Abstract
Drug-elicited head-twitch behavior is a useful model for studying hallucinogen activity at 5-HT2A receptors in the mouse. Chemically diverse compounds active in this assay yield biphasic dose-effect curves, but there is no compelling explanation for the “descending” portion of these functions. A set of experiments was designed to test the hypothesis that the induction of head-twitch behavior is mediated by agonist actions at 5-HT2A receptors, whereas the inhibition of head-twitch behavior observed at higher doses results from competing agonist activity at 5-HT2C receptors. The effects of the phenethylamine hallucinogen R(−)-2,5-dimethoxy-4-iodoamphetamine (DOI) on head-twitch behavior were studied over a range of doses in the mouse, generating a characteristic biphasic dose-response curve. Pretreatment with the selective 5-HT2A antagonist (+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (M100907) shifted only the ascending limb of the DOI dose-effect function, whereas pretreatment with the nonselective 5-HT2A/2C antagonist 3-{2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl}quinazoline-2,4(1H,3H)-dione (ketanserin) produced a parallel shift to the right in the DOI dose-response curve. Administration of the 5-HT2C agonist S-2-(chloro-5-fluoro-indol-l-yl)-1-methylethylamine (Ro 60-0175) noncompetitively inhibited DOI-elicited head-twitch behavior across the entire dose-effect function. Finally, pretreatment with the selective 5-HT2C antagonists 6-chloro-5-methyl-1-[(2-[2-methylpyrid-3-yloxy]pyrid-5yl)carbamoyl]indoline (SB242084) or 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro[4,5]decane-2,4-dione hydrochloride (RS 102221) did not alter DOI-elicited head-twitch behavior on the ascending limb of the dose-response curve but shifted the descending limb of the DOI dose-response function to the right. The results of these experiments provide strong evidence that DOI-elicited head-twitch behavior is a 5-HT2A agonist-mediated effect, with subsequent inhibition of head-twitch behavior being driven by competing 5-HT2C agonist activity.
Introduction
Many of the 14 recognized serotonin (5-HT) receptor subtypes are important mediators of the effects of hallucinogenic drugs, and although the 5-HT2A receptor is largely considered a major site of action for these compounds (González-Maeso et al., 2003, 2007; Fantegrossi et al., 2008), the modulatory roles of specific classes of other 5-HT receptors on the behavioral effects of hallucinogens are not well established (Winter et al., 1999). The drug-elicited head-twitch response (HTR) (Corne et al., 1963; Corne and Pickering, 1967) is considered to be a selective behavioral model for hallucinogen activity at 5-HT2A receptors in the mouse (González-Maeso et al., 2003, 2007), and several previous studies have established that direct and indirect 5-HT agonists induce this effect (Peroutka et al., 1981; Colpaert and Janssen, 1983; Green et al., 1983; Goodwin and Green, 1985; Darmani et al., 1990a, 1990b, 1992; Fantegrossi et al., 2004, 2005, 2006). Furthermore, 5-HT2A receptor antagonists selectively block head-twitch behavior (Lucki et al., 1984; Handley and Singh, 1986; Fantegrossi et al., 2004, 2005, 2006), and their potency in this regard is highly correlated with the antagonist's affinity for 5-HT2A receptors (Peroutka et al., 1981; Ortmann et al., 1982). However, 5-hydroxytryptophan also elicits head-twitch behavior in the mouse (Corne et al., 1963; Schmid et al., 2008), although it lacks hallucinogenic effects in humans. Drug discrimination studies have revealed that the discriminative stimulus effects of phenethylamine and indolalkylamine hallucinogens are mediated by agonist activity at 5-HT2A receptors (Fiorella et al., 1995a) and modulated by agonist activity at 5-HT2C receptors (Fiorella et al., 1995b). Similar findings have been obtained in assays of drug-elicited head-twitch behavior, because HTR induced by local injection of R(−)-2,5-dimethoxy-4-iodoamphetamine (DOI) into the frontal cortex is attenuated by pretreatment with the selective 5-HT2A antagonist (+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (M100907) but is not altered by prior administration of the 5-HT2C/2B antagonist (+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate (SDZ-SER 082) in the rat (Willins and Meltzer, 1997).
