Abstract
Luminal ATP increases duodenal bicarbonate secretion (DBS) via brush border P2Y receptors. Because ATP is sequentially dephosphorylated to adenosine (ADO) and the brush border highly expresses adenosine deaminase (ADA), we hypothesized that luminal [ADO] regulators and sensors, including P1 receptors, ADA, and nucleoside transporters (NTs) regulate DBS. We measured DBS with pH and CO2 electrodes, perfusing ADO ± adenosine receptor agonists or antagonists or the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor CFTRinh-172 on DBS. Furthermore, we examined the effect of inhibitors of ADA or NT on DBS. Perfusion of AMP or ADO (0.1 mM) uniformly increased DBS, whereas inosine had no effect. The A1/2 receptor agonist 5′-(N-ethylcarboxamido)-adenosine (0.1 mM) increased DBS, whereas ADO-augmented DBS was inhibited by the potent A2B receptor antagonist N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS1754) (10 μM). Other selective adenosine receptor agonists or antagonists had no effect. The A2B receptor was immunolocalized to the brush border membrane of duodenal villi, whereas the A2A receptor was immunolocalized primarily to the vascular endothelium. Furthermore, ADO-induced DBS was enhanced by 2′-deoxycoformycin (1 μM) and formycin B (0.1 mM), but not by S-(4-nitrobenzyl)-6-thioinosine (0.1 mM), and it was abolished by CFTRinh-172 pretreatment (1 mg/kg i.p). Moreover, ATP (0.1 mM)-induced DBS was partially reduced by (1R,2S,4S,5S)-4–2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt (MRS2500) or 8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine (PSB603) and abolished by both, suggesting that ATP is sequentially degraded to ADO. Luminal ADO stimulates DBS via A2B receptors and CFTR. ATP release, ecto-phosphohydrolases, ADA, and concentrative NT may coordinately regulate luminal surface ADO concentration to modulate ADO-P1 receptor signaling in rat duodenum.
Introduction
Adenosine (ADO) is a purinergic signaling molecule that profoundly affects gut function, including motility, ion secretion, and the modulation of inflammation (Antonioli et al., 2008; Ye and Rajendran, 2009). Most of the research regarding extracellular ADO signaling in the gut has addressed enteric neurons, smooth muscle, afferent neurons, and the immune system, with relatively few studies addressing its effect on epithelial secretory function. Of these, even fewer have reported the effect of luminal ADO on ion secretion in intact epithelia in vivo, because most published studies reported short-circuit current (Isc) measured in intestinal T84 monolayers (Barrett et al., 1989; Strohmeier et al., 1995) or mounted mammalian intestinal tissues (Dobbins et al., 1984; Grasl and Turnheim, 1984; Ghanem et al., 2005). Because apical or basolateral ADO stimulated Isc, ADO receptors were predicted to be expressed on both epithelial surfaces (Barrett et al., 1989; Strohmeier et al., 1995). Luminal AMP and ADO increase glucose transport in intact mouse small intestine in vitro and in vivo, consistent with apical ADO receptors (Kimura et al., 2005).
The duodenal enterocyte actively secretes bicarbonate as part of a system that protects the mucosa from acid injury. We have reported that duodenal bicarbonate secretion (DBS) is regulated by a luminal purinergic regulatory system comprised of the brush border intestinal alkaline phosphatase (IAP), nonlytic ATP release, and enterocyte P2Y receptors, which, when activated, augment epithelial DBS (Mizumori et al., 2009). Although not directly tested, this purinergic regulatory system probably functions to regulate duodenal surface microclimate pH, which prior studies had identified with the use of microelectrodes (Flemström et al., 1982; Flemström and Kivilaakso, 1983). We have also demonstrated in vivo that IAP activity at the epithelial surface is correlated with the rate of DBS (Akiba et al., 2007), further suggesting the importance of IAP in surface microclimate pH regulation. Despite strong evidence supporting the involvement of P2Y receptors in this regulatory system, potent P2Y receptor antagonists only partially inhibit DBS augmented by the perfusion of exogenous ATP or the inhibition of IAP (Mizumori et al., 2009). Because surface phosphohydrolases, including IAP and the combination of ecto-nucleoside triphosphate diphosphohydrolase (ENTPDase or CD39) and 5′-nucleotidase (CD73), hydrolyze ATP to ADO, another known purinergic signaling compound, endogenously produced ADO may also mediate intestinal ion secretion. Unlike ATP, which activates P2Y Gq/11 receptors followed by the increase of cellular Ca2+, ADO activates P1 Gi receptors (A1 and A3) or Gs receptors (A2A and A2B). The latter receptors increase adenylate cyclase activity, which elevates cellular cAMP, directly activating the cystic fibrosis transmembrane regulator (CFTR), an important component of active DBS (Hogan et al., 1997; Seidler et al., 1997; Hirokawa et al., 2004; Akiba et al., 2005). There is, however, no published study addressing the effect of luminal ADO on bicarbonate secretion.
An ADO-degrading enzyme adenosine deaminase (ADA) is highly expressed and active in the enterocyte brush border (Witte et al., 1991; Mohamedali et al., 1993). Furthermore, luminal ADO is absorbed via the enterocyte nucleoside transporters (NTs), such as concentrative NT (CNT or SLC28) and the equilibrative NT (ENT or SLC29) (Pastor-Anglada et al., 2007). These observations suggest that luminal ADO signaling activity may be affected by ADA and NT activity, as reported in the airway epithelium (Hirsh et al., 2007). Furthermore, apical CNT activity is thought to mediate ADO uptake into the epithelial cells, where it can be recycled to ATP, metabolized, or released across the basolateral membrane (Ritzel et al., 1998).
On the basis of these observations, we formulated a hypothesis that, in addition to ATP-P2Y purinergic signaling, DBS is regulated by a related ADO-based system, in which ADO is generated from released ATP by brush border phosphohydrolases and interacts with P1 receptors on the enterocyte brush border. Brush border ADA and NT activity may regulate luminal surface ADO concentration, affecting luminal surface ADO signaling. We thus studied the presence and mechanism of a luminal surface ADO signaling system in rat duodenum, testing the hypothesis that DBS is regulated by ADO. We further hypothesized that ADO-P1 signaling complements ATP-P2Y signaling in the coregulation of DBS. Because physiological, endogenous ADO signaling in the gut has not been reported previously and orally ingested P1 receptor ligands are of current interest in the therapy of intestinal inflammation, an integrative study of intestinal brush border ADO signaling is likely to be of current interest.
Materials and Methods
Chemicals and Animals.
CFTRinh-172 was synthesized by Dr. Samedy Ouk in the Department of Chemistry, University of California, Los Angeles (Akiba et al., 2005). Formycin B (ForB) was purchased from Berry and Associates, Inc. (Dexter, MI). 2′-Deoxycoformycin (DCF), (1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt (MRS2500), and 8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine (PSB603) were obtained from Tocris Bioscience (Ellisville, MO). ADO, AMP, ATP, inosine (INO), N6-cyclopentyladenosine (CPA), 5′-(N-ethylcarboxamido)-adenosine (NECA), N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (IB-MECA), 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS21680), 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX), 8-(3-chlorostyryl)caffeine (CSC), S-(4-nitrobenzyl)-6-thioinosine (NBTI), N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS1754), 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate (MRS1523), HEPES, and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Krebs' solution contained 136 mM NaCl, 2.6 mM KCl, 1.8 mM CaCl2, and 10 mM HEPES at pH 7.0. All studies were performed with the approval of the Veterans Affairs Institutional Animal Care and Use Committee. Male Sprague-Dawley rats weighing 200 to 250 g (Harlan, Indianapolis, IN) were fasted overnight, but had free access to water.
Measurement of DBS.
Duodenal loops were prepared and perfused as described previously (Mizumori et al., 2006; Akiba et al., 2007). Under isoflurane anesthesia (1.5–2.0%), the proximal duodenal loop (perfused length 2 cm) was perfused with pH 7.0 Krebs buffer by using a peristaltic pump (Thermo Fisher Scientific, Waltham, MA) at 1 ml/min. The perfusate was bubbled with 100% O2 and stirred and warmed at 37°C with a heating stirrer (Barnstead International, Dubuque, IA). To eliminate the buffer action of agonists or antagonists, which would overestimate or underestimate the titration volume by using pH-stat, two sets of flow-through pH and CO2 electrodes were connected in the perfusion loop where pH and CO2 concentration ([CO2]) were simultaneously and continuously measured. Because the input (perfusate) [CO2] is ∼0, the effluent [CO2] and pH were used to calculate the total CO2 output equivalent to the secreted HCO3− as described previously (Mizumori et al., 2006; Akiba et al., 2007). After stabilization with continuous perfusion of pH 7.0 Krebs buffer for ∼30 min, the time was set as t = 0. The duodenal loop was perfused with pH 7.0 Krebs buffer from t = 0 min until t = 10 min (basal period). The perfusate was then changed to pH 7.0 Krebs buffer containing agonists or antagonists from t = 10 min until t = 35 min (challenge period), with or without agonists or antagonists.
Experimental Protocol.
We first examined the effect of perfusion of AMP, ADO, or INO on DBS. The duodenal loop was perfused with AMP, ADO, or INO (0.1 mM), the same concentration used for ATP-induced stimulation of DBS (Mizumori et al., 2009), dissolved in pH 7.0 Krebs buffer during the challenge period. Some animals were pretreated with the potent selective CFTR inhibitor CFTRinh-172 (1 mg/kg i.p) 1 h before the experiments. Pretreatment with CFTRinh-172 at this dose eliminates acid-induced HCO3− secretion in rat duodenum (Akiba et al., 2005).
To determine which P1 adenosine receptor subtype (A1, A2A, A2B, or A3) is involved in DBS, we examined the effect of perfusion of P1 receptor agonists at concentrations close to the ED50 for each receptor on DBS: a selective A1 receptor agonist CPA (0.1 mM), a potent A2A receptor agonist CGS21680 (10 μM), a nonselective A1/A2 receptor agonist NECA (0.1 mM), or a selective A3 receptor agonist IB-MECA (10 μM). Furthermore, a potent P1 receptor antagonist was coperfused with ADO (0.1 mM), a selective A1 receptor antagonist DPCPX (0.1 mM), a selective A2A receptor antagonist CSC (0.1 mM), a potent A2B receptor antagonist MRS1754 (10 μM), or a selective A3 receptor antagonist MRS1523 (10 μM). Antagonist concentrations were chosen to be at concentrations near the ID50 of each receptor.
To test the contribution of the ADO-degrading enzyme ADA and the ADO-absorbing CNT or ENT to DBS, we perfused a highly potent ADA inhibitor DCF (1 μM), a CNT inhibitor ForB (0.1 mM), or an ENT inhibitor NBTI (0.1 mM) with or without ADO (0.1 mM).
Because we have shown that luminally released ATP from duodenal mucosa stimulates HCO3− secretion partially via P2Y1 receptor activation (Mizumori et al., 2009) and ATP is degraded to ADO by IAP and ENTPDase/5′-nucleotidase (Zimmermann, 2000), we examined the effect of a highly potent P2Y1 receptor antagonist MRS2500 (1 μM) or a highly selective A2B receptor antagonist PSB603 (10 μM) on ATP (0.1 mM)-induced HCO3− secretion to clarify the contribution of ATP-P2Y and ADO-P1 signals to the ATP-induced DBS.
Expression of P1 Receptor Subtypes in Rat Duodenum.
Immunofluorescence staining was performed as described previously (Akiba et al., 2006) on the cryostat sections of proximal duodenum fixed with 4% paraformaldehyde, using primary antibodies for A1, A2A, A2B, and A3 receptors (rabbit polyclonal, 1:100; Alomone Labs, Jerusalem, Israel), CFTR (M3A7 mouse monoclonal, 1:50; Thermo Fisher Scientific, Waltham, MA), or ADA (goat polyclonal, 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Negative controls were examined by omitting the primary antibody or preincubating antibody with the immunized peptide. The sections were observed under a fluorescence microscope (Carl Zeiss GmbH, Jena, Germany), and the images were captured and recorded with a charge-coupled device color video camera (Hamamatsu Photonics, Hamamatsu, Japan) with imaging software, Simple PCI (Compix Inc. Imaging Systems, Cranberry Township, PA) or a Zeiss confocal laser scanning microscope (LSM 710).
Statistics.
All data are expressed as means ± S.E.M. Data were derived from six rats in each group. Comparisons between groups were made by one-way analysis of variance followed by Fischer's least significant difference test. P values <0.05 were taken as significant.
Results
Effect of ADO on Duodenal HCO3− Secretion.
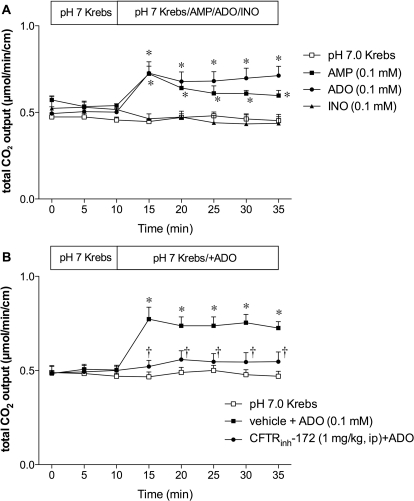
To determine nucleotide or nucleoside specificity, we initially examined the effect of AMP, ADO, or INO (0.1 mM) on DBS. During perfusion of pH 7.0 Krebs buffer, HCO3− secretion (measured as total CO2 output) was stable over time (Fig. 1). AMP and ADO uniformly increased HCO3− secretion, whereas INO had no effect (Fig. 1A), suggesting that ADO is a predominant signaling molecule among the three for HCO3− secretion.
Fig. 1.
Effect of ADO on duodenal HCO3− secretion in rats. A, duodenal HCO3− secretion was measured as total CO2 output with flow-through pH and CO2 electrodes. Perfusion of AMP (0.1 mM) or ADO (0.1 mM) similarly increased total CO2 output, whereas INO (0.1 mM) had no effect. Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group. B, CFTR was inhibited by CFTRinh-172 (1 mg/kg i.p) 1 h before the experiment. CFTR inhibition abolished the ADO effect. Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group; †, p < 0.05 versus ADO group.
To test the role of CFTR in ADO-induced DBS, rats were pretreated with CFTRinh-172 (1 mg/kg i.p). CFTR inhibition abolished ADO-induced HCO3− secretion (Fig. 1B), suggesting that ADO-induced DBS is mediated via CFTR.
Effect of P1 Receptor Agonists or Antagonists on Duodenal HCO3− Secretion.
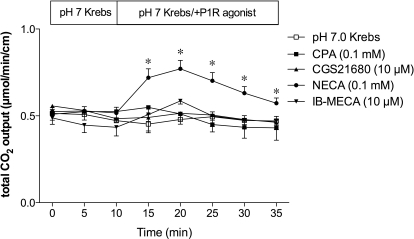
Next, we examined the effect of P1 receptor agonists on DBS. The A1/A2 receptor agonist NECA (0.1 mM) increased HCO3− secretion, whereas CPA (A1, 0.1 mM), CGS21680 (A2A, 10 μM), or IB-MECA (A3, 10 μM) had no effect (Fig. 2).
Fig. 2.
Effect of P1 receptor agonists on duodenal HCO3− secretion in rats. Perfusion of CPA (0.1 mM), CGS21680 (10 μM), or IB-MECA (10 μM) had no effect, whereas NECA (0.1 mM) increased HCO3− secretion. Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group.
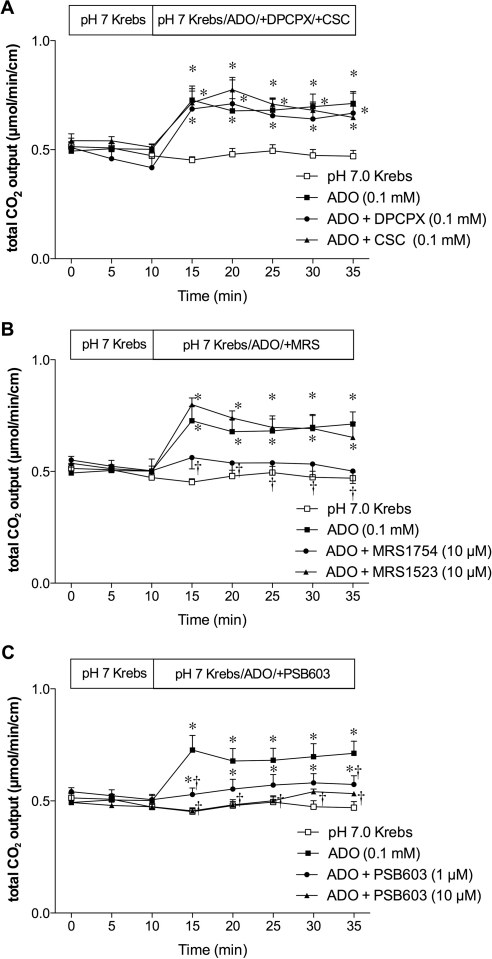
We also examined the effect of P1 receptor antagonists on ADO-induced HCO3− secretion. The selective A1 receptor antagonist DPCPX (0.1 mM) or selective A2A receptor antagonist CSC (0.1 mM) failed to affect ADO-induced HCO3− secretion (Fig. 3A). The potent A2B receptor antagonist MRS1754 (10 μM) abolished ADO-induced HCO3− secretion, whereas the selective A3 receptor antagonist MRS1523 (10 μM) had no effect (Fig. 3B). To confirm the inhibitory selectivity for A2B receptors, a highly selective A2B receptor antagonist PSB603 (1 or 10 μM) was perfused with ADO. PSB603 dose-dependently inhibited ADO-induced HCO3− secretion (Fig. 3C). These results suggest that A2B receptor is involved in ADO-induced DBS.
Fig. 3.
Effect of P1 receptor antagonists on ADO-induced augmented HCO3− secretion in rat duodenum. A, coperfusion of DPCPX (0.1 mM) or CSC (0.1 mM) had no effect on ADO-induced increase of HCO3− secretion. B, coperfusion of MRS1754 (10 μM) abolished the ADO effect, but MRS1523 (10 μM) had no effect. C, coperfusion of PCB603 (1 or 10 μM) inhibited the effect of ADO. Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group; †, p < 0.05 versus ADO group.
Expression of P1 Receptors in the Duodenal Epithelium.
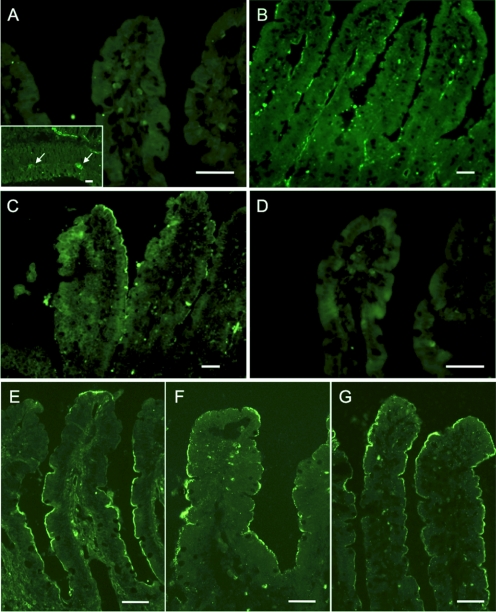
Immunofluorescence for P1 receptors showed that A2B receptor was expressed on the brush border membrane of duodenal villous cells (Fig. 4C). A2A receptor was recognized predominantly in the endothelium in the lamina propria of villi (Fig. 4B). A1 or A3 receptor was not observed in the villi (Fig. 4, A and D), whereas A1 receptor was recognized in the myenteric plexus (Fig. 4A inset). This result supports our hypothesis that luminal ADO stimulates duodenal HCO3− secretion via the A2B receptor. To further demonstrate the presence of the components of luminal ADO-P1 signaling on the duodenal brush border, immunostaining for CFTR, A2B receptor, and ADA was also examined. The brush border membranes of duodenal villous cells expressed CFTR (Fig. 4E), A2B receptor (Fig. 4F), and ADA (Fig. 4G), further supporting our hypothesis.
Fig. 4.
Expression of P1 receptors, CFTR and ADA, in rat duodenal mucosa. A–D, conventional microscopic images. E–G, confocal laser scanning microscopic images. Cryostat sections of fixed rat duodenum were reacted with primary antibodies for P1 receptors, CFTR and ADA. A and D, no specific staining was observed for A1 (A) or A3 (D) receptor in the villi. A1 receptor-like immunoreactivity was recognized in the myenteric plexus (arrows, A, inset). B, A2A receptor-like immunoreactivity was observed mainly on the endothelial cells of vasculature. C and F, A2B receptor-like immunoreactivity was recognized on the brush border membranes of villous cells. E–G, CFTR (E), A2B receptor (F), and ADA (G) were coexpressed on the enterocyte brush border. Bars, 50 μm.
Effect of Inhibition of ADA or NT on DBS.
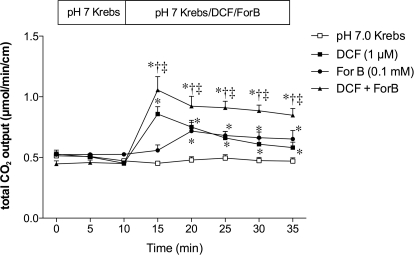
The highly potent ADA inhibitor DCF (1 μM) increased HCO3− secretion, whereas a CNT inhibitor ForB (0.1 mM) gradually increased HCO3− secretion (Fig. 5). Furthermore, coperfusion of DCF and ForB additively enhanced HCO3− secretion (Fig. 5). This result suggests that endogenous ADO stimulates HCO3− secretion and luminal surface ADO concentration is regulated by the brush border ADA activity and CNT.
Fig. 5.
Effect of inhibitors of ADA or CNT on duodenal HCO3− secretion in rats. DCF (1 μM), ForB (0.1 mM), or both was perfused. DCF and ForB augmented HCO3− secretion. *, p < 0.05 versus pH 7.0 Krebs group; †, p < 0.05 versus DCF group; ‡, p < 0.05 versus ForB group.
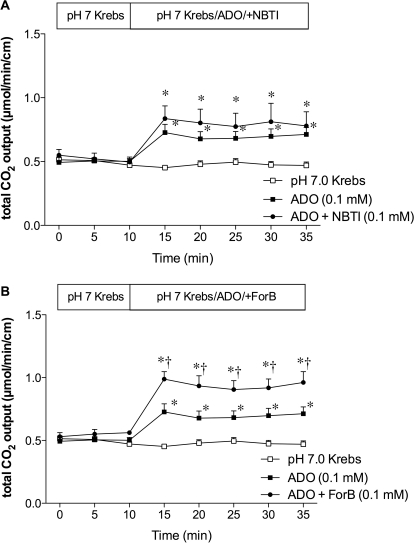
Furthermore, we examined the role of ENT and CNT in ADO-induced HCO3− secretion. The ENT inhibitor NBTI (0.1 mM) had no effect on ADO-induced HCO3− secretion (Fig. 6A), whereas the CNT inhibitor ForB (0.1 mM) enhanced the effect of ADO (Fig. 6B), further suggesting that CNT, not ENT, regulates luminal surface ADO-induced HCO3− secretion in rat duodenum.
Fig. 6.
Effect of ENT or CNT on ADO-induced HCO3− secretion in rat duodenum. A, the ENT inhibitor NBTI (0.1 mM) had no effect on ADO-induced HCO3− secretion. B, ADO-induced HCO3− secretion was enhanced by the addition of ForB (0.1 mM). Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group; †, p < 0.05 versus ADO group. Data represent mean ± S.E.M. (n = 6 rats).
Effect of P2Y1 or A2B Receptor Antagonist on ATP-Induced DBS.
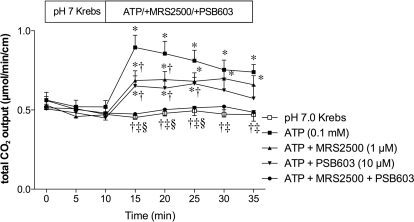
We next tested our hypothesis that endogenous ADO is produced by brush border phosphohydrolases, because ATP is released from the duodenal mucosa in response to physiological secretory stimuli such as luminal acid perfusion, or ATP release is unmasked under the inhibition of IAP or ENTPDase (Mizumori et al., 2009). