Abstract
We have proposed that the mechanisms of alloengraftment and variable acquired tolerance can be facilitated by minimum posttransplant immunosuppression. It was further suggested that the efficacy of minimalistic treatment could be enhanced by preoperative recipient conditioning with an antilymphoid antibody preparation. A total of 76 adults (38 hepatitis C virus [HCV]−, 38 HCV+) were infused with 30 mg alemtuzumab before primary cadaveric liver transplantation and maintained afterward on daily monotherapy unless breakthrough rejection mandated additional agents. In stable patients, the intervals between tacrolimus doses were lengthened (“spaced weaning”) after approximately 4 months. Eighty-four contemporaneous nonlymphoid-depleted liver recipients (58 HCV−, 26 HCV+) were treated with conventional postoperative immunosuppression. The overall incidence of rejection was similar with the two strategies of immunosuppression. With follow-ups of 14 to 22 months, patient and primary graft survival in HCV− cases are 97% and 90%, respectively, with alemtuzumab depletion plus minimal immunosuppression versus 71% and 70%, respectively, under conventional immunosuppression. In HCV+ recipients, current patient and graft survival in the alemtuzumab-pretreated group are 71% and 70% versus 65% and 54%, respectively, under conventional treatment. With both strategies of immunosuppression, the adverse effect of preexisting HCV on survival parameters and graft function already was significant at the 1-year milestone, but its extent was not evident until the second year. With or without HCV, 62% of the 64 surviving lymphoid-depleted patients are on spaced immunosuppression, and four patients receive no immunosuppression. Lymphoid depletion with alemtuzumab and minimalistic maintenance immunosuppression is a practical strategy of liver transplantation in HCV− recipients but not HCV+ recipients.
In 2001, we proposed that the heavy multidrug immunosuppression given in most centers from the time of organ transplantation could erode the seminal engraftment mechanism of clonal exhaustion-deletion (Fig. 1A) (1). Avoidance of this self-defeating consequence of treatment by the use of minimum posttransplant immunosuppression has been counterintuitive because of concern about a potentially higher rate of acute rejection. To avoid this penalty, we suggested the joint application of a second therapeutic principle, that is, reduction of the anticipated antidonor response into a more readily deletable range by lymphoid depletion of the recipient before exposure to donor antigen (Fig. 1B)) (1). Encouraging results were obtained in our first series of organ recipients in whom both therapeutic principles were applied. The lymphoid depletion was performed in the first cases with rabbit antithymocyte globulin (Thymoglobulin) (2, 3).
FIGURE 1.

Proposed effect of immunosuppression on tolerogenic mechanisms (1). (A) Subversion of clonal exhaustion-deletion by excessive posttransplant immunosuppression. (B) The stepwise effect of pretreatment (1) and minimal posttransplant immunosuppression (2) in reducing the antigraft response into a more deletable range (see text). (C) Alloengraftment achieved with minimal post-transplant immunosuppression alone.
Here, we report the use of alemtuzumab (Campath) instead of antithymocyte globulin for the pretreatment of 76 recipients of cadaveric livers. Alemtuzumab, which was developed by Hale, Waldmann, and Dyer (4), causes profound depression of the lymphocyte lineages without causing major declines in platelets. Its efficacy in kidney transplantation was first reported by Calne et al. (5) and subsequently confirmed by Stuart et al. (6), Knechtle et al. (7), and Kirk et al. (8). Alemtuzumab also has been used by Tzakis et al. (9) for intestinal and liver transplantation. In most of the foregoing experience, two doses of alemtuzumab were given, the first intraoperatively and the second several days later. In our patients, a single dose was given before transplantation.
