Between 1955 and the end of 1967, the framework of clinical organ transplantation that exists today was established in a small number of centers in continental Europe, Great Britain, and the North America. Here, I will describe the events during this period that led to human liver replacement. These efforts were influenced by, and in turn contributed to, the development of other kinds of organ transplantation, and especially that of the kidney.
The genesis of liver transplantation
As described by the immunologist, Leslie Brent,1 and the Glasgow surgeon-historian David Hamilton,2 transplantation of all the major organs except the liver can be traced back to the early 1900s. In contrast, liver transplantation was not mentioned in the literature until 1955. The first report was in a journal called Transplantation Bulletin, the forerunner of the current Transplantation.
The auxiliary liver concept
In this one-page article, C Stuart Welch of Albany Medical College (Fig. 1), described the insertion of a hepatic allograft in the right paravertebral gutter of dogs, without disturbing the native liver.3 A more complete report was published in Surgery the following year.4 The auxiliary livers were revascularized by anastomosing the graft hepatic artery to the recipient aortoiliac system, and by end-to-end anastomosis of the portal vein to the host inferior vena cava (Fig. 2). The transplanted organs underwent dramatic shrinkage, a finding that was incorrectly considered for most of the next decade to be a special feature of liver rejection.
Figure 1.

C Stuart Welch (1909–1980), the author of the first article in the literature on liver transplantation. Information about Welch’s tragic personal life can be found in: Starzl TE: The Puzzle People: Memoirs of a Transplant Surgeon. PittSburgh, PA: University of Pittsburgh Press; 1992.
Figure 2.
Auxiliary liver homotransplantation in dogs (the Welch procedure). Note that the reconstituted portal venous inflow is from the inferior vena caval bed rather than from the splanchnic organs. Biliary drainage was with cholecystoduodenostomy. a, artery; v, vein. (From: Starzl TE, Marchioro TL. Rowlands DT Jr, et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg 1964;160:411–439; with permission .)
In 1957, Welch gave a lecture on his experimental operation during a visiting professorship at the University of Miami Medical School, where I was a general surgery residen. Because he had provided the auxiliary grafts with high flow input of systemic venous blood from the recipient inferior vena cava, Welch was convinced that his transplanted livers were optimally revascularized.
Contrary to this assumption, I had been exploring the possibility that the first pass delivery of endogenous insulin from the pancreas to the liver by portal blood was important in metabolic crossregulation of the two organs. Evidence consistent with this hypothesis had come from studies of Eck’s fistula (portacaval shunt) and reverse Eck’s fistula.5,6 If the hypothesis was correct, the Welch procedure was physiologically flawed.
Liver replacement
To pursue the metabolic studies, I had developed a new method of total hepatectomy.7 The unique feature of the procedure was preservation of the retrohepatic inferior vena cava, as is done in today’s piggy-back modification of liver transplantation8-10 (Fig. 3). Reimplantation (autotransplantation) of the excised specimen was soon envisioned as an ideal way to study the portal physiology of an unequivocally denervated liver that was devoid of cryptic collateral arteries. Welch had obviated the need to anastomose multiple hepatic veins by including as part of his auxiliary allografts the short length of vena cava into which all of these hepatic veins empty and by connecting the upper end of the caval stump to the recipient vena cava (Fig. 2).
Figure 3.

Hepatic fossa after removal of the liver with preservation of the retrohepatic inferior vena cava. Duod., duodenum; P.v., portal vein; V.c., vena cava. (From: Starzl TE, Bernhard VM, Benvenuto R, Cortes N. A new method for one-stage hepatectomy in dogs. Surgery 1959; 46:880–886; with permission)
For liver replacement, it was easiest to excise the host retrohepatic vena cava along with the native liver, and to replace it with the comparable caval segment of an allograft (Fig. 4). Restoration of caval continuity required end-to-end anastomoses: one at the diaphragm and the other below the liver. The performance of everting anastomoses in a confined space without the need for long vascular cuffs was made feasible by perfection of the intraluminal continuous suture technique used today (Fig. 5). Hepatic arterial and biliary tract anastomoses were done with conventional methods.11
Figure 4.
Canine liver allograft. The graft includes the segment of retrohepatic inferior vena cava into which the hepatic veins empty. G.b., gallbladder; H.a., hepatic artery; P.v., portal vein. (From: Starzl T, Kaupp H Jr, Brock D, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733–743, with permission.)
Figure 5.
Intraluminal everting suture technique for close-quarter vena caval anastomoses. Ant., anterior; Post., posterior. (From: Starzl T, Kaupp H Jr, Brock D, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733-743, with permission.)
At first, none of the animals survived the operation. This finally was accomplished in June, 1958, a few days after I moved from Miami to Northwestern University in Chicago. During the rest of the summer, the different kinds of liver revascularization studied in Miami in non-transplant models were systematically tested in allografts (Fig. 6). Any alteration of the portal supply resulted in reduced survival. Although the findings were congruent with the original hypothesis that splanchnic venous blood contains liver-modulating factors, this issue was not fully resolved for another 15 years.
Figure 6.
Alternative methods of portal vein revascularization. (A) reverse Eck fistula. (B) with small side-to-side portacaval shunt. (C) anatomically normal. Survival was best with C. P.v., portal vein. (From: Starzl T, Kaupp H Jr, Brock D, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733-743, with permission.)
Two administrative steps were taken at the end of that summer that ensured crucial research support for at least 5 years. The first was submission of a four-page NIH grant request for continued investigation of the liver’s role in insulin and carbohydrate metabolism that included liver transplantation. In addition, my North-western chairman, Loyal Davis, nominated me for a Markle Scholarship; the purpose of these awards was to keep young faculty members in academic medicine in pursuit of some stipulated career objective. My proposal was the development of clinical liver transplantation. Just before Christmas, 1958, I was notified that the grant would be fully funded, and that I was to be a Markle scholar.
Other early investigations
Unknown to the in 1958, our attempts at liver replacements had been preceded by those of a UCLA surgeon named Jack Cannon (Fig. 7). Cannon, now 83 years old, later practiced in Phoenix where he has retired. In collaboration with William P Longmire Jr, Cannon already had made important basic observations about spontaneous tolerance in a neonatal chick model of skin transplantation, and the facilitation of such tolerance with adrenal corticosteroids.12 The significance of this neglected work is discussed elsewhere.13 His liver transplant experiments were mentioned in a one-page review14 entitled “Brief Communication,” published in 1956 in the same Transplantation Bulletin as Welch’s report of the year before.
Figure 7.

Jack A Cannon (1919–). the first surgeon to attempt experimental liver replacement. The work was done in 1956 at the then new medical school of the University of California Los Angeles (UCLA).
Cannon’s description did not specify the species studied (presumably dog), and did not contain any specific information. Cannon acknowledged Welch’s report as his inspiration, and alluded to “…several successful operations… (liver replacements) without survival of the ‘patient’… .“ In a prophetic comment, he suggested that … ”the liver undoubtedly has a great deal to do with the production of the homograft reaction and probably with the inception and maintenance of tissue specificity. Replacement transplantation of intact liver, therefore, might well lead to interesting results.”
In early 1959, I learned that a team headed by the late Francis D Moore (Fig. 8) had begun the development of canine liver transplantation at the Peter Bent Brigham Hospital in June or July 1958, at the same time as my own first successful experiments. By the end of the summer of 1958, the Boston team had done six liver replacements. These were reported in a 1959 issue of Transplantation Bulletin.15 I first met Moore at the 1960 meeting of the American Surgical Association, where I discussed his presentation.16 By then, the cumulative total of canine liver replacements in the 2 laboratories had increased to 111—31 in Boston and 80 in Chicago. The results were published separately in 1960 in different journals.11,16
Figure 8.

Francis D Moore (1913–2001), professor of surgery at Harvard Medical College, and chief of surgery at the Peter Bent Brigham Hospital, Boston, Moore instituted a program of canine liver replacement in the summer of 1958, and attempted one of the human procedures done in 1963.
Prerequisites for canine liver replacement
The rwo prerequisites for perioperative survival of canine recipients were identified in both the Boston and Chicago laboratories. The first requirement was prevention of ischemic injury to the allograft. This was accomplished in Boston by immersing the liver in iced saline, a method independently used for preservation of intestinal and cardiac allografts by Lillehei and colleagues17 and by Lower and Shumway,18 respectively.
Our exploitation of hypothermia in Chicago reflected the influence of F John Lewis, professor of surgery at Northwestern, who, with his Fellow, Norman Shumway, had pioneered total body hypothermia for open heart surgery at the University of Minnesota (Fig. 9). The livers were cooled by intravascular infusion of chilled solutions (Fig. 10), using thermal probes to monitor core temperatures (Fig. 11). Interestingly, this now universal practice had never been done before, apparently because of fear of damaging the microcirculation. Better liver preservation was later obtained with infusates of differing osmotic, oncotic, and electrolyte composition: eg, the Collins,19 Schalm,20 and University of Wisconsin (UW) solutions21-23 that originally were developed for kidney transplantation.24,25
Figure 9.

