We have previously described the nephrotoxic qualities of FK 506,1–4 which were particularly evident in liver transplant recipients switched to FK 506 after previous treatment with cyclosporine (CyA).1, 2 The probability that these two drugs are synergistically nephrotoxic has precluded their use together,1 a decision that has support from studies in a rat model of renal ischemia.5 However, FK 506 also can be nephrotoxic when given from the outset after transplantation.3 Since the magnitude of drug nephrotoxicity is difficult to assess after renal transplantation, we have studied instead the postoperative renal function in cohorts of primary liver and thoracic organ recipients. This approach of investigating renal function in nonrenal organ recipients was used during the last decade to define the nephrotoxic liability of CyA.6–8
CASE MATERIAL
Thoracic Organ Transplantation
The patients, who were recently reported by Armitage et al,3 received the following transplants: 22 hearts, 2 double lungs, and one heart-lung. The transplantations were performed between October 1989 and May 1990. Only two patients (both heart recipients) were eliminated from this analysis. One, an adult, died 3.5 days postoperatively from right ventricular failure. The other, a 22-day-old infant who survived, had acute renal failure 3 weeks postoperatively at the same time as total thrombosis of the abdominal inferior vena cava up to retrohepatic levels occurred; the kidneys eventually recovered completely with the development of venous collaterals. The clinical features of the 25 analyzed patients are summarized in Table 1. There were four infants or children and 20 adults.
Table 1.
Thoracic Transplants Under FK 506
| Sex (21 M/4 F) |
| Age range (22 d-54 y) |
| Causes of heart failure |
| Ischemic (10) |
| Idiopathic (7) |
| Congenital (5) |
| Valvular (2) |
| Circulatory support 8 |
Adapted from Armitage et al.3
Liver Transplantation
The 120 patients who were recently reported by Todo et al9 underwent primary liver replacement between September 8, 1989 and February 4, 1990. Of the starting group, 112 (93.3%) are alive with follow-ups of 6 to almost 12 months. Of the 112 survivors, 8 have required retransplantation. These patients were eliminated from the analysis because of the complexity of their courses and the nearly invariable need for prolonged intensive care and nephrotoxic antibiotics. These factors and the rest of the spectrum of potential renal insults under conventional CyA immunosuppression have been described elsewhere.10 This left 104 patients who have had continuous function of their original liver grafts for 6 to 11.5 months. This subset was further reduced by eliminating three patients from analysis because they were on chronic dialysis at the time of liver transplantation. The clinical features of the 101 patients included in the final analysis are summarized in Table 2. Eighty-eight of these patients were adults and 13 were infants or children.
Table 2.
Liver Transplant Patients Under FK 506
| Sex (58 M/47 F) |
| Age (mean) (45 ± 11.5 y) |
| Causes of liver failure |
| Adults |
| Nonalcoholic cirrhosis (42) |
| Alcoholic cirrhosis (26) |
| Cholestatic disease (28) |
| Tumor (3) |
| Fulminant failure (3) |
| Miscellaneous (3) |
| Children |
| Sex (9 M/6 F) |
| Biliary atresia (8) |
| Cirrhosis (4) |
| Tumor (2) |
| Fulminant failure (1) |
| Total 120 |
Adapted from Todo et al.9
Immunosuppression
For the liver and heart recipients, IV doses of 0.075 mg/kg or 0.15 mg/kg were infused over 4 hours in the operating room and repeated at a 0.075 mg/kg dose every 12 hours until oral intake was started. The conversion from IV to oral doses of 0.15 mg/kg every 12 hours frequently overlapped for 1 day. The patients either were started on a 5-day burst of IV prednisone. starting at 200 mg on the first day and ending at 20 mg on the sixth day, or in many cases, the starting dose was 20 mg/d. If rejection occurred, the therapeutic response was with an increase of FK 506 or steroids, or sometimes both. OKT3 was used in 10 (9.9%) of the patients who had severe rejection.
