In contrast to kidney and heart, liver transplantation does not seem to benefit from HLA-matching in most patients. Furthermore, lower graft survival rates have been reported when HLA compatibility between donor and recipient is present (1–5). Since HLA mismatching contributes to liver allograft rejection, we have proposed a dualistic role of HLA in liver transplantation (1). HLA-matching reduces acute cellular rejection but augments other immunologic mechanisms of allograft injury, especially those mediated by major histocompatibility complex (MHC)*-restricted lymphocytes. In support of this concept, we have recently reported a higher incidence of CMV hepatitis in HLA-DR matched liver transplants, which was also associated with a higher frequency and earlier onset of chronic rejection (6).
Hepatitis B and C virus (HBV, HCV) infections are a major cause of end-stage liver disease requiring liver transplantation. However, both viruses are difficult to eradicate, ensuring that the viral infection will persist and that recurrent hepatitis will occur in many recipients (7, 8). In the present study we investigated whether HLA matching affects the recurrence of HBV and HCV hepatitis after liver transplantation.
From January 1991 to July 1992, 98 adult patients underwent orthotopic liver transplantation (OLTx) because end-stage liver disease secondary to HBV and/or HCV infections. Of these, 9 (9%) died during the first month after OLTX. The remaining 89 patients constituted the study group. Thirty-one patients (35%) had hepatitis B, 53 (59%) had hepatitis C, and 5 (6%) had B and C hepatitis. All hepatitis B patients were HBsAg-positive and HBV DNA-negative before transplantation. HBeAg was available for 23 patients, and 17 of them (74%) tested positive. Hepatitis C diagnosis was performed by the presence of anti-HCV antibodies in the serum by a first-generation ELISA (Abbot Laboratories, Abbot Park, IL) and HCV RNA detection in the original liver by reverse transcriptase polymerase chain reaction (PCR). HLA typing of patients and donors was done by standard serological techniques based on lymphocytotoxicity testing with sera against HLA-A, B, DR, and DQ antigens.
Primary immunosuppression included in all patients tacrolimus (FK506) and steroids. Tacrolimus was initially given in a continuous infusion at 0.1 mg/kg/day, which was converted to an oral dose of 0.15 mg/kg/day every 12 hr with the return of bowel function. Subsequent dosage adjustments were guided by the quality of the graft, the presence of rejection, toxicity, and tacrolimus plasma trough level (normal value: <2 ng/ml). Rejection episodes were treated with a 1-g pulse of methylprednisolone or a 5-day taper of high-dose steroids from 200 to 20 mg. Steroid-resistant rejection episodes were treated with a 5-day course of OKT3.
During a follow-up of between 18 and 24 months, 45 of the 89 (51%) liver transplant recipients had recurrence of hepatitis at (mean ± SD) 259 ± 174 days after transplantation. Recurrence of hepatitis was defined by the presence of portal and parenchymal mononuclear infiltration and isolated hepatocyte necrosis, as evidenced by acidophilic bodies in histopathology. In addition, diagnosis hepatitis B recurrence required the expression of HBsAg and HBcAg by liver cells, determined by immunoperoxidase techniques, along with positive HBsAg in the serum. The diagnosis of hepatitis C recurrence required the presence of anti-HCV antibodies by a second-generation ELISA (Abbot Laboratories, Abbot Park, IL), and detection of HCV RNA in the liver biopsy. In 13 of the 31 patients with recurrent hepatitis C, HCV RNA was also investigated in serum specimens, and all them were positive. Other causes of liver dysfunction, such as rejection, drug toxicity, or viral infections by the herpesvirus group (CMV, EBV and herpes simplex), were excluded.