Left: Norman Shumway (1923–) and the late F John Lewis (1916-1993) at an American College of Surgeons congress in the 1970s.
Figure 10.
Cooling of canine hepatic allograft by infusion of chilled lactated Ringer’s solution into the donor portal vein. The animals were simultaneously exsanguinated. G.b. gallbladder; H.a., hepatic artery; P.v., portal vein. (From: Starzl T. Kaupp H Jr. Brock D, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733–743. with permission.)
Figure 11.

Rewarming curve at room temperature of a canine liver that had been cooled by an infusion of chilled lactated Ringer’s solution. The temperature probe was placed at the center of the allograft. (From: Starzl T. Kaupp H Jr. Brock D. et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733–743, with permission.)
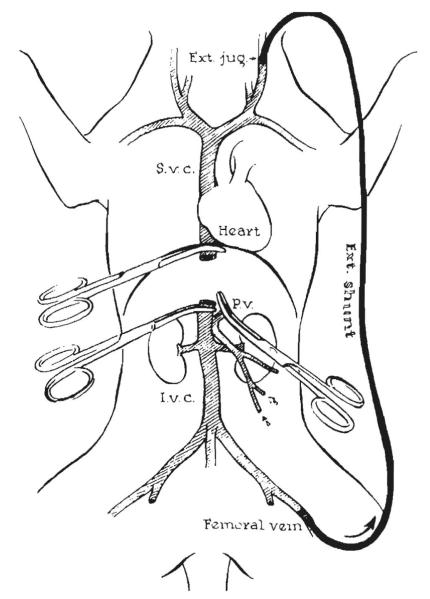
The second prerequisite for successful canine liver transplantation was avoidance of damage to the recipient splanchnic and systemic venous beds, the drainage of which was obstructed during host hepatectomy and graft implantation. This was accomplished in both the Boston and Chicago laboratories with decompressing external venovenous bypasses (Fig. 12).
Figure 12.
Decompression of the portal and vena caval beds. This was done in Chicago with a single femoral vein to external jugular bypass after preliminary portacaval anastomosis.11 In Boston, separate external shunts were used for individual decompression of the 2 venous systems15 as shown in Figure 32. Ext., external; P.v., portal vein; S.V.C., superior vena cava. (From: Starzl T, Kaupp H Jr, Brock D, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960;111:733–743, with permission .)
The pathology of liver rejection
Until 1960, the kidney had been the only organ allograft whose unmodified rejection had been systematically studied. Most transplanted canine livers were destroyed in 5 to 10 days. The survival of our dogs that lived for at least 4 days is shown in Fig. 13. The histopathologic studies were done in Chicago by Donald Brock26 (now retired in Northville, MI) and in Boston by the late Gustav Dammin27 (Fig. 14). Typically, a heavy concentration of mononuclear cells was seen in the portal triads, and within and around the central veins. Hepatocyte necrosis was extensive.
Figure 13.
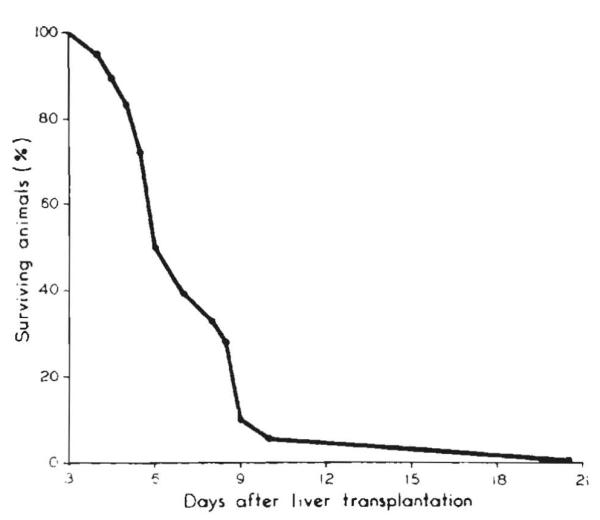
Survival in dogs who lived more than 4 days after liver replacement. Note: high mortality between 5th and 10th day. (From: Starzl TE, Kaupp HA Jr. Brock DR. Linman JW. Studies on the rejection of the transplanted homologous dog liver. (From Surg Gynecol Obstet 1961;112:135–144.)
Figure 14.

(A) Donald Brock (1926–). As chief of pathology at the Northwestern VA (Research) Hospital in 1958–1960, Brock did the histopathologic analyses for the Chicago liver transplant studies in unmodified dogs. (B) Gustav Dammin (1911–1991) already was an acknowledged expert in kidney transplant pathology when he was recruited by Francis D Moore for the Brigham canine liver transplant initiative.
A curious observation was made, however, in our 63rd liver replacement experiment.26 The recipient’s serum bilirubin reached a peak at 11 days and then progressively declined (Fig. 15, dashed line). The predominant histopathologic findings in the allograft by the 21st day were more those of repair and regeneration than of rejection. This was, to my knowledge, the first recorded exception to the existing dogma (based on skin graft research) that rejection, once begun, was inexorable. Five years later, the London pathologist, KA Porter (Fig. 16), described similar findings in allografts of the first long-surviving canine liver recipients whose rejections had been reversed under immunosuppression in Denver.28 In 1969, he extended his observations to human liver allografts.29
Figure 15.

Serial blood sugars and serum bilirubins in a nonimmunosuppressed canine liver recipient who survived for 3 weeks. Note the decline of bilirubin after the 11th day. This evidence of the spontaneous reversal of rejection was consistent with the histopathology of the autopsy liver at 21 days. (From Starzl T, Kaupp HA, Brock DR, Linman JW. Studies on the rejection of the transplanted homologous dog liver. (Surg Gynecol Obstet 1961;112:135–144, with permission.)
Figure 16.

(A) KA Porter (1925–). In 1963. Porter, then a reader (and subsequently chairman) of pathology at St. Mary’s Hospital and Medical School, began a 35-year transatlantic collaboration with me. Porter’s interests, which previously had been focused on the kidney,30 shifted to the liver in the mid 1960s.29 (B) Anthony J Demetris (1956–), founding chief of the Division of Transplantation Pathology at the University of Pittsburgh. After Porter’s retirement, Demetris emerged as the doyen in the new field of transplantation pathology.
Because Porter’s previous principal research had been kidney transplantation,30 he was now able to sort out features of rejection that were common to both organs (and various other allografts) in unmodified and immunosuppressed recipients, and to distinguish these changes from those that were specific to the different kinds of organ allografts. Under the leadership of AJ Demetris at the University of Pittsburgh (Fig. 16), the field of clinical transplantation pathology rose from the base laid by the earlier workers.
Variant liver transplant procedures
The studies completed in Boston and Chicago defined, almost to the last detail, the liver replacement operation soon to be performed in humans. (Fig. 17). By the end of 1959, we also had developed the operation of multivisceral transplantation. Here, the allograft consisted of the liver and all of the other intraperitoneal organs (Fig. 18)31,32. Essentially all of this work, and the development ofliver transplantation, was done with the help of Harry A Kaupp Jr, a skillful general surgery resident, who practiced vascular surgery in Allentown, PA, until his retirement. Two medical students (Robert Lazarus and Robert Johnson) rounded out the team.
Figure 17.
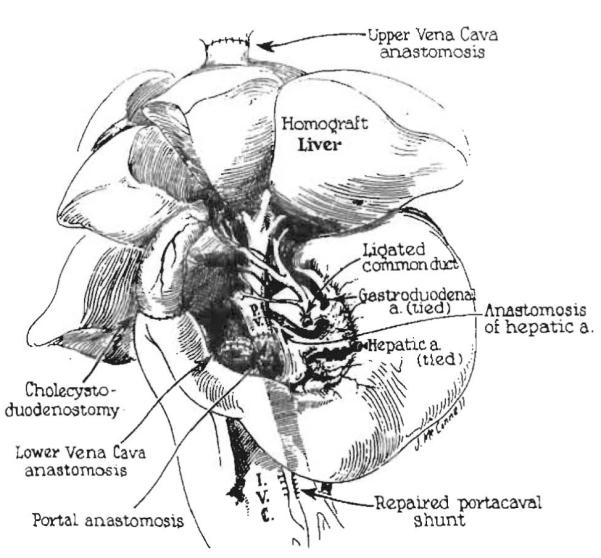
Completed liver replacement in the dog. The fact that the recipient was a dog rather than a human is identifiable only by the multilobar appearance of the liver. a., artery. (From: Brettschneider L, Daloze P, Porter K, et al. The Use of combined preservation techniques for extended storage of orthatopic liver homografts . Surg Gynecol Obstet 1968;26:263–274; with permission).
Figure 18.
Canine multivisceral transplantation. The organs of the composite allograft are not shaded. (From: Surg Forum 11:28–30, 1960; with permission).
Two further observations about rejection were made in the multivisceral experiments that were validated much later in rodent studies33,34 and in humans.35 First, rejection of the different organs transplanted with the liver was less than rejection of the individual organs transplanted alone. Second, there was histopathologic evidence of a widespread graft versus host reaction in recipient tissues without resulting in overt graft versus host disease.
Multivisceral transplantation and its modifications (Fig. 19) were applied in humans 30 years later36-39 and are now part of the conventional armamentarium of advanced organ transplant centers. When the operation was first presented at the Surgical Forum of the American College of Surgeons in October 1960,31 it was lampooned. In fact, all surgical research in transplantation of the 1958 to 1960 era was considered naïve or wasteful by many critics and especially by basic immunologists, most of whom viewed the immune barrier as impenetrable.
Figure 19.