METHODS
Immunosuppression
Alemtuzumab-Tacrolimus
Seventy-six adults underwent primary cadaveric liver transplantation between September 2002 and April 2003 after a 30-mg infusion of alemtuzumab; 1 or 2 g of methylprednisolone was given simultaneously to prevent cytokine reactions. Within 1 day, the lymphocyte counts decreased from 1,056+712 mm3 to 130±135 mm3. Recovery from the profound depression began by 1 to 2 weeks. At 3 and 6 months, the mean lymphocyte counts were 619±522 mm3 and 685±578 mm3, respectively. Even at 1 year, there was still a residual depression of CD4+ and to a lesser extent CD8+ cells. As other investigators have observed, the rebound of B cells preceded that of the other mononuclear lineages and was restored to above baseline at 3 months. Platelet counts were not significantly depressed in comparison with those in conventionally immunosuppressed liver recipients.
Twice-daily tacrolimus was begun on the day after transplantation, with a target trough level of 10 ng/mL. With suspected drug toxicity (most commonly nephrotoxicity or neurotoxicity), cyclosporine or sirolimus was substituted for tacrolimus in a few patients. If rejection occurred in the first 4 months, additional immunosuppression was added to the monotherapy, but only for as long as necessary to restore stable graft function. At approximately 4 months, the twice-daily doses of tacrolimus (or the substitute monotherapeutic agent) were consolidated to one dose per day in patients who had been stable on the single drug therapy for at least 60 days. One to 2 months later, dose frequency was reduced to every other day and subsequently to longer intervals when possible. If rejection occurred while weaning, daily treatment with the baseline drug was resumed, and other agents were temporarily added. After recovery from the rejection, a search was reinstituted for the lowest possible daily immunosuppression.
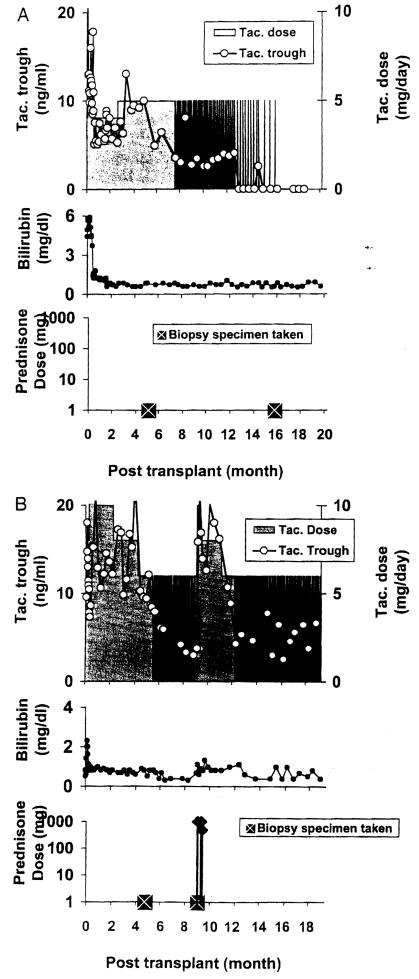
Examples of application of the algorithm are shown in Figure 2. Every other day dosing in the patient depicted in Figure 2A was begun 7.5 months after liver transplantation on September 12, 2002. By 14 months, the tacrolimus dosing had been reduced to once per week, and at 15 months treatment was stopped. There was no evidence of rejection at any time during the 22 months of follow-up. In the case depicted in Figure 2B, rejection developed after dosing frequency was reduced to two times per week. The patient was given two boluses of prednisone, and daily tacrolimus was temporarily resumed. After 3 additional months, he was weaned to 3 doses per week and has been stable for the ensuing 9 months.
FIGURE 2.
(A) Course of alemtuzumab-pretreated recipient. Spaced weaning from tacrolimus monotherapy was started at 7.5 months and reduced progressively thereafter. After 15 months, treatment was stopped. (B) Interruption of weaning when rejection occurred after doses were reduced to 2 times/week. The patient was treated with three boluses of prednisone and temporarily returned to daily tacrolimus. Three months later, he was weaned to three doses per week and has been stable since.