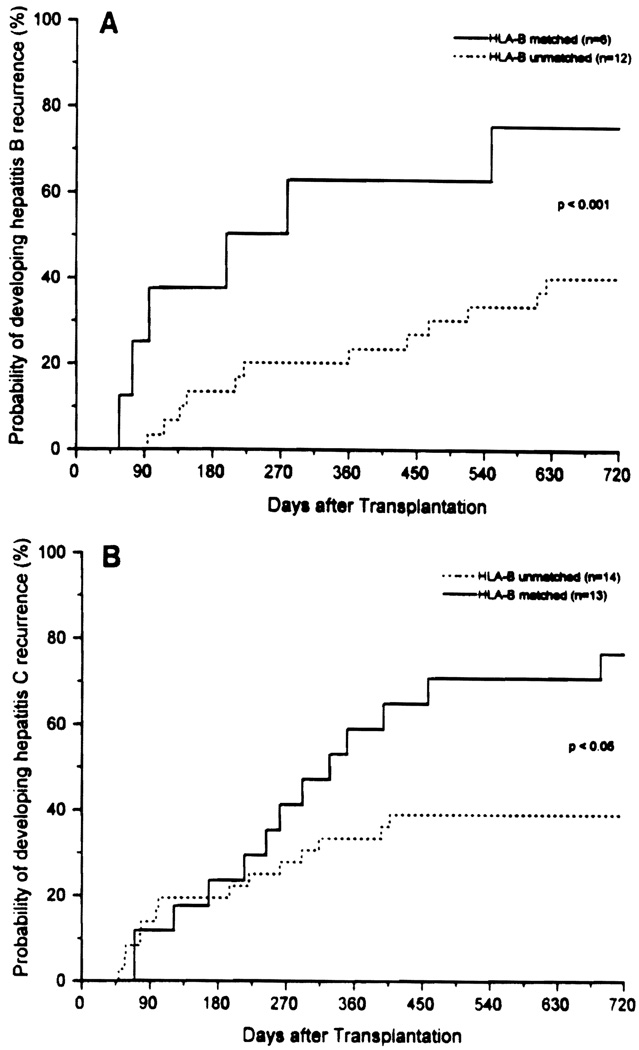
Fourteen of 31 patients (45%) with HBV infection—27 of 53 (51%) with HCV and 4 of 5 (80%) with HBV and HCV infections—had recurrent hepatitis. The incidence of recurrence of hepatitis was significantly higher for HLA-B-compatible liver transplants recipients. Nineteen of the 45 patients (42%) with recurrent hepatitis shared at least one HLA-B antigen with the donor. In contrast, HLA-B sharing was found in 9 of 44 patients (20%) without recurrent hepatitis (P<0.05). One or two HLA-B antigen matching increased the relative risk (Odds ratio) of recurrent hepatitis 2.8 times. There seemed to be no preferential sharing of any particular HLA-B antigen in the recurrent hepatitis group. There were no differences between HLA-B-matched and -unmatched patients in terms of severity of recurrent hepatitis. No significant associations were found between recurrence of hepatitis and donor-recipient sharing of HLA-A, HLA-DR, and HLA-DQ antigens. Kaplan-Meier analysis showed that HLA-B matching significantly increased the probability of developing recurrent hepatitis from 40% to 75% at two years in patients with HBV infection (patients with HBV and HCV infection are included in this group) (Fig. 1A), and from 39% to 76% in patients with HCV infection (Fig. 1B).
FIGURE 1.
The probability of developing hepatitis recurrence in HLA-B-matched and -unmatched liver transplant recipients with HBV (A) and HCV (B) infection.
These observations suggest that HLA-B sharing between donor and recipient promotes the recurrence of HBV and HCV hepatitis in liver transplant recipients. Our results confirm previous studies showing that HLA class I matching between donor and recipient is associated with the development of hepatic lesions in recurrent HBV infection after liver transplantation (9). Infection of the liver allograft by HBV and HCV is a frequent event in liver transplant recipients who are HBV or HCV carriers. However, a dissociation between the virus load and replication and the severity of hepatic lesions has been observed in liver transplant recipients (10, 11). This occurs even in the presence of increasing viral titers after liver transplantation (12). These observations are similar to the existence of an HBV or HCV carrier state for many years without evidence of liver damage (13). Both suggest that the viruses are not cytopathic by themselves and that immune-mediated mechanisms are the most important factor in liver injury.
Our results suggest that MHC restriction of antigen-specific lymphocyte reactivity might be involved in the allograft damage by HBV and HCB. It has been shown that HBV and HCV infections increase the expression of HLA class I antigens on hepatocytes surface (14, 15). Although exogenous antigens and viral particles are in general associated with a class II MHC–restricted response, there is evidence that HBV and HCV are among the few antigens presented in association with class I molecules (16). Thus, liver-infiltrating HBV and HCV specific lymphocytes might be HLA class I–restricted (17, 18). Infection of a transplanted liver by HBV or HCV leads to the expression of viral antigens, which—in context with MHC class I molecules on the cell surface—could be recognized by recipient T lymphocytes. HLA matching would permit a more efficient MHC-restricted antigen presentation, thereby augmenting cell-mediated immune responses toward HBV- and HCV-infected liver allografts. Thus, theoretically immunosuppressive therapy should reduce liver injury mediated by immune mechanisms (19). However, it also increases virus replication, which may be associated with an increased antigen expression and greater spread through the allografted liver.