The original canine multivisceral allograft (bottom center) and its variations (arrows). All are used clinically today.
Immunosuppression by host cytoablation
Just as this kind of surgical research in unmodified animals was losing momentum, it was dramatically revitalized by six successful human kidney transplantations performed between January 1959 and February 1962, first by Joseph Murray in Boston,40 and then by the teams of Jean Hamburger41 and Rene Kuss42 in Paris (Fig. 20). Although ”success“ was defined as survival for at least 1 year, the first two recipients (both of fraternal twin kidneys) had continuous graft function for more than two decades without post-transplant immunosuppression. All six patients had been conditioned before transplantation with sublethal doses of 450R tOtal body irradiation (Table 1, above dashed line).
Figure 20.
Pioneers in kidney transplantation. Left to right: Joseph E Murray (1919–), Boston surgeon and 1990 Nobel Laureate; Jean Hamburger (1909–1992), Paris nephrologist; and Rene Kuss (1913–), Paris urologist.
Table 1.
Kidney Transplantation: Survival of 6 Months or More as of March 1963 (Above Dotted Line)
| Patient No. | City (Ref) | Date | Donor | Survival (mo) |
|---|---|---|---|---|
| 1 | Boston (40) | 1-24-59 | Fraternal twin | >50 |
| 2 | Paris (41) | 6-29-59 | Fraternal twin | >45 |
| 3 | Paris (42) | 6-22-60 | Unrelated* | 18 (died) |
| 4 | Paris (41) | 12-19-60 | Mother* | >12 (died) |
| 5 | Paris (42) | 3-12-61 | Unrelated* | 18 (died) |
| 6 | Paris (41) | 2-12-62 | Cousin* | >13 |
| 7 | Boston (54) | 4-5-62 | Unrelated | 11 |
Boston: JE Murray (patients 1 and 7).
Paris: J Hamburger (patients 2, 4 and 6), R Kuss (patients 3 and 5).
Adjunct steroid therapy.
In an extension of the host preconditioning concept, the urologist, Willard Goodwin (Fig. 21), performed six human kidney transplantations at UCLA in 1960-1961 in which host cytoablation was done with myelotoxic doses of cytoxan and methotrexate instead of total body irradiation.43 Although five of the six recipients came to an early death, Goodwin successfully reversed several rejections with prednisone during the 143-day survival of his third patient, whose kidney was donated by her mother in September 1960. This crucial observation was not reported until 1963 and was not known to us until then.
Figure 21.

Willard Goodwin (1915–1999), Founding Chief, Division of Urology, University of California Los Angeles (UCLA) and pioneering kidney transplant surgeon.
In any event, it quickly became apparent that the Boston and French successes with cyroablation for kidney transplantation, remarkable though they were, would not be a bridge to liver transplantation. In our hands, total body irradiation precluded even perioperative, much less extended, survival of canine liver recipients.44
Drug immunosuppression for clinical kidney transplantation
A sea change occurred with the arrival of the drug 6-mercaptopurine (6-MP). The key observation was that immune depression under 6-MP did not depend on overt bone marrow depression.45 The potential value of the drug in transplantation was first demonstrated in a rabbit skin graft model by Schwartz and Dameshek46 at Tuft’s Medical School in Boston, and by the research team of Robert Good at the University of Minnesota.47 Prolongation of survival of canine kidney allografts under 6-MP was reponed soon after by Roy Calne (in London)48 and Charles Zukoski (in Richmond)49 (Fig. 22).
Figure 22.
Photograph in the 1980s of Roy Calne (1930–) and currently of Charles Zukoski (1926–). Both men tested 6-MP in the canine kidney transplant model in 1960 and reported encouraging results in the same year.48,49
By the end of 1960, Calne (by now in Boston with Murray)50,51 and Zukoski (with David Hume in Richmond)52 obtained survival of canine kidney recipients for 100 days or more under treatment with 6-MP. Even better results soon were reported by Calne50 with azathioprine an imidazole derivative of 6-MP. When clinical kidney transplant trials with the new drugs were begun in Boston in 1960 and 1961, the possibility of transplanting the human liver seemed close at hand.
Before William R Waddell (Fig. 23) left the Massachusetts General Hospital to become chair of surgery at the University of Colorado in Denver on July 1, 1961, where I joined him from Northwestern a few months later, we settled on clinical liver transplantation as our highest priority. The plan was shelved in early 1962 when we learned of disappointing results in the clinical trials of kidney transplation in Boston53,54 and England.54 ray of hope could be found, however, in a report by the future Nobel laureate, Murray, in the September, 1962, issue of the Annals of Surgery.53
Figure 23.

William R Waddell (1919–), Chairman. Department of Surgery, University of Colorado, 1961–1972, during the early days of clinical liver transplantation. He is retired in Silver City, NM.
The article contained a description of a kidney that had functioned under azathioprine therapy for 120 days, from the time of its transplantation from an unrelated donor on April 5, 1962. That kidney still functioned at 11 months,54 and was destined to suppon dialysis-free life of the recipient for 17 months. Although this was the only patient who lived for as long as 6 monrhs, he became the 7th human to survive more than 1 year after kidney transplantation, and the first to do so without total body irradiation (Table 1, below the dashed line).
In the meantime, we had obtained our own supply of azathioprine in the spring of 1962, and systematically evaluated it with the simpler canine kidney model rather than with liver transplantation. Many of the experiments were done with Tom Marchioro (Fig. 24), subsequently a revered professor of surgery at the University of Washington (1967–1995). As in other laboratories, our yield of 100-day canine kidney transplant survivors was small.
Figure 24.

Thomas L Marchioro (1928–1995). Assistant and Associate Professor of Surgery. University of Colorado from 1962 to 1967.
But two crucial findings were clinically relevant. First, canine kidney rejection developing under azathioprine invariably could be reversed with the addition of large doses of prednisone.56 The second key observation was that a mean survival of 36 days in dogs treated with azathioprine was almost doubled when the animals also were pretreated with the drug for 7 to 30 days57 (Table 2). We committed to clinical trials of kidney and liver transplantation, in that order. Daily doses of azathioprine were to be given for 1 to 2 weeks before and after transplantation, with the addition of prednisone only to treat rejection (Fig. 25). The renal program was opened in the autumn of 1962.
Table 2.
Influence of Pretreatment with Azathioprine on Post-transplant Longevity in Dogs
| n | Mean survival, d |
SD | SE | |
|---|---|---|---|---|
| Controls | 21 | 10.8 | 2.75 | .06 |
| Acute azathioprine | 21 | 36 | 13.7 | 3.0 |
| Azathioprine pretreatment | 18 | 69.1 | 68.1 | 16.1 |
Comparison of groups 2 and 3 (p < 0.001).
The rationale for the practice of pretreatment with azathioprine is based on the 60-dog experiments in which homotransplantation and bilateral nephrectomy were performed. Untreated animals lived for 10.8 ± 0.06 (SE) days. Animals that received treatment with azathioprine, started on the day of operation, lived 36 ± 3.0 (SE) days. Animals that received preoperative treatment wirh azathioprine for 7 to 30 days, and postoperative therapy, lived for 69 ± 16.1 (SE) days. The difference between the twO groups was highly significant (p < 0.001). (From Experience In Renal Transplantation. Philadelphia, PA: WB Saunders Company; 1964.)
Figure 25.