Although HTR and drug discrimination are in many ways complementary assays that can be used in parallel to characterize hallucinogen effects in vivo (Fantegrossi et al., 2008), there are important differences between these two procedures. It is noteworthy that drug discrimination dose-effect curves are asymptotic, whereas we have found dose-effect curves derived from HTR data to be biphasic (Fantegrossi et al., 2005, 2006). The ubiquity of biphasic dose-effect curves in behavioral pharmacology should not detract from the fact that there is no compelling explanation for the “descending” portion of HTR curves. Phenethylamine hallucinogens typically have similar affinities for 5-HT2A and 5-HT2C receptors (McKenna and Peroutka, 1989; Appel et al., 1990). Thus, it is possible that these distinct 5-HT receptor subtypes interact to generate the biphasic nature of HTR dose-effect curves. An analogous situation has been described in the interaction of dopamine D2 and D3 receptors in the mediation of yawning behavior in the rat (Collins et al., 2005). Specifically, administration of D2-like agonists induces dose-dependent yawning until some maximally effective dose is reached, beyond which higher doses inhibit yawning behavior. The ascending limbs of these biphasic curves have been attributed to agonist activity at D3 receptors, whereas the descending limbs have been shown to result from D2 agonist activity (Collins et al., 2005). We speculated that 5-HT2A and 5-HT2C receptors may similarly interact to mediate head-twitch behavior, such that the biphasic nature of HTR curves elicited by the phenethylamine hallucinogen DOI might be attributable to selective 5-HT2A agonist activity at low doses (the ascending limb) and the recruitment of 5-HT2C agonist activity at high doses (the descending limb). Thus, we established dose-effect functions for DOI using the head-twitch assay in mice in the absence and presence of the selective 5-HT2A antagonist M100907 and the nonselective 5-HT2A/2C antagonist 3-{2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl}quinazoline-2,4(1H,3H)-dione (ketanserin). Likewise, the selective 5-HT2C agonist S-2-(chloro-5-fluoro-indol-l-yl)-1-methylethylamine (Ro 60-0175) was administered in combination with DOI to gauge the involvement of 5-HT2C receptor activation in the mediation of DOI-elicited head-twitch behavior. Finally, the effects of the selective 5-HT2C antagonists 6-chloro-5-methyl-1-[(2-[2-methylpyrid-3-yloxy]pyrid-5yl)carbamoyl]indoline (SB242084) and 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido) phenyl-5-oxopentyl]1,3,8-triazaspiro[4,5] decane-2,4-dione hydrochloride (RS 102221) on DOI-elicited head twitches were also determined. The hypotheses tested were that 1) the selective 5-HT2A antagonist would shift the ascending limb of the DOI curve to the right but have no effects on the descending limb; 2) the nonselective 5-HT2A/2C antagonist would produce a parallel rightward shift in the entire dose-effect function; 3) the selective 5-HT2C agonist would noncompetitively inhibit DOI-elicited twitch behavior, resulting in a flattening of the dose-effect function; and 4) pretreatment with selective 5-HT2C antagonists would produce a parallel rightward shift in the descending limb of the DOI curve in the absence of effects on the ascending limb.
Materials and Methods
Animals.
Male NIH Swiss mice (Harlan, Inc., Indianapolis, IN) and Swiss-Webster mice (Harlan, Inc., and Charles River Laboratories, Wilmington, MA) weighing 20 to 25 g on delivery were housed 12 animals per cage in temperature-controlled rooms maintained at an ambient temperature of 22 ± 2°C at 45 to 50% humidity at the University of Michigan, the Yerkes National Primate Research Center, and the University of Arkansas for Medical Sciences, respectively. At all institutions, lights were set to a 12-h light/dark cycle, and animals were fed ad libitum with standard rodent chow (Laboratory Rodent Diet 5001; Purina, St. Louis, MO) and water until immediately before testing. Animals were not used in experiments until at least 2 days after arrival at the respective institutions. Each animal was used in only one experimental observation and was sacrificed immediately after use.
Procedure.
On experimental days, NIH Swiss mice at the University of Michigan and Swiss-Webster mice at the Yerkes National Primate Research Center and University of Arkansas for Medical Sciences were weighed, marked, and returned to the home cage. Doses were then calculated and prepared for intraperitoneal injection. Individual animals were then removed from the home cage, injected with saline or various doses of M100907, ketanserin, Ro 60-0175, SB242084, or RS 102221 and placed into an observation cage containing fresh bedding. Ten minutes after the pretreatment injection, mice were injected with saline or various doses of DOI and returned to the observation cage. Five minutes after this second injection, an overhead camera was activated and behavior was recorded for 10 min. Videotapes were later scored for drug-elicited head twitches (defined as a rapid rotational jerk of the head that can be distinguished from species-appropriate grooming or scratching behaviors) by at least one blind observer. At the University of Michigan, head-twitch experiments were conducted in the colony room; at the Yerkes National Primate Research Center and the University of Arkansas for Medical Sciences, head-twitch experiments were conducted in a separate behavioral laboratory proximal to the vivarium. In all cases, neither food nor water was available during the experiments.
