Abstract
Purpose
To study results of radiation on the local control of triple receptor negative breast cancer (negative estrogen (ER), progesterone (PR) and HER-2/neu receptors).
Materials and Methods
Conservative surgery and radiation were used in 753 patients with T1–T2 breast cancer. Three groups were defined by receptor status: ER or PR (+) group 1; ER and PR (−) but HER-2 (+) group 2; and triple negative (TN) group 3. Factors analyzed were age, menopause, race, stage, tumor size, node status, presentation, grade, extensive in-situ disease, margins, and systemic therapy. The primary endpoint was 5-year local-regional recurrence (LRR) isolated or total with distant metastases.
Results
ER and PR negative patients were statistically significantly more likely to be black, T2, have tumors detectable on both mammogram and physical exam, grade 3, and receive chemotherapy. There were no significant differences in ER and PR negative patients by Her-2 status. There was a significant difference in rates of first distant metastases (3%, 12% and 7% for groups 1, 2 and 3, respectively, p=0.009). However, the isolated 5-year LRR was not significantly different (2.3%, 4.6%, and 3.2%, respectively, p=0.36) between the 3 groups..
Conclusions
Patients with TN breast cancer are not at significantly increased risk for isolated LRR at 5-years so remain appropriate candidates for breast conservation.
Keywords: Breast Cancer, Radiation Therapy, Hormone Receptor Negative, HER-2/neu, Basal-like Breast Cancer
Introduction
Radiation therapy following conservative surgery is a standard part of breast preservation therapy for invasive breast cancer1, 2. The addition of radiation therapy significantly reduces the risk of local recurrence in prospective randomized trials with or without adjuvant systemic therapy3, 4. There are relatively few contraindications to breast conservation in general or radiation therapy in particular - a history of prior therapeutic irradiation to the breast, inability to obtain negative resection margins, pregnancy, or multicentric breast cancer1. For example, Morrow et al5 reported that of 216 consecutive patients initially thought to be candidates for breast-conservation, it was successfully carried out in 210 (97.2%). In the past, identification of factors associated with an increased risk for local recurrence after radiation has usually been limited to clinical, pathologic or treatment-related factors. For example, there is evidence for increased rates of local recurrence in patients with young age or positive resection margins6.
Now, breast tumors of different gene expression profiles have been identified that are associated with different clinical behaviors. In particular, basal subtype is associated with a more aggressive clinical behavior and worse prognosis7–10. Without a validated clinical-use assay to identify these tumors prospectively, these different classes of tumors can be roughly distinguished more easily in clinical practice by expression of currently standard ER, PR and HER2 receptor testing. Luminal A and B subtypes are associated with positive ER. ER negative tumors are divided into those positive for HER-2, or those of `basal-like' expression associated with low to absent ER, PR and HER2 receptor expression11. There is an incomplete correlation between basal-like tumors and these receptor expression profiles: from 15–45% of basal-like tumors express at least one of these markers, and only 85% of triple receptor negative tumors are basal-like by expression arrays.10 These previous studies have associated these subtypes with different behaviors in overall recurrence or survival. However, there have been limited series to date about the impact these gene expression profiles have upon local control specifically12–14.
The purpose of this study was to report the local-regional recurrence rates after breast-conserving surgery and radiation for these triple negative breast cancers in order to determine if patients with this subtype remain appropriate candidates for breast conservation.
Methods and Materials
The study population consists of 753 women with American Joint Committee on Cancer stages I–III15 treated by breast conserving surgery and radiation therapy between October 1990 and July 2006. All patients had known ER, PR and HER-2/neu receptor status. Positive HER-2/neu was either immunohistochemical 3+ staining or amplication by flouresence in-situ hybridization. There was no central review or retrospective retesting of receptor status. Exclusion criteria for this study included: male breast cancer, T3 to T4 disease, Stage IV disease, mastectomy or patients treated without radiation therapy. Out of a total of 2,315 patients in the database from October 1990 to July 2006, there were 1,862 with T1–2 breast cancer, breast-conserving surgery and axillary dissection. Of these, 1,109 were excluded for having an unknown ER, PR or HER-2/neu status. The remaining 753 patients make up the study population. Patient information is maintained and updated in a confidential database by a single data manager. The protocol for collection, storage and data retrieval are under compliance with the Hospital Institutional Review Board and Health Insurance Portability and Privacy Act regulations.
All patients underwent breast-conserving surgery and postoperative whole breast irradiation. The median dose of to the whole breast volume was 46 Gy, and the primary tumor bed was boosted in 99% of patients. The total tumor bed dose with the boost was generally determined by the extent of surgery and final margin status. The total dose combining the whole breast and boost doses was a median of 60 Gy for all patients (range 22 – 66 Gy). Patients with a negative margin were treated to a median 60 Gy (range 2200–6600) and close or positive margins 64 Gy (range 5000–6600). There were only 8 patients treated to a dose less than 60 Gy.
