in these PVG recipients were consistent with microchimerism, but this diagnosis could not be made conclusively with only 2 animals available for study and the sparse number of ACI-positive cells.
DISCUSSION
In our recent study,1 we cited evidence from the literature 12 as well as from our own observations in heart, liver, and intestinal recipients5,11 (and unpublished observation) that the BN rat was a universal donor. However, when grafting was done the other way around under FK 506, BN recipients of LEW intestines almost invariably developed GVHD,1 similar to observations using cyclosporine by DeBruin et al13 with WAG (RT1u) to BN. The GVHD syndrome resembled that of the classical parent to F1 hybrid experiments of Monchik and Russell.2 However. because BN to LEW and LEW to BN experiments did not yield mirror image results, these could not fit into the same conventional genetic analyses.1
In our earlier report,1 the disparate data could not be explained by the responder versus nonresponder classification of the BN and LEW rats, the lymphocyte subpopulations of the respective strains, the in vitro reactivity of these strains (each to the other) with or without FK 506 culture enrichment, or differences in the traffic or density of donor passenger leukocytes in recipient lymphoid organs out to 40 days. The most solid clue was evidence in BN rats of a defect in the invariant chain of MHC class II molecules 1,14 with the implication that this could lead to inefficient processing of donor antigen without necessarily implying immune abnormalities in this strain.15,16
Nevertheless, the GVHD propensity of the BN intestinal recipient could imply subtle immunologic perturbations, a possibility strengthened by collateral evidence. For example, the BN rat strain carrying the RT1n haplotype and derived strains are exquisitely sensitive to the induction of autoimmunity precipitated by treatment with mercuric chloride, gold salts, or D-penicillamine.17–20 They are also highly permissive of the trafficking in their tissues of many, but not all, strains of allogeneic lymphocytes.21 In fact, autoimmune reactions similar to those precipitated by heavy metals can also be triggered in BN rats and certain mice strains by an allogeneic GVH reaction.22,23 The characteristics of this autoimmunity include the production of antinuclear antibodies, immune glomerulonephritis, enhanced class II MHC expression on B-cells, increased serum immunoglobulin (Ig)G and IgE, elevated interleukin (IL)-4 activity, and impairment of IL-2 production.17–23 The cytokine profile observed suggests that an active TH 2-type immune response may be important in the pathogenesis of all these manifestations.24 The possibility is under investigation that an autoimmune reaction after transplantation secondary to the ubiquitous distribution of donor hematolymphoid cells could playa role in fostering graft acceptance or allowing a fatal GVHD after intestinal transplantation.
Beyond confirming that BN was the only universal donor among the 4 strains used, the present study also showed that the choice of recipient strain dominated the outcome in all combinations tested (Table 3). Following a 2-week course of FK 506, the LEW recipient did not accept any strain intestine unreservedly, although it may be inferred that longer treatment would have allowed lifetime survival with BN11 and probably also PVG and ACI bowel. In contrast, ACI and PVG rats were universal recipients by clinical criteria under the short-term treatment conditions of these experiments. The ACI recipients had in vitro findings of obtunded but not absent donor recipient reactivity, as well as the ability to permanently accept donor skin grafts. Their allograft intestines were essentially normal and they had convincing evidence of microchimerism.
The PVG recipients whose clinical course was not perceptably different than ACI did not have a donor-specific defect on MLR, and had delayed rejection of donor skin. Systemic microchimerism was minimal, and the intestines had histopathologic findings suggesting subclinical early rejection. Considering the lifespan of rats, the biologic differences between the ACI and PVG recipients may prove to be insignificant. The discordance in both the ACI and PVG recipients between in vivo and in vitro indicators of tolerance (‘split tolerance’) has been observed before in neonatal chimeras25 and after organ transplantation under cyclosporine,26,27 FK 506,28 monoclonal antibodies,29 and as a natural consequence of liver grafting.30,31
The superiority of the ACI recipient can be explained by the previously reported genetically controlled hyporesponsiveness that could be quantitated by in vitro testing.3,4 These tests showed weak alloantibody production and ineffectual cytotoxicity against donor antigens after immunization with lymphocyte injections. Such unresponsiveness could also be used to explain why only ACI donors did not mount vigorous GVH reactions and therefore permitted consistently long survival (median 91 days) without GVHD in the relatively defenseless BN recipient. However, this argument (in both strain direction) loses force by the fact that other successful or semisuccessful strain combinations did not depend either on a low responding donor, recipient, or either for avoidance of GVHD. Instead, we have postulated elsewhere14,32 that a balance without the emergence of dominance of 1 cell population must be accomplished between the coexisting cell populations for successful allotransplantation, particularly for organs with a heavy constituency of passenger leukocytes.