Conventional Treatment
Also between September 2002 and April 2003, 84 cadaver liver recipients did not have alemtuzumab infusion. These 84 recipients were treated with the generic strategy depicted in Figure 1A. Tacrolimus and a 5-day cycle of methyl-prednisolone were begun on the day of operation, beginning with 200 mg on the first day and ending on day 5 at 40 mg. Weaning thereafter was performed slowly. At 2 months, 90% of the surviving patients were still receiving 10 mg or more per day prednisone. In addition, mycophenolate mofetil was given from the time of operation in many cases. Spaced weaning from the baseline drug (usually tacrolimus) was not a primary objective, but an attempt was made to reach the lowest maintenance daily level consistent with stable graft function. In a few cases, spaced weaning was attempted to relieve drug toxicity.
Pathologic Studies of Rejection
Biopsy specimens were obtained for suspicion of rejection or in preparation for weaning. In addition to conventional hematoxylin-eosin histopathology, special stains were used when indicated to study the extent of fibrosis (Masson Trichrome) or to visualize other points of interest. Biopsy findings were entered into standardized categories using the Banff schema (10), Patients with no biopsy samples and no clinical evidence of rejection were considered to be rejection-free.
Statistical Analysis
The populations were described using mean and standard deviation or frequencies and percentages. Survival estimates were obtained using the Kaplan-Meier method. Although this was in no sense a prospective, randomized trial, the significance of differences in survival and graft function at 1 year between the two groups was determined by using a log-rank test and other variables compared by t test and chi-square test. P values less than 0.05 were considered significant.
RESULTS
Case Selection
Of the eight severely ill status 1 patients, seven were members of the conventional immunosuppression group (Table 1). In most of the others in this group, delays in liver allocation had left insufficient time for the alemtuzumab infusion. With elimination of the infusion, the average cold ischemia time of the allografts in the conventional group was similar to the alemtuzumab group (Table 1).
TABLE 1.
Recipient and donor factors
| Alemtuzamab pretreated | Conventional immunosuppression | |
|---|---|---|
| n | 76 | 84 |
| Case accrual | 9/12/02 → 4/30/03 | 9/12/02 → 4/30/03 |
| Recipient age (yr) | 52±10.2 | 53±10.6 |
| Male/female | 27/49 | 29/55 |
| MELD at transplant | 14.2±5.7 | 14.9±7.3 |
| Hepatitis C | 38 (50%) | 26 (31%) |
| Status 1 patients | 1 (1%) | 7 (8%) |
| Donor age | 47±15.9 | 51±18.2 |
| Cold ischemia | 11.9±3.36 hr | 11.8±3.43 hr |
| HLA mismatch | 4.5 ±1.2 | 4.5±1.1 |
HLA, human leukocyte antigen; MELD, Model for End-Stage Liver Disease.
The incidence of hepatitis C virus (HCV)-associated disease was 50% in the alemtuzumab cohort versus 31% in the conventional treatment group (P<0.01) (Table 1).
Patient and Graft Survival
Overall
One-year patient survival of the alemtuzumab-pre-treated patients was 87% versus 80% for the conventionally immunosuppressed patients (P=0.36, not significant [NS]). In the respective cohorts, 1-year primary graft survival was 82% versus 73% (P=0.26, NS). Notably, the results at 1 year and beyond were profoundly influenced by the presence or absence of HCV.
Hepatitis C Virus Effect
With follow-ups of 14 to 22 months (to July 1, 2004), the lymphoid-depleted recipients without HCV continue to have the same 97% and 90% patient and primary graft survival recorded at 1 year (Table 2).
TABLE 2.