Analysis for significant differences was done by global and pairwise comparisons. Factors analyzed included clinical (age, menopause status, race, tumor stage, tumor size, method of detection, and use of prior tamoxifen or estrogen replacement therapy prior to diagnosis); pathologic (nodal stage, grade, necrosis, lymphovascular invasion, extensive intraductal component (EIC), and final margin status); and treatment (use of systemic therapy). Differences in patient characteristics between subgroups were compared using the Chi-square test. The endpoints of the study were 5-year local-regional recurrence (LRR) as an isolated event or with distant metastases. Only first patterns of recurrence were include, not LRR after distant metastases. Other endpoints were disease-free survival and overall survival. Univariate analyses of 5-year outcomes were conducted by using Kaplan-Meier methodology and the log rank test.
Results
The initial analysis of patient characteristics and outcomes showed no significant differences between patients that were ER positive or PR positive by HER-2 status, so they were combined into a single group (group 1). Patients who were ER and PR negative and HER-2/neu positive were in group 2, and patients with all 3 receptors negative were in group 3. Patient characteristics for each group and results of statistical comparisons are shown in table 1.
Table 1.
Patient characteristics by results of ER, PR and Her-2/neu receptor testing
| ER + or PR + | ER − PR − HER-2 + | ER − PR − HER-2 − | P-value | |
|---|---|---|---|---|
| Patient number | 600 | 55 | 98 | |
| Follow-up (months) | 48 | 41 | 44 | 0.23 |
| Median age (years) | 59 | 54 | 54 | 0.2 |
| Race | 0.03 | |||
| White | 90 | 78 | 82 | |
| Black | 7 | 16 | 12 | |
| Other | 3 | 6 | 6 | |
| Tumor Size | 0.0015 | |||
| T1 | 84 | 78 | 68 | |
| T2 | 16 | 22 | 32 | |
| Node Status | 0.47 | |||
| NO | 71 | 69 | 75 | |
| N1–3 | 25 | 22 | 21 | |
| N4+ | 4 | 9 | 4 | |
| Detection | <0.0001 | |||
| Physical Exam | 13 | 18 | 7 | |
| Mammo | 56 | 40 | 39 | |
| Both | 31 | 42 | 54 | |
| Grade | <0.0001 | |||
| 1 | 15 | 0 | 2 | |
| 2 | 47 | 16 | 10 | |
| 3 | 28 | 78 | 84 | |
| Unknown | 10 | 6 | 4 | |
| EIC | 0.14 | |||
| Positive | 16 | 22 | 10 | |
| Negative | 70 | 56 | 72 | |
| Unknown | 14 | 22 | 17 | |
| Necrosis | 0.01 | |||
| Yes | 18 | 35 | 17 | |
| No | 4 | 5 | 1 | |
| Unknown | 79 | 60 | 82 | |
| LVI | 0.66 | |||
| Yes | 15 | 14 | 17 | |
| No | 50 | 44 | 53 | |
| Unknown | 35 | 42 | 30 | |
| Resection margins | 0.96 | |||
| Negative | 85 | 85 | 87 | |
| Positive | 2 | 4 | 2 | |
| Close | 13 | 11 | 11 | |
| Systemic Therapy | <0.0001 | |||
| Chemotherapy | 2 | 73 | 64 | |
| Tamoxifen | 43 | 5 | 4 | |
| Both | 36 | 0 | 2 | |
| Neither | 19 | 22 | 30 |
(numbers in percentages unless otherwise stated)
Statistically significant differences between ER and PR - patients of groups 2 and 3 versus group 1 were more likely T2, detectable on both mammography and physical, grade 3, presence of necrosis, black and use of chemotherapy. Trastuzumab was given to 14 patients (2%) in group 1 and 12 patients (22%) in group 2 who were HER-2/neu positive in later years of the study period. There were no significant differences between group 2 versus 3 patients. There were no significant differences between patients by median age, menopausal status, use of tamoxifen or estrogen replacement therapy prior to diagnosis, node status, EIC, lymphovascular invasion, or margin status.
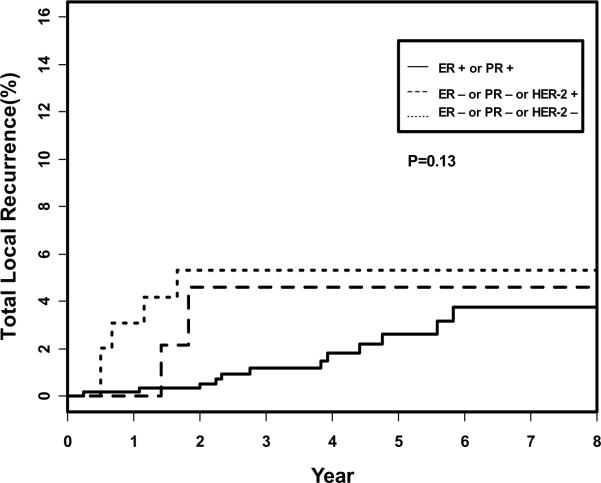
Outcomes are shown in Table 2. The isolated 5-year LRR was not significantly different between groups 1 (2.3%), 2 (4.6%) or 3 (3.2%), p=0.36. Figure 1 shows the actuarial total local-regional recurrence between the three groups. There was no overall difference between the three groups (p=0.13). There was a higher observed rate in group 2 of distant metastases (11.9%) that translated into a lower relapse-free survival (84%). However, the total LRR (5.3%) for TN patients in group 3 was of borderline significance compared to group 1 patients (2.6%, p=0.05) because of the greater numbers of patients with simultaneous local and distant metastases.