Different lines of inquiry may be needed than those pursued in the past to learn how this cellular balance is accomplished with or without the aid of immunosuppression. Little attention has been given to the role that alloreactive NK cells33,34 or other natural cytotoxicity mechanisms may have in solid organ transplantation biology.35,36 Such non-T cells are genetically defined, facilitate or inhibit allogeneic progenitor cell engraftment,35 and are responsible for the rapid elimination within hours of mature allogeneic lymphocytes, long before classical allogeneic immunity comes into play.36 Consistent with this line of reasoning. Sheng-Tanner and Miller37 have shown that anti-NK cell therapy fosters the development of hematolymphoid chimerism and prolongs the survival of allografts.
It will be useful for other investigations of intestinal transplantation to have a catalogue of strain combinations with and without immunosuppression. Our study provided 12 in the treatment as well as in the non treatment category, each including 5 combinations already reported by us or others. Thus, we have added 7 to both groups. There have been 4 additional strain combinations in the 2 categories, adding up at present to a total of 16 in each. This framework of previously published and new information is summarized in Tables 6 and 7.
Table 6.
Recipient Survival After Fully Allogeneic Small Bowel Transplantation Without Immunosuppression (Published Through 1993)
Table 7.
Recipient Survival After Fully Allogeneic Small Bowel Transplantation With Immunosuppression (Published Through 1993)
| Strain Combinations | ||||
|---|---|---|---|---|
| Recipient | Donor | Immunosuppression | Survival (d) | Reference(s) |
| LEW | ACI | CyA 15 mg/kg/d, IM (d 0–>28) | 130.3 | 39 |
| CyA 40 mg/kg/d, IM (d −2–>7) | 34.3 | 47 | ||
| FK 506 0.1–0.5 mg/kg/d, IM (d 0–>29) | 13.6–34.6 | 45 | ||
| FK 506 2.0->1.0 mg/kg/d, IM (d 0–>30) | 50.6 | 47 | ||
| LEW | BN | CyA 5 mg/kg/d, IM (d 0–>13) | > 100 | 41 |
| CyA 15 mg/kg/d, IM (d 0–>28) | > 360 | 38, 39 | ||
| CyA 15 mg/kg/d, IM (d 0–>6) | > 200 | 53 | ||
| FK 506 0.15 mg/kg/d, IM (d 0–>13) | 42.0 | 1 | ||
| FK 506 0.32–0.64 mg/kg/d, IM (d 0–>13) | > 100 | 1, 6, 11 | ||
| FK 506 2.0 mg/kg/d, IM (d 0–>4) | > 180 | 38 | ||
| RS 61443 30 mg/kg/d, PO (d 0–>6) | 10.7 | 46 | ||
| Rapamycin 0.8–1.6 mg/kg/d, civ (d 0–> 13) | 21.6–31.0 | 48 | ||
| LEW | F344 | CyA 30 mg/kg/d, IM (d 0–> 13) | > 150 | 40 |
| FK 506 0.32 mg/kg/d, IM (d 0–>13) | > 150 | 40 | ||
| Deoxyspergualin 3 mg/kg/d, IM (d 0–>11) | > 80 | 40 | ||
| BN | LEW | FK 506 0.15–1.0 mg/kg/d, IM (d 0–>13) | 22.5–28.5 (GVHD) | 1 |
| BN | WAG | CyA 5–25 mg/kg/d, IM (d 0, 1, 2, 4, 6) | 18.7–92.7 (GVHD) | 13, 50 |
| FK 506 2.0 mg/kg/d, IM (d 0, 1, 2, 4, 6) | 28.5 (GVHD) | 49 | ||
| Rapamycin 1.0 mg/kg/d, IM (d 0, 1, 2, 4, 6) | 10.0 | 50 | ||
| WAG | BN | CyA 5–25 mg/kg/d, IM (d 0, 1, 2, 4, 6) | 9.6–200 | 13 |
| Rapamycin 1.0 mg/kg/d, IM (d 0, 1, 2, 4, 6) | 11.3 | 50 | ||
| WFu | BUF | Rapamycin 0.8 mg/kg/d, civ (d 0–>13) | 25.0 | 48 |
| PVG | DA | CyA 15 mg/kg/d, IM | > 100 | 44 |
| DA | PVG | CyA 15 mg/kg/d, PO | > 56 | 54 |
Abbreviations: CyA, cyclosporine A; IM, intramuscular injection; PO, oral administration; civ, continuous intravenous infusion.