Two recipients of double lungs and a heart-lung recipient were treated differently in that no steroids or other drugs were added to FK 506 monotherapy. In these 3 patients. FK 506 doses were increased until there was evidence of renal dysfunction. Optimal use of FK 506 required surveillance of serum potassium, and if this increased, it was corrected with administration of fludrocortisone acetate (Florinef). The usual starting doses of fludrocortisone acetate were 0.1 mg/bid. This therapy was initiated when serum potassium was >5.2–5.5 mEq/L.
Hypertension was treated if the systolic arid diastolic pressures consistently were over 160 and 90 mm Hg. respectively. Centrally acting agents (clonidine) or calcium channel blockers were used preferentially. Beta blockers and ACE inhibitors that might exacerbate the hyperkalemia or block renal autoregulation generally were avoided. However, when these agents were used, no adverse reactions were noted.
Parameters Examined
The serum creatinine and blood urea nitrogen were recorded preoperatively. Postoperatively, the mean results of these same tests were obtained for consecutive 5-day postoperative periods for 30 days, at approximately 60 days, and at the last patient visit in July 1990 (5 to 10 months postoperatively. Plasma levels of FK 506 were measured with the enzyme immunoassay technique of Tamura et al.11
The incidence of arterial hypertension was assessed by the need for antihypertensive drugs and by whether single or multiple agents were given. In addition, untreated patients whose diastolic blood pressures persistently were >95 mm Hg in absence of treatment were sought. None were found.
Statistical Analyses
Values for pooled data were expressed as mean ± SD. When appropriate, Student’s t test or ANOVA were used to test the difference between means as appropriate. Values were considered to be significant when P < .05.
RESULTS
After Thoracic Organ Transplantation
The evolution of the creatinine in the 25 heart recipients is shown in Fig 1. Almost every recipient developed an increase in serum creatinine perioperatively, but these usually resolved by the end of the first postoperative month. One patient who had cardiac arrest and massage while on the way to the operating room required five hemodialysis treatments postoperatively. The perioperative renal dysfunction was more prooounced in the eight (32%) patients who were on assist devices prior to transplantation. Serum creatinine at latest follow-up for all patients was 1,9 ± 0.6 mg/dL.
Fig 1.
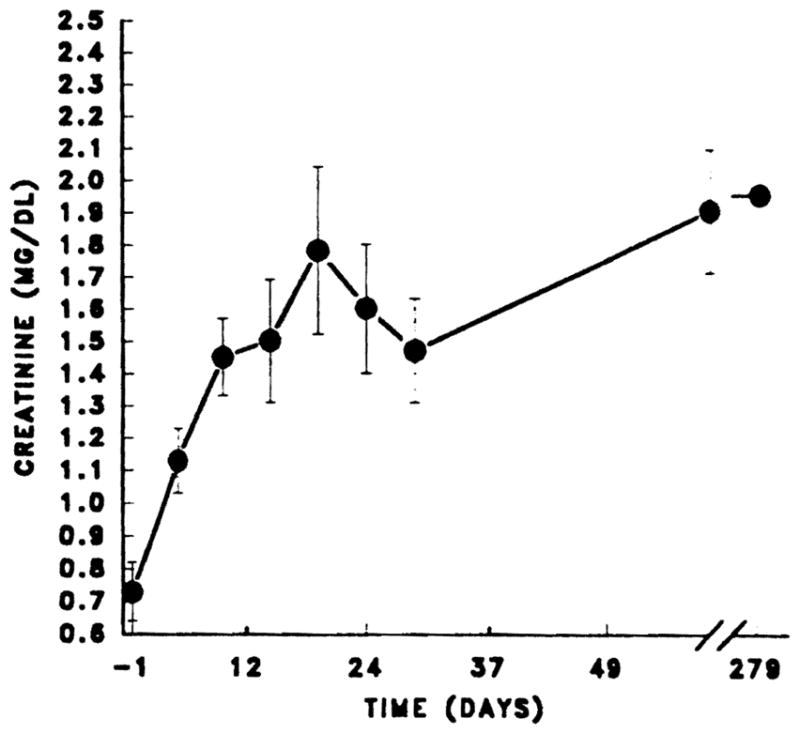
Scr in heart transplants. Mean ± standard error for heart transplant patients. Values from day 0 to 30 are expressed as mean ± standard error at 5-day intervals.