Data Analysis.
Data are presented as mean ± S.E.M. for groups [n = 6, except for one instance (0.3 mg/kg DOI after a pretreatment with 3.0 mg/kg RS 102221) in which n = 5]. In this case, a single animal exhibited more than 30 head twitches in the 10-min observation period and thus appeared to be an outlier. The Grubbs maximum normed residual test (NIST/SEMATECH e-Handbook of Statistical Methods, http://www.itl.nist.gov/div898/handbook/) was used to statistically confirm with 95% confidence that the behavioral response from this animal was more than 2 S.D. outside the grand mean. Thus, this animal was dropped from the study. Statistical analyses were conducted using one-way analysis of variance, and post hoc pairwise multiple comparisons on significant effects and interactions were performed after the method of Holm-Sidak. In one instance (studies involving DOI and M100907), data were not normally distributed, so a one-way analysis of variance was run on ranks, and post hoc pairwise multiple comparisons on significant effects and interactions were accomplished using Tukey's honestly significant difference test. All statistical tests were executed using commercially available software, and significance was judged at P < 0.05. In all figures, points without error bars indicate instances in which the variance is contained within the data point.
Drugs.
DOI, SB242084, and ketanserin were purchased from Sigma-Aldrich (St. Louis, MO). Ro 60-0175 and RS 102221 were purchased from Tocris Bioscience (Ellisville, MO). M100907 was synthesized in the Laboratory of Medicinal Chemistry at the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases and was supplied as a generous gift from Dr. Kenner C. Rice (National Institute on Drug Abuse, Bethesda, MD). Saline vehicle and all other experimental supplies were obtained from standard commercial sources.
Results
Strain Differences and Interinstitution Variability.
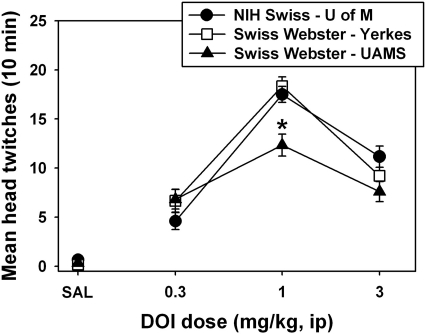
After saline injection, very low levels of head-twitch behavior were observed in all groups of mice (Fig. 1), and there were no group differences in baseline head-twitch behavior (P > 0.05). DOI elicited a significant dose-dependent HTR in all cohorts of mice. In all cases, 1.0 mg/kg DOI elicited maximal head-twitch behavior, whereas higher doses defined the descending limbs of the dose-effect curves. Post hoc testing detected no differences between NIH Swiss mice at the University of Michigan and Swiss-Webster mice at the Yerkes National Primate Research Center across equivalent doses of DOI (t = 1.643, P = 0.111 for 0.3 mg/kg DOI; t = 0.540, P = 0.593 for 1.0 mg/kg DOI; t = 1.275, P = 0.212 for 3.0 mg/kg DOI). There were thus no significant strain differences between the NIH Swiss mice used at the University of Michigan and the Swiss-Webster mice used at the Yerkes National Primate Research Center, in terms of sensitivity to the effects of DOI on head-twitch behavior. Swiss-Webster mice at the University of Arkansas for Medical Sciences expressed less total head-twitch behavior than the other two cohorts at the maximally effective DOI dose (t = 4.387, P = 0.0005 for the comparison against the Swiss-Webster mice used at the Yerkes National Primate Research Center; t = 3.778, P = 0.00182 for the comparison against the NIH Swiss mice used at the University of Michigan), but the shape and position of the dose-effect curves was similar at all three institutions.
Fig. 1.
Dose-dependent effects of DOI on head-twitch behavior in NIH Swiss mice at the University of Michigan (●), the Yerkes National Primate Research Center (□), and the University of Arkansas for Medical Sciences (■). Abscissa, dose of DOI expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences among groups.
Selective 5-HT2A Antagonist.