ACKNOWLEDGMENTS
Supported by Project Grant No. DK 29961 and AI 16869 from the National Institutes of Health, Bethesda, Maryland.
FK 506 was a gift from Fujisawa Pharmaceutical Co, Ltd, Osaka, Japan.
REFERENCES
- 1.Murase N, Demetris AJ, Woo J, Tanabe M, Furuya T, Todo S, Starzl TE. Transplantation. 1993;55:1. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monchik GJ, Russell PS. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 3.Butcher GW, Howard JC. Transplantation. 1982;34:161. [PubMed] [Google Scholar]
- 4.Butcher GW, Corvalan JR, Licence DR, Howard JC. J Exp Med. 1982;155:303. doi: 10.1084/jem.155.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 6.Murase N, Demetris AJ, Furuya T, Todo S, Fung JJ, Starzl TE. Transplant Proc. 1992;24:1143. [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer D, Diflo T, Love W, Clowes GHA, Maki T, Monaco AP. Surgery. 1988;104:518. [PubMed] [Google Scholar]
- 8.Hoffman RA, Langrehr JM, Billiar TR, Curran RD, Simmons RL. J Immunol. 1990;145:2220. [PubMed] [Google Scholar]
- 9.Milton AD, Fabre JW. J Exp Med. 1985;161:98. doi: 10.1084/jem.161.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferies WA, Green JR, Williams AF. J Ex-p Med. 1985;162:117. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murase N, Demetris AJ, Matsuzaki T, Yagihasi A, Todo S, Fung JJ, Starzl TE. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 12.Guillaume J, Niessen GJCM, Marquet RL, Bijnen AB, Obertop H, Jeekel JJ. Surgery. 1982;91:339. [PubMed] [Google Scholar]
- 13.De Bruin RWF, Saat RE, Heineman E, Jeekel J, Marquet RL. Transplant Proc. 1990;22:2472. [PubMed] [Google Scholar]
- 14.Demetris AJ, Murase N, Fujisaki S, et al. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 15.Chicz RM, Urban RG, Lane WS, Gorga JC, Stern LJ, Vignali ADD, Stromiger JL. Nature. 1992;358:764. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 16.Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. Science. 1992;256:1817. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 17.Tournade H, Pelletier L, Pasquier R, Vial MC, Mandet C, Druet P. Clin Exp Immunol. 1990;81:334. doi: 10.1111/j.1365-2249.1990.tb03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aten J, Bosman CB, Rozing J, Stijnen T, Hoedemaeker PJ, Weening JJ. Am J Pathol. 1988;133:127. [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson CJG, Balazs T, Egorov IK. Toxicol Appl Pharmacol. 1986;86:159. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- 20.Tournade H, Pelletier L, Pasquier R, Vial MC, Mandet C, Druet P. J Immunol. 1990;144:2985. [PubMed] [Google Scholar]
- 21.McNeilage LJ, Heslop BF, Heyworth MR, Gutman GA. Cell Immunol. 1982;72:340. doi: 10.1016/0008-8749(82)90482-8. [DOI] [PubMed] [Google Scholar]
- 22.Via CS, Shearer GM. Immunol Today. 1988;9:207. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 23.Rozendaal L, Pals ST, Gleichmann E, Melief CJM. Clin Exp Immunol. 1990;82:527. doi: 10.1111/j.1365-2249.1990.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman M, Druet P, Gleichmann E. Immunol Today. 1991;12:223. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 25.Streilein JW, Strome P, Wood PJ. Transplantation. 1989;48:630. [PubMed] [Google Scholar]
- 26.Westra AL, Prop J, Kuijpers KC, Wildevuur CRH. Transplantation. 1990;49:826. [PubMed] [Google Scholar]
- 27.White DJG, Lim SM. Transplantation. 1988;46:118S. doi: 10.1097/00007890-198808001-00022. [DOI] [PubMed] [Google Scholar]
- 28.Murase N, Kim D-G, Todo S, Cramer DV, Fung JJ, Starzl TE. Transplantation. 1990;50:739. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson TC, Darby CR, Bushell AR, West LJ, Morris PJ, Wood KJ. Transplantation. 1993;55:361. doi: 10.1097/00007890-199302000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Houssin D, Gigou M, Franco D, Bismuth H. Transplantation. 1980;29:418. doi: 10.1097/00007890-198005000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Engmann R, Thiede UA, Ruchholtz M, Hamelmann H. Transplant Proc. 1983;15:729. [Google Scholar]
- 32.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaage JT, Dissen E, Ager A, Fossum S, Rolstad B. Eur J Immunol. 1991;21:2167. doi: 10.1002/eji.1830210927. [DOI] [PubMed] [Google Scholar]
- 34.Rolstad B, Berestad HB. Immunology. 1991;74:8. [PMC free article] [PubMed] [Google Scholar]
- 35.Rolstad B, Ford WL. Immunol Rev. 1983;73:87. doi: 10.1111/j.1600-065x.1983.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 36.Olszewski WL. In: In Vivo Migration of Immune Cells. Olszeweski WL, editor. Boca Raton: CRC Press; 1987. p. 179. [Google Scholar]
- 37.Sheng-Tanner X, Miller RG. j Exp Med. 1992;176:407. doi: 10.1084/jem.176.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KKW, Stangl Ml, Todo S, Langrehr JM, Starzl TE, Schraut WM. Transplant Proc. 1990;22:78. [PMC free article] [PubMed] [Google Scholar]
- 39.Langrehr JM, Lee KKW, Wachs ME, Lee TK, Stangl MJ, Venkataramanan R, Kunz HW. Transplant Proc. 1990;22:2533. [PubMed] [Google Scholar]
- 40.Iga C, Okajima K, Takeda Y, Tezuka K. Transplant Proc. 1990;22:1658. [PubMed] [Google Scholar]
- 41.Shimazu R, Grogan JB, Raju S. Transplantation. 1988;46:673. doi: 10.1097/00007890-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Zhong R, He G, Sakai Y, Zhang Z, Garcia B, Li XC, Jevnikar A, Grant D. Transplantation. 1993;56:381. doi: 10.1097/00007890-199308000-00025. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Zhong R, He G, Sakai Y, Quan D, Garcia B, Duff J, Grant D. Transplant Proc. 1992;24:1206. [PubMed] [Google Scholar]
- 44.Lim SML, Isaac JR, Li SQ. Transplant Proc. 1992;24:1509. [PubMed] [Google Scholar]
- 45.Utsunomiya H, Tanaka K, Uemoto S, Kato H, Nishizawa T, Yamamoto E, Ozawa K. Transplant Proc. 1992;24:1191. [PubMed] [Google Scholar]
- 46.Shaffer D, Blakery ML, Gottschalk R, Monaco AP. Transplant Proc. 1992;24:1159. [PubMed] [Google Scholar]
- 47.Hoffman AI, Makowka L, Banner B, Cai X, Cramer DV, Pascualone A, Todo S, Starzl TE. Transplantation. 1990;49:483. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepkowski SM, Chen HF, Wang ME, Daloze p, Kahan BD. Transplantation. 1992;53:258. doi: 10.1097/00007890-199202010-00002. [DOI] [PubMed] [Google Scholar]
- 49.de Bruin RW, HogenEsch H, Heineman E, Jeekel J, Marquet RL. Transplant Proc. 1991;23:3257. [PubMed] [Google Scholar]
- 50.Marquet RL, de Bruin RW, Heineman E, Jeekel J. Transplant Proc. 1993;25:2695. [PubMed] [Google Scholar]
- 51.Sarnacki S, Cerf-Bensussan N, Revillon Y, Calise D, Goulet O, Ricour C, Brousse N. Transplant Proc. 1992;24:1210. [PubMed] [Google Scholar]
- 52.Kort WJ, Westbroek DL, MacDicken I, Lameijer LD. Eur Surg Res. 1973;5:81. doi: 10.1159/000127644. [DOI] [PubMed] [Google Scholar]
- 53.Diflo T, Maki T, Monaco AP. Transplant Proc. 1989;21:2885. [PubMed] [Google Scholar]
- 54.Clark CLI, Price BA, Crane PW, Lear PA, Wood RFM. Br J Surg. 1992;79:424. doi: 10.1002/bjs.1800790517. [DOI] [PubMed] [Google Scholar]