Table 2.
5-year outcomes
| ER + or PR + | ER − PR − HER-2 + | ER − PR − HER-2 − | P-value | |
|---|---|---|---|---|
| LRR isolated | 2.3 | 4.6 | 3.2 | 0.36 |
| Distant metastases | 3 | 11.9 | 6.5 | 0.009 |
| LRR total (+distant) | 2.6 | 4.6 | 5.3 | 0.13 |
| Relapse-free survival | 94 | 84 | 90 | 0.005 |
| Overall survival | 94 | 88 | 90 | 0.15 |
(numbers in percentages)
Figure 1.
Total local-regional recurrence as a first site of recurrence with or without simultaneous distant metastases.
Discussion
Patients with triple receptor negative breast cancers in this study were not at increased risk for isolated LRR at 5 years. This is consistent with our finding of no significant differences in clinical or pathologic factors with this subtype that have been predictive of a higher rate of local recurrence in the past – factors such as young age, positive margins or EIC6. Of the three other largest studies providing information on LRR by subtype, 2 of the three showed an increased risk with basal-like expression.
Haffty et al12 reported a series of 117 triple receptor negative patients treated by breast-conserving surgery and radiation and compared them with 365 non-triple negative controls. Our study is concordant with their findings in that patients with triple negative breast cancer were more likely to be black, have T2 tumor size and receive chemotherapy. There was no difference in breast relapse-free survival at 5 years.
Nguyen et al13 reported a series of 89 patients out of 793 with basal-like receptor expression treated by breast-conserving-surgery and radiation. Basal subtype was associated with younger patient age, high grade, and larger size. The 5-year local recurrence rate was 7.1% in basal-like patients. On multivariate analysis, basal-like subtype was associated with increased local recurrence compared to luminal A type patients. There was also a statistically significant increase in distant recurrence in basal-like tumors on multivariate analysis.
Kyundi et al14 reported a series of 152 patients with triple negative breast cancer treated on prospective randomized trials of mastectomy with or without radiation therapy. These randomized trials had shown improvements in LRR and overall survival in general. These patients with basal-like subtype represented only 15% of all patients tested in this analsyis. There was a statistically significant increase in local-regional recurrence and overall mortality but not distant metastases in multivariate analsysis. When the analysis was restricted to those randomized to no radiation, triple-negatives had a higher local-regional recurrence, distant metastasis, and overall mortality. In contrast, those patients randomized to radiation, triple-negative was only associated with increased LRR. In an analysis testing for interaction between postmastectomy radiation and receptor subtypes, there was no improvement in overall survival with radiation in the triple-negative subgroup.
In our series, ER/PR negative and HER2/neu positive tumors had the highest risk of isolated distant metastases and lowest relapse-free survival. This is most likely reflective of the study period before routine availability of trastuzumab. Trastuzumab was given to only 14 patients (2%) in group 1 and 12 patients (22%) in group 2 in later years of the study period. While the HER-2 positive patients comprising group 2 had a higher risk of distant metastases and lower relapse-free survival, this was not associated with a higher rate of isolated or total LRR compared to other patients. The addition of trastuzumab is now routine management of these patients today but would require study in a very large population to determine if it can significantly further reduce the already low rates of LRR seen in this study.
In conclusion, our current policy based upon the outcomes data in this study is to routinely continue to offer breast-conserving surgery and radiation for triple receptor-negative patients.
Acknowledgment
The authors thank Cindy Rosser for her collection and management of the data for the study population, and Lillian Henry for her assistance in the preparation of the manuscript.
Footnotes
Abstract presentation at the 89th Annual Meeting of the American Radium Society, Amsterdam, Netherlands, May 5–9, 2007.
Conflict of Interest Statement: The authors of this manuscript have no conflicts of interest to report.
References
- 1.National Comprehensive Cancer Network [Accessed August 1, 2008];NCCN practice guidelines for breast cancer. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 2.NIH Consensus Conference Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 3.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Morrow M, Bucci C, Rademaker A. Medical contraindications are not a major factor in the underutilization of breast conserving therapy. J Am Coll Surg. 1998;186:269–274. doi: 10.1016/s1072-7515(97)00153-1. [DOI] [PubMed] [Google Scholar]
- 6.Freedman G, Hanlon A, Fowble B, Anderson PR, Nicolaou N. Recursive partitioning identifies patients at high and low risk for ipsilateral tumor recurrence after breast conserving surgery and radiation. J Clin Oncol. 2002;20:4015–4021. doi: 10.1200/JCO.2002.03.155. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia S, Dalès JP, Charafe-Jauffret E, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 12.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 14.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 15.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York: 2002. American Joint Committee on Cancer. [Google Scholar]