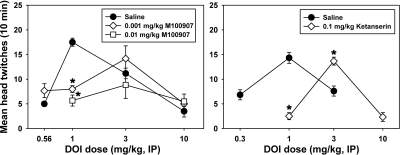
Administration of M100907 significantly altered HTR induced by DOI in NIH Swiss mice at the University of Michigan (χ2 = 33.333, df = 8, P < 0.001) (Fig. 2, left). M100907 pretreatment doses of 0.001 mg/kg (q = 4.696, P < 0.05) and 0.01 mg/kg (q = 5.367, P < 0.05) significantly suppressed the HTR elicited by 1.0 mg/kg DOI. Neither dose of M100907 significantly altered HTR on the descending limb of the DOI dose-effect curve.
Fig. 2.
Left, effects of the selective 5-HT2A antagonist M100907 on head-twitch behavior elicited by DOI in NIH Swiss mice at the University of Michigan. Right, effects of the nonselective 5-HT2A/2C antagonist ketanserin on head-twitch behavior elicited by DOI in Swiss-Webster mice at the University of Arkansas for Medical Sciences. Abscissae, dose of DOI, expressed in milligrams per kilogram on a log scale. Ordinates, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls.
Nonselective 5-HT2A/2C Antagonist.
Treatment with 0.1 mg/kg ketanserin induced a significant rightward shift in the HTR curve elicited by DOI in Swiss-Webster mice at the University of Arkansas for Medical Sciences (F = 16.898, P < 0.001) (Fig. 2, right). At a dose of 1.0 mg/kg DOI, ketanserin significantly suppressed HTR (t = 6.677, P < 0.01), whereas HTR elicited by 3.0 mg/kg DOI was significantly increased by ketanserin pretreatment (t = 2.898, P < 0.01). The maximally effective doses of DOI elicited HTRs (1.0 mg/kg after saline pretreatment, 3.0 mg/kg after ketanserin pretreatment) were not statistically different from each other (t = 0.504, P = 0.619), indicating that the rightward shift produced by ketanserin was parallel.
5-HT2C Agonist.
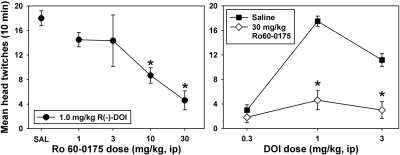
Pretreatment with Ro 60-0175 significantly and dose-dependently attenuated the HTR induced by the peak dose (1.0 mg/kg) of DOI in NIH Swiss mice (F = 5.821, P = 0.002) (Fig. 4, left). Subsequent experiments combined the most effective dose of Ro 60-0175 (30 mg/kg) with multiple doses of DOI to gauge the effects of 5-HT2C receptor stimulation on the entire DOI dose-effect curve. Pretreatment with this dose of Ro 60-0175 significantly suppressed the HTR curve elicited by DOI (F = 29.945, P < 0.001). This attenuation was not surmounted by the administration of higher doses of DOI (Fig. 4, right), as 30 mg/kg Ro 60-0175 significantly reduced head-twitch behavior elicited by both 1.0 mg/kg DOI (t = 8.055, P < 0.01) and 3.0 mg/kg DOI (t = 5.102, P < 0.01) to a similar extent.
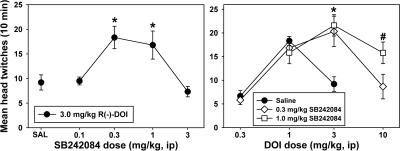
Fig. 4.
Left, biphasic dose-dependent effects of the 5-HT2C antagonist SB242084 on head-twitch behavior elicited by 3.0 mg/kg DOI in Swiss-Webster mice at the Yerkes National Primate Research Center. Abscissa, pretreatment with saline or SB242084, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls. Right, Parallel rightward shifts in the descending limb of the DOI dose-effect curve for head-twitch behavior induced by SB242084 at the Yerkes National Primate Research Center. Abscissa, dose of DOI, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls, whereas hash marks indicate significant differences between 0.3 and 1.0 mg/kg SB242084 pretreatments.
5-HT2C Antagonists.
Prior administration of SB242084 to Swiss-Webster mice had significant (F = 4.402, P = 0.005) biphasic dose-dependent effects on the HTR elicited by subsequent injection of 3.0 mg/kg DOI (Fig. 5, left). A low dose of SB242084 (0.1 mg/kg) did not alter DOI-elicited twitches, but doses of 0.3 and 1.0 mg/kg SB242084 significantly potentiated the HTR induced by this dose of DOI (t = 2.850, P = 0.007 for 0.3 mg/kg SB242084; t = 2.059, P = 0.047 for 1.0 mg/kg SB242084). Doses higher than 1.0 mg/kg SB242084 reduced the HTR elicited by DOI back toward control levels. Subsequent studies combined the two active SB242084 doses (0.3 and 1.0 mg/kg) with multiple doses of DOI to assess the effects of 5-HT2C antagonism on the entire dose-effect curve. SB242084 significantly altered the HTR curve elicited by DOI (F = 8.595, P < 0.001) (Fig. 5, right). Neither dose of SB242084 altered the HTR elicited by DOI doses on the ascending limb of the dose-effect curve, but both doses of the 5-HT2C antagonist produced parallel rightward shifts in the descending limb of the curve. At a dose of 3.0 mg/kg DOI, both 0.3 mg/kg SB242084 (t = 3.977, P < 0.001) and 1.0 mg/kg SB242084 (t = 4.429, P < 0.001) significantly increased head-twitch behavior compared with saline pretreated controls. Likewise, at a dose of 10.0 mg/kg DOI, mice pretreated with 1.0 mg/kg SB242084 exhibited significantly more head-twitch behavior than did mice pretreated with 0.3 mg/kg SB242084 (t = 2.548, P = 0.014), illustrating the dose-dependent effects of the 5-HT2C antagonist on the descending limb of the DOI dose-effect curve.
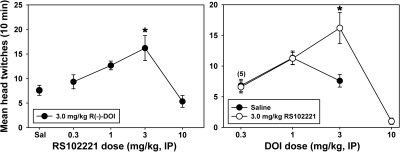
Fig. 5.
Left, biphasic dose-dependent effects of the 5-HT2C antagonist RS 102221 on head-twitch behavior elicited by 3.0 mg/kg DOI in Swiss-Webster mice at the University of Arkansas for Medical Sciences. Abscissa, pretreatment with saline or RS 102221, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls. Right, rightward shift in the descending limb of the DOI dose-effect curve for head-twitch behavior induced by 3.0 mg/kg RS 102221 at the University of Arkansas for Medical Sciences. Abscissa, dose of DOI, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls. The number above the points at the 0.3 mg/kg dose of DOI indicates an n = 5 for the group pretreated with 3.0 mg/kg RS 102221. See Data Analysis for further information.
Parallel studies were performed with the structurally distinct 5-HT2C antagonist RS 102221. Administration of RS102221 to Swiss-Webster mice had significant biphasic dose-dependent effects on the HTR elicited by subsequent injection of 3.0 mg/kg DOI (F = 7.674, P < 0.001) (Fig. 5, left). At 0.3 and 1.0 mg/kg RS 102221, DOI-elicited twitches were not altered, but 3.0 mg/kg RS 102221 significantly potentiated the HTR induced by this dose of DOI (t = 4.341, P < 0.001). Doses higher than 3.0 mg/kg RS 102221 suppressed the HTR elicited by DOI to saline-like levels. Further studies combined 3.0 mg/kg RS 102221 with multiple doses of DOI to assess the effects of 5-HT2C antagonism on the entire dose-effect curve. RS 102221 significantly altered the HTR curve elicited by DOI (F = 7.865, P < 0.001) (Fig. 6, right). It is noteworthy that RS 102221 pretreatment did not alter DOI doses on the ascending limb of the dose-effect curve, but antagonist pretreatment induced a parallel rightward shift in the descending limb of the curve. At a dose of 3.0 mg/kg DOI, 3.0 mg/kg RS 102221 significantly increased head-twitch behavior compared with saline-pretreated controls (t = 3.951, P < 0.001).
Discussion
These experiments were designed to investigate the pharmacological underpinnings of the “inverted-U” shape of dose-effect curves generated in studies of drug-elicited head-twitch behavior. These studies, like the preponderance of previous research using the head-twitch model, focused on DOI because this compound exhibits perhaps the greatest selectivity for 5-HT2A over 5-HT2C receptors among the commercially available phenethylamine hallucinogens. The data reported here support the theory that DOI-induced head-twitch behavior in mice is mediated by agonist activation of the 5-HT2A receptor, whereas the inhibition of head-twitch behavior observed at higher doses results from a competing agonist activation of the 5-HT2C receptor. Four specific hypotheses were tested in these experiments, and support was found for each one.
First, we proposed that the selective 5-HT2A antagonist M100907 would inhibit the ascending limb of the DOI curve but would have no effects on the descending limb. This is exactly what was observed (Fig. 2, left). Indeed, M100907 antagonized the induction of head-twitch behavior, eliciting a significant, downward and rightward shift of the ascending limb of the HTR dose-response curve, but did not alter the descending limb of the dose-response curve for DOI-induced head-twitch behavior. This effect is consistent with data we have previously published regarding the capacity of similar doses of M100907 to antagonize the induction of head-twitch behavior elicited by the phenethylamine hallucinogen 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) (Fantegrossi et al., 2005). Likewise, we hypothesized that the nonselective 5-HT2A/2C antagonist ketanserin would produce a parallel rightward shift in the entire dose-effect function for DOI-elicited head-twitch behavior. Again, the data collected in these studies support this notion (Fig. 2, right). Previous studies have reported that ketanserin and M100907 suppress head-twitch behavior elicited by DOI (Schreiber et al., 1995; Vickers et al., 2001), but only single doses of DOI were used in those experiments. The presently reported results extend those findings by assessing the effects of ketanserin and M100907 against a range of DOI doses, revealing a dissociation between the antagonist effects of these two compounds with important implications for the underlying pharmacology of DOI-elicited head-twitch behavior.
Another hypothesis tested in this study was that selective 5-HT2C agonists would noncompetitively inhibit DOI-elicited twitch behavior, resulting in a flattening of the dose-effect curve and a functionally insurmountable antagonism. This prediction was confirmed using Ro 60-0175 (Fig. 3). Pretreatment with the 5-HT2C agonist dose-dependently suppressed subsequent head-twitch behavior elicited by 1.0 mg/kg DOI (the dose representing the peak of the dose-effect function for DOI). Furthermore, the dose of the 5-HT2C agonist that was maximally effective in terms of suppressing head-twitch behavior induced by this reference dose of DOI also completely blocked head-twitches elicited by higher DOI doses, suggesting a noncompetitive inhibition. The present use of Ro 60-0175 to insurmountably block DOI-elicited head-twitch behavior provides a reasonable attempt to gauge the involvement of 5-HT2C stimulation in the expression of DOI-induced head-twitch behavior, but the in vitro selectivity of this compound for 5-HT2C over 5-HT2A receptors is underwhelming (Knight et al., 2004). There is clearly a continuing need for the development of truly selective 5-HT2C agonists, and a replication of these studies using such a compound would seem warranted.
Fig. 3.
Left, dose-dependent effects of the 5-HT2C agonist Ro 60-0175 on head-twitch behavior elicited by 1.0 mg/kg DOI in NIH Swiss mice at the University of Michigan. Abscissa, pretreatment with saline or Ro 60-0175, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Right, functionally insurmountable antagonism of DOI-elicited head-twitch behavior induced by Ro 60-0175 pretreatment in NIH Swiss mice at the University of Michigan. Abscissa, dose of DOI, expressed in milligrams per kilogram on a log scale. Ordinate, mean head twitches recorded over a 10-min observation period. Asterisks indicate significant differences from saline controls.
Finally, we proposed that pretreatment with selective 5-HT2C antagonists would produce a parallel rightward shift in the descending limb of the DOI curve, in the absence of effects on the ascending limb. We observed exactly that in studies involving the effects of SB242084 and RS102221 with DOI (Figs. 4 and 5, respectively). In each case, pretreatment with the 5-HT2C antagonists dose-dependently increased subsequent head-twitch behavior elicited by 3.0 mg/kg DOI (a dose on the descending limb of the dose-effect curve for DOI), then reduced DOI-elicited head-twitch behavior back to control levels at higher doses. These biphasic effects presumably reflect antagonist effects at 5-HT2A receptors at high doses of SB242084 and RS102221. Nevertheless, doses of SB242084 and RS102221 that significantly potentiated head-twitch behavior elicited by 3.0 mg/kg DOI failed to alter head-twitch behavior elicited by DOI doses on the ascending limb of the DOI dose-effect curve. In the case of SB242084, these selective effects on DOI-elicited head-twitch behavior along the descending limb of the dose-effect function were dose-dependent. Previous reports of the effects of SB242084 against DOI-elicited head-twitch behavior have produced conflicting results. Vickers et al. (2001) studied this drug combination, but only at DOI doses on the ascending limb of the dose-effect curve. That study found no significant interaction between the 5-HT2C antagonist and DOI, which would be expected given the present findings. More recently, Canal et al. (2010) reported that pretreatment with either SB242084 or the 5-HT2C antagonist SB206553 