Twenty-two (88%) of the 25 heart recipients did not require any antihypertensive drugs, and the other 3 have mild hypertension and are (or were) on single agent therapy. One of these three patients had a previous history of hypertension that persisted postoperatively. A second patient is talking verapamil for cardiac indications and had diastolic pressures of 90–96 mm Hg prior to therapy.
Ten (40%) of the recipients were treated with fludrocortisone acetate at some time postoperatively and 8 (32%) continue to require this agent at latest follow-up for control of hyperkalemia.
The two double lung and the heart-lung recipients were managed solely with FK 506. In these patients, the FK 506 dose was deliberately pushed to toxicity. Figure 2 shows the interrelations of FK 506 dose, plasma FK 506, and renal function over follow-ups of 5 to 9 months. Management of the second double lung recipient was complicated by a cytomegalovirus (CMV) lung infestation and the need for intensive Gancyclovir therapy. He also was treated with Bactrim. Now after 8 to 10 months, the serum creatinine in the two double lung and heart-lung recipients is 1.9 ± 0.9 mg/dL. All are normotensive without medication.
Fig 2.
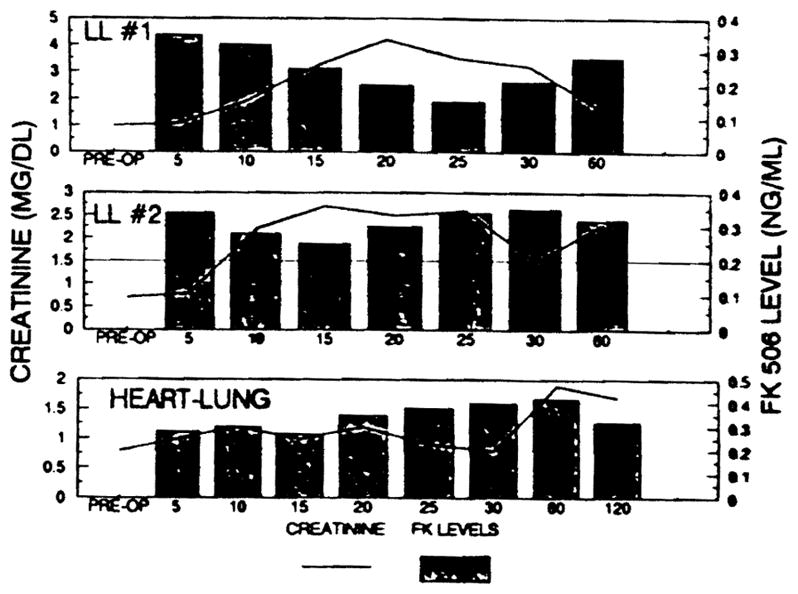
Scr in heart transplants. Individual clinical courses of a heart-lung and two double lung recipients who were treated with FK 506 only.
Liver Transplantation
Early renal dysfunction was also common in these liver recipients whose degree of nutritional and metabolic dis-equilibrium was generally more severe than in the thoracic organ recipients. Nine (7.5%) of the patients required dialysis for the first time postoperatively, and in two cases, this was prolonged. Both cases were complicated postoperatively by recurrent hypotension and a need for nephrotoxic antibiotics. For the remainder, the renal dysfunction was highly reversible, with downward adjustments of the FK 506 dosage, particularly if good liver function was obtained. Renal function was well preserved at latest follow-up with mean serum creatinine 1.4 ± 0.6 mg/dL (Fig 3).
Fig 3.