Effect of hepatitis C virus on patient and primary graft survival after alemtuzumab pretreatment versus conventional immunosuppression after follow-up of 13 to 21 monthsa
| Alemtuzumab N=76 | Conventional N=84 | |
|---|---|---|
| Patient survival | ||
| Overall | 64/76 (84%) | 67/84 (80%) |
| Without HCV | 37/38 (97%) | 50/58 (86%) |
| With HCV | 27/38 (71%) | 17/26 (65%) |
| Graft survival | ||
| Overall | 60/76 (79%) | 60/84 (71%) |
| Without HCV | 34/38 (90%) | 46/58 (79%) |
| With HCV | 26/38 (70%) | 14/26 (54%) |
A statistical analysis of the effect of HCV on survival and allograft function at the 1-yr milestone is contained in the text.
HCV, hepatitis C virus.
In contrast, two of the HCV+ recipients died at 14 and 21 months of hepatic failure caused by recurrent disease. Thus, the current patient and graft survival in HCV+ cases are 71% and 70%, respectively.
Viral replication was frequently associated with alemtuzumab infusion. In the patient whose course is depicted in Figure 3, a striking increase in viral titer occurred 2 months after alemtuzumab pretreatment and transplantation. The viral load then declined for the next half year, but after a repeat dose of alemtuzumab, there was a similar wave of viral replication (peak 92 million HCVIU). The patient is clinically well at present, but the 14-month biopsy revealed bridging fibrosis.
FIGURE 3.
Viral replication to 37.7 million IU/mL 2 months after alemtuzumab pretreatment and liver transplantation. The viral load (open squares) returned to baseline for the next half year coincident with drug weaning. Then, a repeat dose of alemtuzumab was followed by a viral crisis (92 million IU) that subsequently subsided. Although the patient is clinically well and back on spaced weaning, the 14-month biopsy shows bridging fibrosis.
The deleterious consequences of HCV were equally apparent under conventional immunosuppression. In the HCV− recipients, the 1-year patient and graft survival of 86% and 79% remain the same after 14 to 22 months. The 65% patient and 58% primary graft survival in the HCV+ cohort already were inferior at 1 year. Although no further deaths have occurred in this cohort, one of the 1-year survivors underwent retransplantation after 14 months. Thus, current graft survival is only 54%.
Causes of Death
The proximate causes of death under the two kinds of immunosuppression were much the same. Common themes were sepsis and multiple organ failure.
Graft Function
Alemtuzumab-Pretreated Cohort
At the 1-year milestone, the mean serum bilirubin of 1.04±0.97 mg/dL of the HCV− recipients was half the 2.5±6.55 mg/dL of the surviving HCV+ patients bearing primary grafts (P<0.01). The current mean bilirubin is 0.92±0.76 mg/dL in the HCV− group versus 1.65±2.85 mg/dL in the HCV+ cohort.
Conventional Immunosuppression Cohort
The current mean serum bilirubin of 0.68±0.44 mg/dL in the HCV− cohort is half the 1.24±1.35 mg/dL in the HCV+ subgroup.
Frequency and Pathology of Rejection
No grafts were lost to acute or chronic rejection in either the pretreatment or conventional treatment series. The incidence of chronic rejection in both groups was zero. During the first 4 months, the overall rate of first onset acute rejection was 10% in the alemtuzumab-pretreated patients compared with 20% in the conventionally treated patients (P=0.56, NS). Only 6% of the HCV+ recipients pretreated with alemtuzumab developed first rejection during the first 4 months after transplantation compared with 30% in the conventional group (P=0.10).
The major histopathologic features of acute rejection in the patients treated with alemtuzumab and minimum immunosuppression were similar to those in the conventionally treated recipients. Mixed portal tract or perivenular inflammation containing blastic lymphocytes, eosinophils, and plasma cells, and similar subendothelial infiltration of portal vein branches and hepatic venules and bile duct inflammation and damage were the characteristic features. In some patients maintained on little or no steroids, eosinophils comprised a significant proportion of the inflammation.
In several long-term survivors, at least two of whom demonstrated HCV, intense plasmacytic interface activity was noted that resembled autoimmune hepatitis. In these cases, it was difficult to distinguish among viral immunity, alloimmunity, and autoimmunity. Although findings of antibody-mediated rejection were not conspicuous in most rejection episodes, a systematic investigation of antidonor antibodies and C4d deposits in the liver allografts is currently under investigation.
It is noteworthy that class I and II antibodies did not develop de novo in 32 of 41 alemtuzumab-pretreated recipients of crossmatch negative recipients in whom sequential serum samples were obtained. The other nine patients had one or more samples that contained new class I or II antibodies, or both. The new antibodies were detected before spaced weaning in some cases and afterward in others. One of the nine patients died of recurrent HCV. Three of the other eight patients are on spaced weaning, whereas five are on daily doses.
Incidence of Weaning
Of the surviving 64 alemtuzumab-pretreated recipients, only 5 (7.8%) are receiving more than one drug 14 to 22 months after transplantation. Sixteen patients (25%) are receiving daily monotherapy (14 receiving tacrolimus and 2 receiving cyclosporine). Another 39 patients (62%) are receiving spaced doses of monotherapy (35 receiving tacrolimus, 3 receiving cyclosporine, and 1 receiving sirolimus) at intervals ranging from every other day to once every 2 weeks. Four patients are not receiving immunosuppression (Table 3). The incidence of spaced or complete weaning in the severely reduced HCV+ recipient population is approximately the same as that in the nearly intact HCV− cohort (67% vs. 65%). Weaning was not systematically attempted in the conventionally treated patients (Table 3).
TABLE 3.
Incidence and extent of weaning
| Immunosuppression dosing regimen | Alemtuzumab pretreated |
Conventional immunosuppression |
||
|---|---|---|---|---|
| HCV+ | HCV− | HCV+ | HCV− | |
| Total alive/entered | 27/38 (71.5%) | 37/38 (97%) | 17/26 (65%) | 50/58 (86%) |
| Daily multidrug therapy | 2 | 3 | 6 | 17 |
| Daily monotherapy | 6 | 10 | 10 | 32 |
| Every other day | 10 | 8 | 1 | 0 |
| 3/wk | 3 | 7 | 0 | 1 |
| 2/wk | 4 | 4 | 0 | 0 |
| 1/wk | — | — | 0 | 0 |
| Every 2 weeks | 1 | 2 | 0 | 0 |
| Off immunosuppression | 1 | 3 | 0 | 0 |
DISCUSSION
The management plan into which alemtuzumab was incorporated was based on the concept that immunosuppression-assisted organ engraftment is a form of variable donor-specific tolerance (1, 11). In this paradigm, the seminal mechanism of alloengraftment is activation and exhaustion-deletion of the clonal response induced in the first few postoperative weeks by migration of the transplanted organ’s passenger leukocytes to the recipient’s lymphoid organs. The purpose of lymphoid depletion in advance of transplantation was to reduce global immune reactivity before exposing the recipient to alloantigen and to thereby bring the anticipated donor specific response into a more readily deletable range (Fig. 1B).
The superior qualities and safety of antilymphoid preparations for pretransplant conditioning have been known since the 1960s (12–14). The experience compiled elsewhere (5–9) and reported here has established alemtuzumab as a premiere antilymphoid antibody agent. But neither the optimal timing nor the long-term consequences of lymphoid depletion have been fully clarified (15, 16). In the other centers, two doses of alemtuzumab were given: the first intraoperatively and the second in the posttransplant period. With this strategy, a low incidence of rejection and a reduced dependence on maintenance immunosuppression were observed (5–9). In the frame of reference shown in Figure 1A, it is conceivable that administration of alemtuzumab in the postoperative period could variably erode the seminal mechanism of clonal exhaustion-deletion in the same way as multidrug therapy. If so, late weaning may be precluded.
The more immediate question that has emerged from the experience reported here concerns the effect of immunosuppression in patients with HCV. The combined use of pretransplant lymphoid depletion with alemtuzumab and minimalistic posttransplant immunosuppression provided excellent results and allowed a high rate of spaced weaning in patients whose original disease was not caused by HCV. Although spaced weaning also could be performed in the majority of surviving HCV+ patients, a large number of HCV+ patients either died early from a variety of non-HCV infections or of HCV-associated graft dysfunction that frequently followed viral replication. The adverse consequences of HCV also were apparent to a greater extent than reported in some other centers (17, 18) in our patients treated with conventional immunosuppression. Although the excessive mortality in our conventionally treated HCV+ patients may have been associated with the liberal use of steroids and other adjunct agents (17, 19), the major risk factor in the alemtuzumab-pretreated HCV+ recipients clearly was lymphoid depletion.
Because HCV-associated disease has become the single most frequent indication for liver transplantation, finding the best treatment for these recipients has become a high priority. Having concluded from the experience reported here that pretransplant lymphoid depletion may contribute to HCV+ recurrence, we are currently exploring the strategy of using the principle of minimal immunosuppression alone (Fig. 1C). Although the incidence of rejection predictably will be higher, acute rejection per se has been shown not to degrade the late results in the large Pittsburgh series of liver recipients (20). Whether the same is true of the HCV+ subgroup of patients in this collection remains to be determined.
Acknowledgments
We thank Ms. Terri L. Mangan for superb administrative and secretarial assistance.
Supported by National Institutes of Health grant DK 64207-01.
References
- 1.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro R, Jordan M, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pre-treatment and low-dose postoperative immunosuppression, with subsequent weaning. Ann Surg. 2003;238:520–527. doi: 10.1097/01.sla.0000089853.11184.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale G, Waldmann H, Dyer M. Specificity of monoclonal antibody Campath-1. Bone Marrow Transplant. 1988;3:237–239. [PubMed] [Google Scholar]
- 5.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative Campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 6.Stuart FP, Leventhal JR, Kaufman DB, et al. Alemtuzumab facilitates prednisone free immunosuppression in kidney transplant recipients with no early rejection. Am J Transplant. 2002;2 (suppl 3):397. [Google Scholar]
- 7.Knechtle SJ, Pirsch JDH, Fechner J, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 9.Tzakis AG, Kato T, Nishida S, et al. Preliminary experience with Campath 1H (C1H) in intestinal and liver transplantation. Transplantation. 2003;75:1227–1231. doi: 10.1097/01.TP.0000065192.53065.50. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous: Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodruff MFA, Anderson NF. Effect of lymphocyte depletion by thoracic duct fistula and administration of anti-lymphocytic serum on the survival of skin homografts in rats. Nature (London) 1963;200:702. doi: 10.1038/200702a0. [DOI] [PubMed] [Google Scholar]
- 13.Monaco AP, Wood ML, Gray JG, et al. Studies on heterologous anti-lymphocyte serum in mice-II, effect on the immune response. J Immunol. 1966;96:229. [PubMed] [Google Scholar]
- 14.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auchincloss H. No tolerance for depletion. Nat Med. 2004;10:21–23. doi: 10.1038/nm0104-21. [DOI] [PubMed] [Google Scholar]
- 17.Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226–231. doi: 10.1097/01.TP.0000101738.27552.9D. [DOI] [PubMed] [Google Scholar]
- 18.Ghobrial RM, Steadman R, Gombein J, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234:384–394. doi: 10.1097/00000658-200109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everson GT. Impact of immunosuppressive therapy on recurrence of hepatitis C. Liver Transpl. 2002;8 (suppl 1):S19–S27. doi: 10.1053/jlts.2002.35852. [DOI] [PubMed] [Google Scholar]
- 20.Demetris AJ, Ruppert K, Dvorchik I, et al. Real time monitoring of acute liver allograft rejection using the Banff Schema. Transplantation. 2002;74:1290–1296. doi: 10.1097/00007890-200211150-00016. [DOI] [PubMed] [Google Scholar]