Empirically developed immunosuppression used for kidney transplant recipients in 1962-1963. Note the reversal of rejection with the addition of prednisone to azathioprine. More than a third of a century later, it was realized that the timing of drug administration had been in accord with the tolerogenic principles of immunosuppression described under “ Mechanisms of Engraftment Tolerance: History Revisited.”
The two features of the adaptive immune response to allografts that eventually would make transplantation of all kinds of organs feasible were promptly recognized. These were described in the title of the report of the first 10 Colorado kidney cases: the reversibility of rejection, and more importantly, the subsequent development of donor specific tolerance.58 ”Tolerance,“ which referred to the time-related decline of need for maintenance immunosuppression, proved to be the correct word.
Nine of the 46 recipients of kidney allografts from live-related donors (20%) remained dialysis-free for 4 decades, all but one with normal renal function throughout. Seven of the nine became immunosuppression-free for periods ranging from 21/2 to 38 years (Fig. 26). One of the nine was recently murdered in a love triangle and had a normal transplanted kidney at coroner’s autopsy. Those remaining bear eight of the nine longest surviving kidney allografts in the world today, including the four longest.59
Figure 26.

Nine (19%) of the 46 live donor kidney recipients treated at the University of Colorado over an 18-month period beginning in the autumn of 1962. The solid portion of the horizontal bars depicts the time off immunosuppression. Note that the current serum creatinine concentration (CR) is normal in all but one patient. *Murdered: kidney allograft normal at autopsy.
Human liver replacement: 1963
Although the maximum followup of our first human renal recipient was only 6 months in the spring of 1963, our kidney experience triggered the decision to go forward with the infinitely more difficult trial of liver transplantation. The first attempt on March 1, 1963 was in an unconscious and ventilator-bound child with biliary atresia who bled to death during operation. The next two recipients, both adults, died 22 and 7.5 days after their transplantations on May 5 and June 3, 1963 for the indication of primary liver malignancies (Table 3). The two adults were found at autopsy to have extrahepatic micrometastases. The three failed cases were described in the December 1963 issue of Surgery, Gynecology, and Obstetrics,60 2 months after the optimistic kidney report in the same journal.58
Table 3.
The First Seven Human Liver Recipients
| Age (y) |
Date | City (ref) | Liver disease |
Survival (d) |
Main cause of death |
|---|---|---|---|---|---|
| 3 | 3-1-63 | Denver (60) | Biliary atresia | 0 | Intraoperarive bleeding |
| 48 | 5-5-63 | Denver (60) | Hepatoma, cirrhosis | 22 | Pulmonary emboli, sepsis |
| 68 | 6-3-63 | Denver (60) | Duct cell carcinoma | 7.5 | Pulmonary emboli |
| 52 | 7-10-63 | Denver (64) | Hepatoma, cirrhosis | 6.5 | Gastrointestinal bleeding, pulmonary emboli/edema, liver failure |
| 58 | 9-16-63 | Boston (65) | Colon metastases | 11 | Pneumonitis, hepatic abscesses, failure |
| 29 | 10-4-63 | Denver (64) | Hepatoma | 23 | Sepsis, bile peritonitis, pulmonary emobli |
| 75 | 1-?-64 | Paris (66) | Colon metastases | 0 | Intraoperative hemorrhage |
Immunosuppression was the same as that used for our kidney recipients. Pretreatment was begun with azathioprine with or without small doses of prednisone. The same therapy was continued after transplantation. With evidence of rejection, a high-dose course of prednisone was added (Fig. 27). Rejections, which were monitored by serial serum bilirubin concentrations, were easily reversed (Fig. 28). The transplanted livers retrieved at autopsy were remarkably free of rejection, as noted in the caption describing the histopathology of the 22-day graft (Fig. 29, right).
Figure 27.

Immunosuppression with azathioprine (imuran.) and prednisone in first patient to survive orthotopic liver transplantation (patient 2). ACTl-C: doses of actinomycin C. (From: Strarzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676, with permission.)
Figure 28.

Rise in serum bilirubin to 12.8 mg% in the patient shown in Figure 27, 9 days after transplantation. The bilirubin declined after institution of high-dose prednisone therapy. (From: Strarzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676, with permission.)
Figure 29.
Original photomicrograph and verbatim caption published in 1963. ”Specimens in Patient 2—A. left: Patient’s own liver, showing hepatoma. B. right: Liver homograft obtained at autopsy 22 days after operation. Note good preservation of architecture. There was slight periportal fibrosis, which is thought to have antedated transplantation. Note mild cholestasis and fatty metamorphosis. A few aggregates of mononuclear cells were present in the periportal areas. Hematoxylin and eosin , × 19.“ (From: Strarzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676, with permission.)
Efficient allograft preservation was accomplished by transfemoral infusion of a chilled perfusate into the aorta of the nonheart-beating donors after cross-clamping the aorta at the diaphragm61 (Fig. 30). The procedure was the same in principle as that of first stage of the ”flexible“ multiple organ procuremem operarion currently used worldwide.62 There was very little ischemic damage to the allografts during their postmortem intervals of 2½ to 8 hours, as indicated by modest increases in the liver injury tests (Fig. 31).
Figure 30.
Extracorporeal perfusion of the cadaver donors reported in 1963. “The venous deainage was from the inferior vena cava and the arterial inflow was through the aorta after insertion of the catheters through the femoral vessels. Note clamp on thoracic aorta to perfuse the lower half of the corpse selectively. A glucose primed pump oxygenator was used with a heat exchanger.” (From: Starzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676, with permission.)
Figure 31.

Liver injury tests after hepatic transplantation in patient 2 of the 1963 report. “Note modest rise in serum glutamicoxaloacetic transaminase (SGOT) with subsequent decline. lactic dehydrogenase (LDH) and serum glutamic pyruvic acid transaminase (SGPT) were similarly affected.” (From: Starzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676. with permission.)
The various anastomoses were performed in the same way as in the canine experiments (Fig. 32). The lethal mistake in the human cases was the use of passive venovenous bypasses (Fig. 32). Emboli formed in the bypass tubing, migrated to the lungs, and caused or contributed to the deaths of all 1963 Denver recipients who survived the operation (Table 3). Overzealous correction of clotting abnormalities may have contributed to the complication. In much the same way as today, coagulation had been monitored with serial thromboelastograms and corrected with blood components and with epsilon amino caproic acid (an analogue of the currently used aprotinine).
Figure 32.
Details of human liver replacement. (A) External bypasses for decompression of the inferior vena caval and splanchnic venous beds. (B) Anastomosis of suprahepatic inferior vena cava. Note that the cuff of the homograft is actually a confluence of the hepatic veins and the vena cava. (C) Subhepatic operative field after completion of all anastomoses. Note that gallbladder has been removed and that the T tube is inserted through a stab wound in the recipient portion of the composite common duct. (From: Starzl TE. Marchioro TL. Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–676, with permission.)
The supreme irony was that the venous decompression that had been critical in the dog experiments is not mandatory in most human liver recipients. The motordriven venovenous bypass system, which was introduced in Pittsburgh in the 1980s,63 made the procedure easier. In some centers, however, it now is used only selectively.
The aftermath
During the last half of 1963, two more attempts at liver transplantation were made in Denver,64 and one each in Boston65 and Paris,66 (Table 3). Clinical activity then ceased for 3½ years. The worldwide moratorium was voluntary. The decision to stop was reinforced, though, by widespread criticism of attempting to replace an un-paired vital organ with an operation that had come to be perceived as too difficult to ever be tried again.
In contrast, kidney transplantation thrived at the University of Colorado. In 1964, a textbook was produced based on our first 70 cases, emphasizing that renal transplantation had reached the level of a bona fide clinical service.67 At the beginning of 1963, the only three clinically active kidney transplant programs in the United States were at the long-standing Brigham center and the two opened in 1962: ours and David Hume’s in Richmond, VA. One year later, nearly 50 kidney teams had started or were gearing up. A similar proliferation was going on throughout Europe.
The liver moratorium
Advances were made between January 1964, and the summer of 1967, most of which were applicable to all organs.
Role of HLA matching
In a clinical collaboration with Paul Terasaki of UCLA (Fig. 33), it was shown that the quality ofHLA matching short of perfect compatibility had little association with kidney transplant outcome.68-70 By inference, desperately ill liver, heart, and other transplant candidates who could not wait for a well-matched organ would not suffer a significant penalty by receiving a mismatched one.
Figure 33.