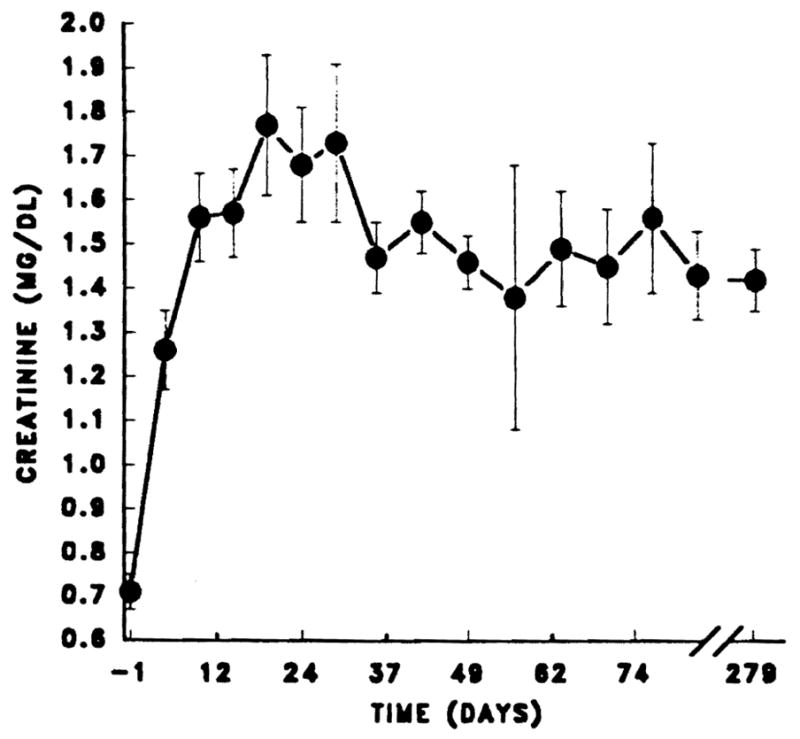
Serum creatinine in 101 primary liver transplant recipients. Values from day 0 to 30 are expressed as mean ± standard error at 5-day intervals.
Eight-two (82%) of the 101 patients are normotensive. Sixteen of the 19 patients requiring treatment are receiving one agent and three require two agents at latest follow-up. Six of the patients requiring antihypertensive drug had a previous history of chronic hypertension. Two of these patients, both with 20-year histories of hypertension, reduced the number of antihypertensive drugs from 3 to 2 after transplantation. An additional patient required nitroprusside acutely for blood pressure control prior to transplantation. One of the patients developed hypertension when treated with dextroamphetamine sulfate (Dexadrine) for presumed narcolepsy.
DISCUSSION
In spite of the management complexity of thoracic organ and liver transplant recipients, a very clear picture of the nephrotoxicity of FK 506 can be pieced together from these observations. At the drug doses used and within the context of these major operative interventions, early postoperative dysfunction was a common, but usually not serious, factor in convalescence. With good function of the heart, lung, and liver grafts, it was possible to make downward adjustments of FK 506 dosage in response to increases in creatinine, and usually without countervailing increases of steroids or the addition of other agents such as azathioprine or antilymphoid preparations. Either with such adjustments or spontaneously, the creatinine could be expected to drift back to baseline. In the lung and heart-lung recipients who were kept steroid free, the highly reversible nephrotoxicity was used as a guide to establish the dose ceiling. Use of FK 506 in high doses frequently depended upon control of hyperkalemia with the mineralocorticoid, fludrocortisone acetate, even in patients with normal renal function.
As has been noted before,3,9,12 the relative freedom from hypertension of patients on FK 506 was noteworthy. Until recently, the hypertension that has been associated with CyA use was considered to be a nephrotoxic manifestation. However, there is evidence in animals13 and humans14, 15 that renal dysfunction and hypertension induced by CyA may be disassociated. If so, our finding of a low incidence of hypertension is less surprising. The contribution of prior history of hypertension undoubtedly predisposes these patients to hypertension after transplantation.
Detailed further studies are needed to study the influence of FK 506 on renal function, and the mechanisms of this influence. During the last year, it has been learned that the FK 506 cytosolic binding site, while distinct from the binding site of CyA (cyclophilin), has in common with cyclophilin a high constituency of the enzyme peptidylprolyl isomerase (PPlase).16, 17 This enzyme, which is presumably inhibited as the central immunosuppressive action of both drugs, is heavily represented in the kidney and was actually discovered there.18 Since PPlase is ubiquitously distributed throughout virtually all tissues, its inhibition by these immunosuppressive drugs will have multiple ramifications. For example, the hyperkalemia that is mineralocorticoid responsive is thought to be due to a selective deficiency of aldosterone and renin. This could have its origin in the adrenal gland or the juxtaglomerular apparatus in the kidneys.
In prospective studies of the effect of FK 506 on renal function, patients with nonhepatic autoimmune diseases should provide the most reliable information since the artefacts caused by major surgical intervention will be eliminated. Such studies (it will be important to know the status of the liver studies presented elsewhere at this meeting) have shown how remarkably the pharmacokinetics (the absorption, bioavailability, and elimination) of FK 506 are altered by liver function, and how different these alterations are from those seen with CyA under similar circumstances. With either drug, studying renal function under the swiftly changing hepatic dynamics of liver transplantation will require more sophisticated pharmacologic monitoring than has been previously used.
We reported earlier that most patients who switched from CyA to FK 506 had significant increases in serum creatinine, and we concluded that these two agents were synergistically nephrotoxic.1, 2 Further documentation of this phenomenon has been presented at this meeting.19 In an ischemic rat model, Nalesnik et al5 have shown that FK 506 causes some of the same changes as CyA including tabular vacuolization, but to a lesser degree.
SUMMARY
Formal studies have not been published on the nephrotoxicity of FK 506 when the drug was used from the outset. This kind of information was sought in 101 recipients of primary livers, 24 hearts, and 3 double lungs or heart-lung. Perioperative renal dysfunction was commonly seen, which appeared to be related to FK 506 doses and plasma levels, particularly when the drug was given IV. This was reversible. Late renal function has been generally satisfactory in all three cohorts of patients, and the incidence of hypertension has been low. The therapeutic index of FK 506 is a good one, as revealed by these observations in patients whose most notable achievement was a low mortality.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant no. 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCauley J, Fung JJ, Jain A, et al. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JM, Kormos RL, Fung J, et al. Transplantation. in press. [Google Scholar]
- 4.Fung JJ, Todo S, Tzakis A, et al. Transplantation. in press. [Google Scholar]
- 5.Nalesnik M, Lai HS, Murase N, et al. Transplant Proc. 1990;22:87. [PMC free article] [PubMed] [Google Scholar]
- 6.Klintmalm GBG, Iwatsuki S, Starzl TE. Lancet. 1981;1:470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers BD, Ross J, Newton L, et al. New Engl J Med. 1984;311:699. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 8.Greenburg A, Egel JW, Thompson ME, et al. Am J Kidney Dis. 1987;9:12. doi: 10.1016/s0272-6386(87)80156-7. [DOI] [PubMed] [Google Scholar]
- 9.Todo S, Fung JJ, Starzl TE, et al. Ann Surg. (in press) [Google Scholar]
- 10.McCauley J, Van Thiel D, Starzl TE, et al. Nephron. 1990;55:121. doi: 10.1159/000185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura K, Kobayashi M, Hasimoto K, et al. Transplant Proc. 1987;19(suppl 6):23. [PubMed] [Google Scholar]
- 12.Starzl TE, Fung J, Jordan M, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 13.Weidle PJ, Vlasses PH. Drug Intell Clin Pharmacol. 1988;22:443. doi: 10.1177/106002808802200601. [DOI] [PubMed] [Google Scholar]
- 14.Braun W. Kidney Int. 1990;37:1368. doi: 10.1038/ki.1990.123. [DOI] [PubMed] [Google Scholar]
- 15.Bennett WN, Porter GA. Am J Med. 1988;85:131. doi: 10.1016/s0002-9343(88)80330-9. [DOI] [PubMed] [Google Scholar]
- 16.Siekierka JJ, Hung SHY, Poe M, et al. Nature. 1989;341:755. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 17.Harding MW, Galat A, Uehling DE, et al. Nature. 1989;341:758. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 18.Fischer G, Bang J. Biochem Biophys Acta. 1984;823:39. [Google Scholar]
- 19.Mieles L, Gordon RD, Mintz D, et al. Transplant Proc. (in press) [PMC free article] [PubMed] [Google Scholar]