In summary, the findings described in this report provide further support for the concept of a dualistic role of HLA in liver transplantation, and help to explain why survival is poorer with better matches. Although HLA matching reduces acute cellular rejection, it increases the risk of CMV hepatitis and recurrence of HBV and HCV hepatitis. A better understanding of all these different HLA-associated immune mechanisms may lead to improved management strategies in hepatic transplantation.
Footnotes
Presented at the 13th Annual Meeting of the American Society of Transplant Physicians, May 16–18, 1994, Chicago, IL.
Abbreviations: CMV, cytomegalovirus; HBV, hepatitis B virus, HCB, hepatitis C virus; MHC, major histocompatibility complex; OLTx, orthotopic liver transplantation.
REFERENCES
- 1.Markus BH, Duquesnoy RJ, Gordon RD, et al. Histocompatibility and liver transplant outcome: does HLA exert a dualistic effect? Transplantation. 1988;46:372. [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson PT, O’Grady J, Portmann B, et al. Evidence for and immune response to HLA class I antigens in the vanishing bile duct syndrome after liver transplantation. Lancet. 1987;1:945. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 3.Yagihashi A, Kobayashi M, Noguchi K, et al. HLA matching effect in liver transplantation. Transplant Proc. 1992;24:2432. [PMC free article] [PubMed] [Google Scholar]
- 4.Thorogood J. Relationship between HLA compatibility and first liver allograft survival. Transplant Proc. 1993;25:2655. [PubMed] [Google Scholar]
- 5.Gunson BK, Hathaway M, Buckels JAC, et al. HLA matching in liver transplantation: a retrospective analysis. Transplant Proc. 1992;24:2434. [PubMed] [Google Scholar]
- 6.Mañez R, White LT, Linden P, et al. The influence of HLA matching on cytomegalovirus hepatitis and chronic rejection after liver transplantation. Transplantation. 1993;55:1067. doi: 10.1097/00007890-199305000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B-virus related liver disease. Hepatology. 1991;13:619. [PMC free article] [PubMed] [Google Scholar]
- 8.Wright T, Donegan M, Hsu H, et al. Hepatitis C viral infection in liver transplant recipients: the importance of detection of viral RNA using the polymerase chain reaction. Gastroenterology. 1992;103:317. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 9.Calmus Y, Hannoun L, Dousset B, et al. HLA class I matching is responsible for the hepatic lesions in recurrent viral hepatitis B after liver transplantation. Transplant Proc. 1990;22:2311. [PubMed] [Google Scholar]
- 10.Demetris AJ, Jaffe R, Sheahan DG, et al. Recurrent hepatitis B in liver allograft recipients: differentiation between viral hepatitis B and rejection. Am J Pathol. 1986;125:161. [PMC free article] [PubMed] [Google Scholar]
- 11.Lauchart W, Muller R, Pichlmayr R. Immunoprophylaxis of hepatitis B virus reinfection. Transplant Proc. 1987;19:2387. [PubMed] [Google Scholar]
- 12.Chazouilleres O, Kim M, Combs C, et al. Quantification of hepatitis C virus RNA in liver transplant recipients. Gastroenterology. 1994;106:994. doi: 10.1016/0016-5085(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 13.Brillanti S, Foli M, Gaiani S, Masci C, Miglioli M, Barbara L. Persistent hepatitis C virus viremia without liver disease. Lancet. 1993;341:464. doi: 10.1016/0140-6736(93)90210-8. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama T, Guilhot S, Klopchin K, et al. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990;248:361. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Buey L, Lopez-Botet M, Garcia-Sanchez A, et al. Variability in the expression of beta 2-microglobulin epitope on hepatocytes in chronic type C hepatitis on treatment with interferon. Hepatology. 1993;17:372. [PubMed] [Google Scholar]
- 16.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I–restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 17.Bertoletti A, Ferrari C, Fiaccadori F, et al. HLA class I–restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci USA. 1991;88:10445. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koziel MJ, Dudley D, Wong JT, et al. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339. [PubMed] [Google Scholar]
- 19.Sjogren MH, Hoofnagle J, Waggoner JG. Effect of corticosteroid therapy on levels of antibody to hepatitis B core antigen in patients with chronic type B hepatitis. Hepatology. 1987;7:582. doi: 10.1002/hep.1840070328. [DOI] [PubMed] [Google Scholar]