Paul Terasaki, PhD (1929–), the basic scientist at UCLA who became a father of tissue matching.
Development of antilymphocyte globulin
A second objective was to improve immunosuppression. Antilymphocyte globulin (ALG) was prepared from antilymphocyte serum (ALS) obtained from horses immunized against dog and human lymphoid cells.71 After its development and testing in dogs between 1963 and 1966, human-specific ALG was introduced clinically in 1966 in combination with azathioprine and prednisone (the “triple drug cocktail”).
In the preclinical canine studies, the efficacy of dogspecific ALG had been demonstrated when it was given before, at the time of, or after kidney and liver transplantation. It was noted that ” … pretreatment [with ALG] did appear to be of value in the canine experiments, and was accordingly made part of the protocol used for patients.“71 The conclusion about the efficacy of pretreatment was consistent with previous rodent studies of ALS by Woodruff and Anderson72 and by Monaco, Wood, Gray, and Russell.73
Demonstration of hepatic tolerogenicity
The goal in Denver of resuming clinical liver transplantation was reflected by a growing kennel population of long surviving canine recipients (Fig. 34), none of whom were treated with more than a 4-month course of azathioprine,8 or a few doses of ALG.71 In presenting the results of 143 canine liver replacements to the Society of University Surgeons in February 1965, I emphasized that
… Although the early recovery after liver homotransplantations has many hazards … the frequency and rapidity with which dogs could be withdrawn from immunosuppression without an ensuing fatal rejection is remarkable …. The consistency of this state of host-graft nonreactivity and the rapidity with which it seemed to develop exceeds that reported after canine renal homotransplantations. 28
Figure 34.
Canine recipient of an orthotopic liver homograft, 5 years later. The operation was on March 23, 1964. The dog was treated for only 120 days with azathioprine and died of old age after 13 years.
A year later, the French surgeon, Henri Garnier, reported (with Cordier) that a significant percentage of untreated outbred pig liver recipients did not reject their allografts.74 This observation promptly was confirmed and extended in England by Calne at Cambridge,75 Peacock and Terblanche in Bristol,76 and us.77 Calne and coworkers78 subsequently demonstrated that the tolerance self-induced by the liver extended to other tissues and organs from the liver donor, but not from third-party pigs.
Reassessment of the auxiliary liver graft
Although our primary focus during the moratorium was on liver replacement, we also evaluated the ostensibly less radical auxiliary liver transplantation (Welch’s operation). After showing that rejection could be completely prevented in some dogs with high doses of azathioprine, it was proved that the acute atrophy of Welch’s auxiliary livers was caused by depriving the allografts of liver supporting constituents of splanchnic venous blood.64,79 At the time of transplantation 45 days previously, the diminutive rejection-free allograft shown in Fig. 35 had been the same size as the recipient’s normally vascularized liver.
Figure 35.

“An auxiliary homograft (right) and the recipient dog’s own liver (left). Note the well-preserved but diminutive structure of the liver homograft. The gallbladder did not shrink proportionately. The specimens were obtained 45 days after transplantation.” (From: Ann Surg 1964;160:411–439, with permission.)
These findings, which finalized the decision to proceed clinically with liver replacement, were not fully explained until the mid-1970s. Eventually, it was established that endogenous insulin was the most important ”hepatotrophic“ factor in normal portal blood.80,81 This was a decisive step in understanding the pathophysiology of Eck’s fistula (portacaval shunt).81,82
Improved organ preservation
The potential pitfall of organ preservation remained. It would still be necessary to obtain livers from nonheartbeating donors. To help surmount this difficulty, we developed an ex vivo perfusion system in 1966 and 1967 that permitted reliable preservation of canine livers for as long as a day. The effort was spearheaded by a young naval surgeon, Larry Brettschneider.83 Now, it was time to try again.
Resumption of human liver replacement
When the liver program reopened in July, 1967, it had another weapon. This was an enthusiastic 2-year NIH-supported Fellow from Stockholm named Carl Groth (Fig. 36). Groth who was determined to succeed, and had no doubt that this would be possible, was a key member of both donor and recipient surgical teams. He also took charge of the post-transplant management team in a continuous vigil that lasted for many months. By the end of the year, multiple examples of prolonged human liver recipient survival had been produced, under triple drug immunosuppression: azathioprine, prednisone, and ALG84 (Fig. 37). The liver transplant beachhead was reinforced by the opening of Roy Calne’s clinical program in Cambridge, England, in February 1968.8 In 1969, a companion to the 1964 kidney transplant book was published, entitled Experience in Hepatic Transplantation,85 based on our first 25 liver replacements and 8 performed elsewhere (4 by Calne).
Figure 36.

Carl Groth (1933–), current president of the International Transplantation Society. After two stints at the University of Colorado (1966–1968 and 1971–1972), Groth returned to Stockholm as the founding chairman of the first Department of Transplantation in the world located at the Huddinge Hospital of the Karolinska Institute, Stockholm. He retired in 2000.
Figure 37.
The first three human recipients to have prolonged Survival after liver replacements in July and August, 1967 . The adult, Carl Groth, was then an NIH-supported Fellow.
Transplantation of other extrarenal organs followed close behind the liver, using similar immunosuppression (Table 4). Hearts were successfully transplanted in 1968 in Capetown by Barnard86 and in Palo Alto by Shumway.87 In 1969, the first prolonged survival after human lung88 and pancreas transplantation89 was accomplished in Ghent and Minneapolis, respectively. But transplantation of the extrarenal organs, and especially of the liver, remained controversial for another decade, because of the high mortality. Swimming against the stream, the German and French teams of Rudolf Pichlmayr and Henri Bismuth entered the field in the early 1970s, as did the Dutch group of Rudi Krom later in the decade.
Table 4.
First Successful Transplantation of Human Allografts (Survival > 6 Months)
| Organ | City | Date | Physician/surgeon | Reference |
|---|---|---|---|---|
| Kidney | Boston | 1/24/59 | Merrill/Murray | 40 |
| Liver | Denver | 7/23/67 | Starzl | 84 |
| Heart | Capetown | 1/2/68 | Barnard/Shumway | 86,87 |
| Lung* | Ghent | 11/14/68 | Derom | 88 |
| Pancreas† | Minneapolis | 6/3/69 | Lillehei | 89 |
Patient died after 10 months; all others in table lived more than 1 year with functioning graft. The first survival longer rhan 1 year of isolated lung recipients was not reponed until 1987.
Kidney and pancreas allografts in uremic patient.
The unusual tolerogenicity of the hepatic allograft previously demonstrated in dogs and pigs was evident in human liver recipients of the 1970s. In 1995, 12 of our 42 patients (28%) surviving from this era already had been off all immunosuppression for 1 to 17 years.90 Since then, many of the remaining 30, who are now out to 33 years post-transplantation, also have stopped drugs and remain well.91,92 Such drug-free tolerance was almost unheard of with the other kinds of cadaveric organs.
Advent of better drugs
Despite such encouraging notations, the widespread use of the liver and other extrarenal organs was precluded for another decade by the high mortality. The outlook for all organs improved after cyclosporine was introduced clinically in England in 1978 by Calne,93 and combined with prednisone in Denver 1 year later.94 Results further improved when tacrolimus was substituted for cyclosporine in the 1990s.95
The increases in liver recipient survival with the two new drugs were particularly striking,96,97 but less dramatic gains were recorded with the other organs. By the end of the 20th century, transplantation of the liver and all of the other vital organs had become an integral part of sophisticated medical practice in every developed country in the world. There was, however, one nagging disappointment.
With better immunosuppressants, drug-free liver recipients were expected to become common. Yet, the ultimate prize of tolerance was achieved less frequently than before, and only when liver transplantation was carried out during three time periods. The first two in-tervals were 1979-1980 and 1989-1990, just after cyclosporine 96 and tacrolimus,95,97 respectively, were introduced clinically as monotherapy, adding prednisone only to treat rejection.
When prophylactic high doses of prednisone and other agents were added at the time of operation in an effort to further reduce the threat of acute rejection, drug-free tolerance was no longer seen. The third cluster of tolerant patients was produced at the turn of the century in Kyoto, Japan, where many pediatric recipients of partial livers from parental donors were successfully weaned from a steroid-sparing tacrolimus-based regimen of immunosuppression98 similar to that originally used in 1989-1990.97
Mechanisms of engraftment-tolerance: History revisited
Despite these ”proof of feasibility“ observations, the goal of deliberate induction of drug-free tolerance would remain out of reach until the mechanisms leading to this state were understood. Insight into the mechanisms began to emerge in 1992 with the study up to 30 years post-transplantation of 5 kidney and 25 liver recipients.
The kidney recipients were survivors from the 1962-1967 era that has been the main focus of this review; most of the liver recipients had undergone transplantation in the 1970s. Samples were obtained of the allografts and of host blood, skin, lymph nodes, and, in some cases, other organs such as bowel and heart. In all 30 patients, including several who had long since stopped all immunosuppression, small numbers of donor hematolymphopoietic cells were demonstrated in blood or tissues,99,100 a condition known as donor leukocyte chimerism.
These findings prompted numerous studies in animal models,101-109 and ultimately led to a collaboration with RolfZinkernagel of Zurich (Fig. 38) whose 1996 Nobel prize was for studies of the adaptive immunity responsible for defense against noncytopathic microorganism and for rejection. 110 At the end, the chimerism-associated mechanisms of organ and bone marrow cell engraftment, and of acquired tolerance, were delineated. 111,112 Only then was it possible to fully explain the meaning of the historical events that I have been describing. More importantly, a map for the future of organ transplantation could be drawn.
Figure 38.

Rolf Zinkernagel (1944–). Swiss physician-immunologist whose discovery (with Peter Doherty) of the mechanisms of the adaptive immune response to noncytopathic microorganisms earned the Nobel prize in 1996.
In essence, organ engraftment is a dynamic process initiated and governed by the migration and localization of the passenger leukocytes of bone marrow origin (ie, hematolymphopoietic cells) that are normal constituents of all tissues and organs. The donor leukocytes migrate preferentially to the host lymphoid organs104,113-115 (Fig. 39, left), where they induce an antigraft T-cell response. Without immunosuppression, this response proceeds to rejection in humans and in most animal models.
Figure 39.

Initial preferential migration of passenger leukocytes from organ allografts to host lymphoid organs (left). After about 30 days, many of the surviving cells move on to nonlymphoid sites (right).
In a few experimental models of spontaneous tolerance, however, or under the appropriate conditions of immunosuppression, the immune response is too weak to eliminate the migratory donor leukocytes and regresses. Collapse of the response occurs when the activated host T-cell clone reaches a proliferation limit and is exhausted and deleted by incompletely understood mechanisms that include apoptosis109 (Fig. 40). Clonal exhaustion-deletion is the seminal mechanism of organ engraftment and of acquired tolerance.99,100,111,112,116 Because the exhaustion is never complete, maintenance of the variable deletional tolerance achieved at the outset depends on the persistence of residual stimulatory donor leukocytes. 111,112 These were the cells discovered up to 30 years after transplantation in our pioneer organ recipients.
Figure 40.
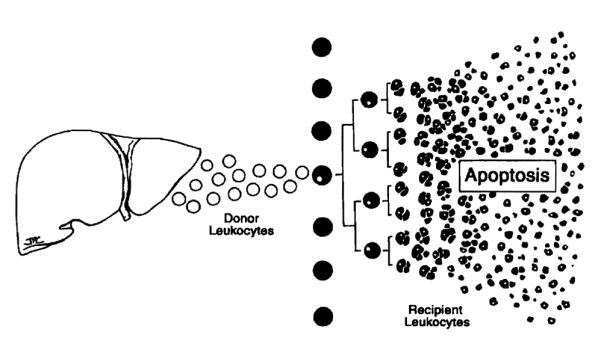
Clonal exhaustion-deletion, the seminal mechanism of organ engraftment, and of acquired tolerance. Persistence of some donor cells is a prerequisite for maintenance of the variable deletional tolerance induced by the initial surge of donor cells.
The important point is that allograft rejection and clonal exhaustion-deletion are products of the same donor-specific immune response. The close relation of rejection and deletional tolerance is exemplified by the results with pig liver transplantation in the mid-1960s (see earlier).74-78 The immune response reported in the majority of untreated pig liver recipients proceeds to rejection (Fig. 41A, ascending thick arrow). In a significant minority of experiments, how-ever, the response is exhausted and deleted (Fig. 41A, decline of the thin-line curve). Unlike the liver, pig kidneys and other porcine organs fail to induce tolerance because they are endowed with a smaller quantity of passenger leukocytes.
Figure 41.
Rather than producing rejection (thick arrows), the donor-specific immune response may be exhausted and deleted as depicted by the fall of the initially ascending continuous thin lines when: (A) The unmodified recipient response is too weak to eliminate the migratory donor cells (spontaneous tolerance). (B) Recipient immune responsiveness is weakened by cytoablation or cytoreduction in advance of transplantation. (C) The recipient response is reduced into the deletable range by immunosuppression (black bar) after transplantation. When excessive immunosuppression (multilayered bars) is given after arrival ofthe allograft. In contrast. panel D shows how over-treatment reduces the efficiency and extent of clonal exhaustion-deletion and is therefore anti-tolerogenic (see text). Horizontal axis: time. Tx: transplantation.
In contrast to its unpredictable occurrence in un-treated pigs, spontaneous tolerance invariably is induced by hepatic allografts in numerous inbred rodent modelS.103,117,118 This is not, however, an exclusive quality the liver. Spontaneous tolerance also can be induced in experimental rodent models by less well leukocyte-endowed heart119,120 and kidney allografts,121,123 albeit less commonly.
In experimental models in which organ allograft rejection by the unmodified recipient is the normal out-come, the passenger leukocyte-induced response can be reduced enough to be deleted by treating the recipient prior to transplantation (Fig. 41B). That almost certainly is what Murray and colleagues40 and Hamburger and associates41 accomplished with pretransplant total body irradiation of their historic fraternal twin kidney recipients of 1959. Less drastic pretreatment is possible with thoracic duct drainage,124,125 total lymphoid irradiation,126 conventional antirejection drugs,57,125 and especially the antilymphoid antibody preparations.71-73,127,128 Prototypes of effective antilymphoid preparations that have been used for pretreatment include the polyclonal ALGs of the kind developed and introduced clinically during the liver transplant moratorium of 1964–1967,70 and the humanized monoclonal ALG, alemtuzumab that has been extensively evaluated clinically by Calne and colleagues.129,130
The antigraft response also can be brought into the deletable range by immunosuppression given after transplantation (Fig. 41 C); This, though, is a doubleedged sword. If antigen-specific immune activation is unduly depressed (Fig. 41D), the derivative event exhaustion-deletion also is eroded. When immunosuppression is reduced later in an initially over-treated recipient, the unde1eted donor-specific clone recovers along with the return of global immune reactivity.106,112 Graft survival is then dependent on permanent immunosuppression.
Two principles of tolerogenic immunosuppression derive from this paradigm.112 The first is the value of recipient pretreatment. The second is the importance of limiting post-transplant immunosuppression to the minimum needed to prevent irreversible immune damage to the transplanted organ. In this view, the primary role of immunosuppression is not to eliminate the antigraft response as has been commonly assumed, but rather to lower it into the deletable range.
Both therapeutic principles had been observed empirically in the early Denver renal transplant experience that resulted in a unique cohort of drug-free kidney recipients and prompted the first liver trial. Pretreatment of these patients as well as minimal post-transplant immunosuppression was given with azathioprine, adding prednisone temporarily only to treat rejection (Fig. 25). No comparable series of tolerant kidney recipients was compiled anywhere in the world during the next 40 years. What had changed?
First, pretreatment of kidney recipients was deemphasized or abandoned in December 1963 after infectious complications occurred in the preoperative period (Fig. 42). Second, high doses of prednisone were instituted at the time of transplantation because of the excessive loss of kidney allografts to uncontrollable rejection (Fig. 42). Although heavy prophylactic immunosuppression has dominated transplantation practice ever since (Fig. 41D), the superior tolerogenic qualities of hepatic allografts continued to result in a handful of drug-free tolerant liver recipients, but only in isolated periods when restrictive posttransplant immunosuppression was used.
Figure 42.

Treatment revisions in immunosuppression made at the University of Colorado in December 1963. that unwittingly violated both principles of tolerogenic immunosuppression shown in Figure 43. Pretreatment was deemphasized or eliminated. and high doses of prednisone were given prophylactically instead of as needed. Acute rejection was frequently prevented. but drug-free tolerance was no longer seen.
Back to the future
With the insight into the mechanisms of engraftment that I have described, we have applied both therapeutic principles in more than 200 cases since July, 2001. Kidney, liver, pancreas, and intestinal recipients were pretreated with a single large dose of a potent ALG, and then administered conservative doses of tacrolimus after transplantation. No other immunosuppression was given unless specifically indicated for rejection (Fig. 43).
Figure 43.