strongly inhibited the expression of DOI-induced HTR in C57BL/6J and DBA/2J mice. This report warrants some comment. First, it is not clear why our results diverge so apparently from those presented by Canal et al. (2010). In some figures, they reported far more head-twitch behavior in response to 1.0 mg/kg DOI (upward of 70 twitches in 10 min) than we observed in the present studies. However, in other figures, Canal et al. presented HTR data that are quite similar to our own DOI dose-effect curves, showing approximately 20 twitches per 10 min in their wild-type mice. It is not readily apparent why the effects of 1.0 mg/kg DOI vary so much across figures in the Canal et al. (2010) publication. Our present studies and those of Canal et al. (2010) used different mouse strains, different forms of DOI (they used the racemic mixture), and different observation cages (they used a 3-l glass beaker). Any or all of those factors could account for the differences observed. It is noteworthy that in both their study and ours, a 3.0 mg/kg pretreatment of SB242084 before 1.0 mg/kg DOI resulted in approximately 18 twitches per 10 min. Our current findings are also consistent with other reports demonstrating that some nonselective 5-HT2C receptor agonists also inhibit DOI-induced head-twitch behavior in the rat (Berendsen and Broekkamp, 1990; Schreiber et al., 1995).
Our findings have important implications for the theory that 5-HT2C agonism is an important component of the hallucinogenic actions of DOI- and d-lysergic acid diethylamide-like hallucinogens (Canal et al., 2010). In this regard, a large-scale clinical trial of the 5-HT2C agonist lorcaserin has recently been published (Smith et al., 2010). In this study, more than 800 subjects self-administered 10 mg of lorcaserin twice per day for 52 weeks, and this dosing regimen was sufficient to induce significantly more weight loss than in subjects in the placebo group. Nevertheless, essentially no psychiatric side effects were reported by any of the subjects taking the 5-HT2C antagonists. In some drug discrimination studies, the discriminative stimulus effects of phenethylamine and indolalkylamine hallucinogens may be blunted by pretreatment with 5-HT2C agonists (Fiorella et al., 1995b), whereas in others, 5-HT2C agonists fail to alter the discriminative stimulus effects of DOI (Schreiber et al., 1994). Finally, behavioral tolerance to DOI has been shown to depend on down-regulation of 5-HT2A receptors, but not 5-HT2C receptors (Smith et al., 1999). All of these findings, and those reported in this article, suggest that 5-HT2C receptor stimulation is not an integral mediator of hallucinogenic effects and may, at least in some instances, oppose the actions of 5-HT2A-mediated hallucinogenesis.
In summary, these studies provide strong evidence supporting the theory that the induction of head-twitch behavior by phenethylamine hallucinogens with mixed agonist effects at 5-HT2A and 5-HT2C receptors is mediated through agonist activity at the 5-HT2A receptor, whereas the subsequent inhibition of head-twitch behavior observed at higher doses is a result of an increasing 5-HT2C agonist activity. In accordance with the current findings, several conclusions follow. First, the ascending limb of a head-twitch dose-response curve defines doses that are functionally selective for 5-HT2A receptors over 5-HT2C receptors, whereas the descending limb described doses that nonselectively activate both 5-HT2A and 5-HT2C receptors. This suggests that an evaluation of doses that induce head-twitch behavior, coupled with an assessment of the maximal amount of head-twitch behavior elicited by a given compound, may be an effective means of determining 5-HT2A potency and efficacy in vivo. Likewise, inhibition of head-twitch behavior may provide useful information regarding in vivo 5-HT2C potency. Finally, the overall shape of the dose-response curve for drug-elicited head-twitch behavior may enable some estimation of in vivo 5-HT2A selectivity of 5-HT2A-preferring 5-HT2A/2C agonists to be made. For example, N-benzyl phenethylamines with increased selectivity for 5-HT2A over 5-HT2C receptors have been described (Braden et al., 2006). On the basis of the present findings, one might expect these novel compounds to elicit head-twitch behavior over a wider range of doses than commercially available analogs with less selectivity. In conclusion, because the current studies provide strong evidence that the induction of head-twitch behavior by phenethylamine hallucinogens such as DOI is mediated by the 5-HT2A receptor, whereas inhibition of head-twitch behavior is mediated by the 5-HT2C receptor, head-twitch behavior may be an important pharmacological effect that can be used to characterize, classify, and discover 5-HT2A and 5-HT2C agonist and antagonist actions in vivo. It may thus be possible to relate other behavioral effects of phenethylamine hallucinogens to their propensity to modulate head-twitch behavior. Future studies to determine whether these findings also apply to tryptamine-based hallucinogens would seem warranted.