Current protocol of immunosuppression in which pretreatment is given with a large dose of a potent ALG (thymoglobulin) followed by tacrolimus monotherapy to which other agents are added only for rejection. See Figure 41 and text for details. P.R.N,. as needed; Tx, transplantation.
Half of these patients have been weaned after 4 months to only one, two, or three doses per week of tacrolimus. Many are expected to become drug free in their second post-transplant year. These results suggest that a. high, if not absolute, degree of sustained donor specific nonreactivity (ie, tolerance) can be expected after the transplantation of all organs. So, the next, and perhaps the most gratifying, phase of transplantation may already have begun. But, of course, that will be a story for future Congresses of the American College of Surgeons.
Postscript
That concludes my remarks except for a postscript. In the early 1960s, Sir Peter Medawar, Nobel laureate and founder of the discipline of transplantation immunology, wrote the following:
Good scientists study the most important problems they think they can solve. It is, after all, their professional business to solve problems, not merely to grapple with them. The spectacle of a scientist locked in combat with the forces of ignorance is not an inspiring one if, in the outcome, the scientist is routed.131
Medawar was referring to the search for the “holy grail” of transplantation, the secret that would allow organ recipients to be rendered drug-free tolerant. Thousands of scientists joined the crusade, driven by the false premise that successful engraftment after organ transplantation occurred by fundamentally different mechanisms than the donor leukocyte chimerism-associated ones of bone marrow transplantation and of acquired tolerance. This assumption was not challenged for the next third of a century.
All the while, the historical observations that I have recounted pointed in a different direction, and in the end these observations were at least as important in arriving at the truth as the results from reductionist investigations. The lesson is clear. History is neither dull nor dead. It is a uniquely human survival tool, aiding those in the present by the ability to draw on the past to meet current needs, and to predict needs yet to come.
Footnotes
Presented at the American College of Surgeons, 88th Annual Clinical Congress, San Francisco, CA, October 2002.
REFERENCES
- 1.Brent L. A history of transplantation immunology. Academic Press; London: 1997. pp. 1–482. [Google Scholar]
- 2.Hamilton D. Towards the impossible. Lippincott Williams & Wilkins; Philadelphia: 2003. (in press) [Google Scholar]
- 3.Welch CS. A note on transplantation of the whole liver in dogs. Transplant Bull. 1955;2:54–55. [Google Scholar]
- 4.Goodrich EO, Jr, Welch HF, Nelson JA, et al. Homocransplanration of the canine liver. Surgery. 1956;39:244–251. [PubMed] [Google Scholar]
- 5.Meyer WH, Jr, Starzl TE. The reverse portacaval shunt. Surgery. 1959;45:531–534. [Google Scholar]
- 6.Meyer WH, Jr, Starzl TE. The effect of Eck and reverse Eck fistula in dogs with experimental diabetes mellitus. Surgery. 1959;45:760–764. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Bernhard VM, Benvenuto R, Cortes N. A new method for one-stage hepatectomy in dogs. Surgery. 1959;46:880–886. [PMC free article] [PubMed] [Google Scholar]
- 8.Calne RY, Williams R. Liver transplantation in man. 1. Observations on technique and organization in five cases. Br Med J. 1968;4:535–540. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Putnam CW. Experience in hepatic transplantation. WB Saunders Company; Philadelphia: 1969. pp. 131–135. with the assistance of. [Google Scholar]
- 10.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Kaupp HA, Jr, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon JA, Longmire WP. Studies of successful skin homografts in the chicken. Ann Surg. 1952;135:60–68. doi: 10.1097/00000658-195201000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE. History of clinical transplantation. World J Surg. 2000;24:759–782. doi: 10.1007/s002680010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon JA. Brief report. Transplant Bull. 1956;3:7. [Google Scholar]
- 15.Moore FD, Smith LL, Burnap TK, et al. One-srage homotransplantation of the liver following total hepatectomy in dogs. Transplant Bull. 1959;6:103–110. doi: 10.1097/00006534-195901000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374–385. (see also discussion of Moore’s presentation by Starzl TE. 1960;152:386.
- 17.Lillehei RC, Goott B, Miller FB. The physiologic response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–560. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lower RR, Shumway NE. Studies in orthotopic homotransplantation of the canine heart. Surg Forum. 1960;11:18–19. [PubMed] [Google Scholar]
- 19.Benichou J, Halgrimson CG, Weil R, III, et al. Canine and human liver preservation for 6-18 hours by cold infusion. Transplantation. 1977;24:407–411. doi: 10.1097/00007890-197712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wall WJ, Calne RY, Herbertson BM, et al. Simple hypothermic preservation for transporting human livers long distances for transplantation. Transplantation. 1977;23:210–216. doi: 10.1097/00007890-197703000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson NV, Sundberg R, Lindell S, et al. Successful 24- to 30-hour preservation of the canine liver: A preliminary report. Transplant Proc. 1988;29(suppl 1):945–947. [Google Scholar]
- 22.Kalayoglu M, Sollinger WH, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 23.Todo S, Nery J, Yanaga K, et al. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 24.Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation: Initial perfusion and 30 hours ice storage. Lancet. 1969;2:1219–1224. doi: 10.1016/s0140-6736(69)90753-3. [DOI] [PubMed] [Google Scholar]
- 25.Schalm SW. Thesis. University of Leyden; Holland: 1968. A simple and clinically applicable method for the preservation ofa liver homograft. [Google Scholar]
- 26.Starzl TE, Kaupp HA, Jr, Brock DR, Linman JW. Studies on the rejection of the transplanted homologous dog liver. Surg Gynecol Obstet. 1961;112:135–144. [PMC free article] [PubMed] [Google Scholar]
- 27.McBride RA, Wheeler HB, Smith LL, et al. Homotransplantation of the canine liver as an orthotopic vascularized graft. Histologic and functional correlations during residence in the new host. Am J Pathol. 1962;41:501–515. [PMC free article] [PubMed] [Google Scholar]
- 28.Strarzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 29.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in hepatic transplantation. WB Saunders Company; Philadelphia: 1969. pp. 422–471. [Google Scholar]
- 30.Porter KA. Pathological changes in transplanted kidneys. In: Starzl TE, editor. Experience In renal transplantation. WB Saunders Company; Philadelphia: 1964. pp. 299–359. [Google Scholar]
- 31.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzi TE, Kaupp HA, Jr, Brock DR, et al. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murase N, Demetris AJ, Kim DG, et al. Rejection of the multivisceral allografts in rats: A sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880–889. [PMC free article] [PubMed] [Google Scholar]
- 34.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 35.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270–282. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 38.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 39.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of a single center experience. Ann Surg. 2001;234:404–416. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray JE, Merrill JP, Dammin GJ, et al. Study of transplantation immunity after total body irradiation: Clinical and experimental investigation. Surgery. 1960;48:272–284. [PubMed] [Google Scholar]
- 41.Hamburger J, Vaysse J, Crosnier J, et al. Renal homotransplantation in man after radiation of the recipient. Am J Med. 1962;32:854–871. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 42.Küss R, Legrain M, Math‘e G, et al. Homologous human kidney transplantation. Experience with six patients. Postgrad Med J. 1962;38:528–531. doi: 10.1136/pgmj.38.443.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodwin WE, Kaufman JJ, Mims MM, et al. Human renal transplantation. I. Clinical experience with six cases of renal homotransplantation. J Urology. 1963;89:13–24. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 44.Starzl TE, Butz GW, Jr, Brock DR, et al. Canine liver homotransplants: The effect of host and graft irradiation. Arch Surg. 1962;85:460–464. doi: 10.1001/archsurg.1962.01310030108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz R, Dameshek W. The effects of 6-mercaptopurine on homograft reactions. J Clin Invest. 1960;39:952–958. doi: 10.1172/JCI104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meeker W, Condie R, Weiner D, et al. Prolongation of skin homograft survival in rabbits by 6-mercaptopurine. Proc Soc Exp Biol Med. 1959;102:459–461. [Google Scholar]
- 48.Calne RY. The rejection of renal homografts: Inhibition in dogs by 6-mercaptopurine. Lancet. 1960;1:417–418. doi: 10.1016/s0140-6736(60)90343-3. [DOI] [PubMed] [Google Scholar]
- 49.Zukoski CF, Lee HM, Hume DM. The prolongation of functional survival of canine renal homografts by 6-mercaptopurine. Surg Forum. 1960;11:470–472. [PubMed] [Google Scholar]
- 50.Calne RY. Inhibition of the rejection of renal homografts in dogs by purine analogues. Transplant Bull. 1961;28:445–461. [PubMed] [Google Scholar]
- 51.Calne RY, Alexandre GPJ, Murray JE. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann NY Acad Sci. 1962;99:743–761. doi: 10.1111/j.1749-6632.1962.tb45358.x. [DOI] [PubMed] [Google Scholar]
- 52.Zukoski CF, Callaway JM. Tolerance to a canine renal homograft induced by 6-methyl mercaptopurine. Surg Forum. 1962;13:62–64. [PubMed] [Google Scholar]
- 53.Murray JE, Merrill JP, Dammin GJ, et al. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337–355. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray JE, Merrill JP, Harrison JH, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. New Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 55.Hopewell J, Calne RY, Beswick I. Three clinical cases of renal transplantation. Brit Med J. 1964;I:411–413. doi: 10.1136/bmj.1.5380.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marchioro TL, Axtell HK, LaVia MF, et al. The role of adrenocortical steroids in reversing established homograft rejec-tion. Surgery. 1964;55:412–417. [PMC free article] [PubMed] [Google Scholar]
- 57.Starzl TE. Experience in renal transplantation. WB Saunders Company; Philadelphia: 1964. pp. 131–133. [Google Scholar]
- 58.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografrs with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 59.Cecka JM, Terasaki PI. Clinical transplants 2001. UCLA Immunogenetics Center; Los Angeles CA: 2002. World transplant records; p. 279. [Google Scholar]
- 60.Starzl TE, Marchioro TL, Von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 61.Marchioro TL, Waddell WR, Starzl TE. Use of extracorporeal cadaver perfusion for preparation of organ homografts. Surg Forum. 1963;14:174–176. [PMC free article] [PubMed] [Google Scholar]
- 62.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Starzl TE, Marchioro TL, Rowlands DT, Jr, et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411–439. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore FD, Smith LL, Shoemaker WC, et al. One-stage homotransplantation of the canine liver after hepatectomy. Societe Internationale de Chirurgie VXIII Congres; Munich, Bruzelles. Imprimerie Medical et Scientifique; 1959. p. 337. [Google Scholar]
- 66.Demirleau, Noureddine, Vignes, et al. Tentative d’homogreffe hepatique. [Attempted hepatic homograft.] Mem. Acad. Chir (Paris) 1964;90:177. [PubMed] [Google Scholar]
- 67.Starzl TE. Experience in renal transplantation. WB Saunders Co; Philadelphia: 1964. pp. 1–383. [Google Scholar]
- 68.Terasaki PI, Marchioro TL, Starzl TE. Histocompatibility testing. National Acad Sci-National Res Council; Washington, DC: 1965. Sero-typing of human lymphocyte antigens: Preliminary trials on long-term kidney homograft survivors; pp. 83–96. [Google Scholar]
- 69.Terasaki PI, Vredevoe DL, Mickey MR, et al. Serotyping for homotransplantation. VI. Selection of kidney donors for thirty-two recipients. Ann NY Acad Sci. 1966;129:500–520. doi: 10.1111/j.1749-6632.1966.tb12873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starzl TE, Porter KA, Andres G, et al. Long-term survival after renal transplantation in humans: With special reference to histocompatibility matching, thymectomy, homograft glomerulonephritis, heterologous ALG, and recipient malignancy. Ann Surg. 1970;172:437–472. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 72.Woodruff MFA, Anderson NF. Effectoflymphocyte depletion by thoracic duct fistula and administration of antilymphocytic serum on the survival of skin homografts in rats. Nature (London) 1963;200:702. doi: 10.1038/200702a0. [DOI] [PubMed] [Google Scholar]
- 73.Monaco AP, Wood ML, Gray JG, Russell PS. Studies on heterologous antilymphocyte serum in mice-II, effect on the immune response. J Immunol. 1966;96:229. [PubMed] [Google Scholar]
- 74.Cordier G, Garnier H, Clot JP, et al. La greffe de foie orthotopique chez le porc. Mem Acad Chir (Paris) 1966;92:799–807. [PubMed] [Google Scholar]
- 75.Calne RY, White HJO, Yoffa DE, et al. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967;2:478–480. doi: 10.1136/bmj.2.5550.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peacock JH, Terblanche J. Orthotopic homotransplantation of the liver in the pig. In: Read AE, editor. The liver. Butterworth; London: 1967. p. 333. [Google Scholar]
- 77.Starzl TE. Experience in hepatic transplantation. WB Saunders Company; Philadelphia: 1969. pp. 184–190. [Google Scholar]
- 78.Calne RY, Sells RA, Pena, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 79.Marchioro TL, Porter KA, Dickinson TC, et al. Physiologic requirements for auxiliary liver homotransplantation. Surg Gynecol Obstet. 1965;121:17–31. [PMC free article] [PubMed] [Google Scholar]
- 80.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 81.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 82.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:687–752. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brettschneider L, Daloze PM, Huguet C, et al. The use of combined preservation techniques for extended storage of orthotopic liver homografts. Surg Gynecol Obstet. 1968;126:263–274. [PMC free article] [PubMed] [Google Scholar]
- 84.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Starzl TE. Experience in hepatic transplantation. WB Saunders; Philadelphia: 1969. pp. 1–553. [Google Scholar]
- 86.Barnard CN. What we have learned about heart transplants. J Thorac Cardiovasc Surg. 1968;56:457–468. [PubMed] [Google Scholar]
- 87.Stinson EB, Griepp RB, Clark DA, et al. Cardiac transplantation in man. VIII. Survival and function. J Thorae Cardiovasc Surg. 1970;60:303–321. [PubMed] [Google Scholar]
- 88.Derom F, Barbier F, Ringoir S, et al. Ten-month survival after lung homotransplantation in man. J Thorac Cardiovasc Surg. 1971;61:835–846. [PubMed] [Google Scholar]
- 89.Lillehei RC, Simmons RL, Najarian JS, et al. Pancreaticoduodenal allotransplantation: Experimental and clinical observations. Ann Surg. 1970;172:405–436. doi: 10.1097/00000658-197009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starzl TE, Demetris AJ, Murase N, et al. The lost chord: Microchimerism. Immunol Today. 1996;17:577–584. 588. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazariegos GV, Reyes J, Marino I, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs; 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 94.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 95.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Starzl TE, Klintmalm GBG, Porter KA, et al. Liver transplantation with use of cyclosporin A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takatsuki M, Uemoto SH, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 99.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 101.Demetris AJ, Murase N, Fujisaki S, et al. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplantation Proceedings. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 102.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terakura M, Murase N, Demetris AJ, et al. Lymphoid/non-lymphoid compartmentalization of donor leukocyte chimerism in rat recipients of heart allografts, with or without adjunct bone marrow. Transplantation. 1998;66:350–357. doi: 10.1097/00007890-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 105.Sakamoro T, Ye Q, Lu L, et al. Donor hemaropoietic progenitor cells in non myeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67:833–840. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ichikawa N, Demetris AJ, Starzl TE, et al. Donor and recipient leukocytes in organ allografts of recipients with variable donor-specific tolerance: with particular reference ro chronic rejection. Liver Transpl. 2000;6:686–702. doi: 10.1053/jlts.2000.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colonystimulating facror. J Exp Med. 1995;182:379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehl S, Aichele P, Ramseier H, et al. Antigen persistence and time ofT-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection. Nat Med. 1998;4:1015–1019. doi: 10.1038/2001. [DOI] [PubMed] [Google Scholar]
- 109.Qian S, Lu L, Fu F, et al. Apoprosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 110.Zinkernagel RM, Doherty Pc. The discovery ofMHC restriction. Immunology Today. 1997;18:14–17. doi: 10.1016/s0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 111.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. New Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Nature Reviews: Immunology. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nemlander A, Soots A, von Willebrand E. Redistribution of renal allograft- responding leukocytes during rejection. II. Kinetics and specificity. J Exp Med. 1982;156:1087–1100. doi: 10.1084/jem.156.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel route for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Demetris AJ, Qian S, Sun H, et al. Early events in liver allograft rejection: Delineation of sites simultaneous intragraft and recipient lymphoid tissue sensitization. Am J Pathol. 1991;138:609–618. [PMC free article] [PubMed] [Google Scholar]
- 116.Bishop GA, Sun J, DeCruz DJ, et al. Tolerance to rat liver allografts. III. Donor cell migration and toletance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156:4925–4931. [PubMed] [Google Scholar]
- 117.Kamada N, Brons G, Davies HffS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 118.Zimmerman FA, Davies HS, Knoll PP, et al. Orthotopic liver allografts in the rat. Transplantation. 1984;37:406–410. doi: 10.1097/00007890-198404000-00019. [DOI] [PubMed] [Google Scholar]
- 119.Corry RJ, Winn HJ, Russell PS. Primary vascularized allografts of hearts in mice: the role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 120.Qian S, Demetris AJ, Murase N, et al. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salaman JR. Renal transplantation between two strains of rats. Nature. 1968;220:930–931. doi: 10.1038/220930a0. [DOI] [PubMed] [Google Scholar]
- 122.Salaman JR. Prolonged survival of renal allografts in rats mismatched at the major histocompatibility locus. Transplantation. 1971;11:63–70. doi: 10.1097/00007890-197101000-00009. [DOI] [PubMed] [Google Scholar]
- 123.Russell PS, Chase CM, Colvin RB, Plate JMD. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term H-2 incompatible transplants. J Exp Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Franksson C, Blomstrand R. Drainage of the thoracic lymph duct during homologouos kidney transplantation in man. Scand J Urol Nephrol. 1967;1:123–131. [Google Scholar]
- 125.Starzl TE, Weil R, III, Koep LJ. The pretreatment principle in renal transplantation as illustrated by thoracic duct drainage. In: Cummings NB, Klahr S, editors. Chronic renal disease. Plenum Publishing Corporation; New York: 1985. pp. 507–515. [Google Scholar]
- 126.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459–467. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 128.Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in many after conditioning by antilymphocytic serum. Brit Med J. 1970;2:131–136. doi: 10.1136/bmj.2.5702.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calne RY, Friend PJ, Moffatt S, et al. Prope tolerance, perioperative campath IH, and low- dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 130.Calne RY, Moffatt SD, Friend PJ, et al. Campath IH allows low- dose cyclosporin monotherapy in 31 cadaveric renal allo-graft recipients. Transplantation. 1999;68:1613–1616. doi: 10.1097/00007890-199911270-00032. [DOI] [PubMed] [Google Scholar]
- 131.Medawar PB. In: A history of transplantation immunology. Brent L, editor. Academic Press; London: 1997. p. 230. [Google Scholar]