Acknowledgments
We thank the animal care staff at the University of Michigan, Yerkes National Primate Research Center, and the University of Arkansas for Medical Sciences for expert animal husbandry services.
This work was supported in part by the Intramural Research program of the National Institutes of Health National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism; the National Institutes of Health National Institute on Drug Abuse [Grant DA020645]; the National Institutes of Health National Center for Research Resources [Grants RR020146, RR00165]; the College on Problems of Drug Dependence; and the American Society for Pharmacology and Experimental Therapeutics [Summer Undergraduate Research Fellowship (to C.M.H.)].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172247.
- 5-HT
- serotonin
- DOI
- R(−)-2,5-dimethoxy-4-iodoamphetamine
- HTR
- head-twitch response
- Ro 60-0175
- S-2-(chloro-5-fluoro-indol-l-yl)-1-methylethylamine
- SB242084
- 6-chloro-5-methyl-1-[(2-[2-methylpyrid-3-yloxy]pyrid-5yl)carbamoyl]indoline
- RS 102221
- 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro[4,5]decane-2,4-dione hydrochloride
- M100907
- (+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol
- ketanserin
- 3-{2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl}quinazoline-2,4(1H,3H)-dione
- SDZ-SER 082
- (+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate
- 2C-T-7
- 2,5-dimethoxy-4-(n)-propylthiophenethylamine.
References
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, De Souza EB. (1990) Autoradiographic characterization of (±)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther 255:843–857 [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. (1990) Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br J Pharmacol 101:667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. (2006) Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol 70:1956–1964 [DOI] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. (2010) The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology 209:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. (2005) Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PA. (1983) The head-twitch response to intraperitoneal injection of 5-hydroxytryptophan in the rat: antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, an LSD antagonist. Neuropharmacology 22:993–1000 [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. (1963) A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother 20:106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11:65–78 [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. (1990a) Withdrawal from chronic treatment with (±)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur J Pharmacol 186:115–118 [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. (1992) Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J Pharmacol Exp Ther 262:692–698 [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. (1990b) Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav 36:901–906 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology 181:496–503 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav 83:122–129 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Van Martin C, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. (2004) Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology 173:270–277 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. (2008) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75:17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Lorrain DS, Rabin RA, Winter JC. (1995b) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology 121:364–372 [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. (1995a) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology 121:347–356 [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452 [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. (2003) Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 23:8836–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Green AR. (1985) A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors.Br J Pharmacol 84:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, O'Shaughnessy K, Hammond M, Schächter M, Grahame-Smith DG. (1983) Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2 antagonist pirenperone. Neuropharmacology 22:573–578 [DOI] [PubMed] [Google Scholar]
- Handley SL, Singh L. (1986) Neurotransmitters and shaking behaviour—more than a “gut-bath” for the brain? Trends Pharmacol Sci 7:324–328 [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. (2004) Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370:114–123 [DOI] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. (1984) Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther 228:133–139 [PubMed] [Google Scholar]
- McKenna DJ, Peroutka SJ. (1989) Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J Neurosci 9:3482–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann R, Bischoff S, Radeke E, Buech O, Delini-Stula A. (1982) Correlations between different measures of antiserotonin activity of drugs. Study with neuroleptics and serotonin receptor blockers. Naunyn Schmiedebergs Arch Pharmacol 321:265–270 [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. (1981) Two distinct central serotonin receptors with different physiological functions. Science 212:827–829 [DOI] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA 105:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. (1994) Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol 264:99–102 [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1995) (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther 273:101–112 [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. (1999) Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology 144:248–254 [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. and Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group (2010) Multicenter, placebocontrolled trial of lorcaserin for weight management. N Engl J Med 363:245–256 [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. (2001) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav 69:643–652 [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. (1997) Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther 282:699–706 [PubMed] [Google Scholar]
- Winter JC, Fiorella DJ, Timineri DM, Filipink RA, Helsley SE, Rabin RA. (1999) Serotonergic receptor subtypes and hallucinogen-induced stimulus control. Pharmacol Biochem Behav 64:283–293 [DOI] [PubMed] [Google Scholar]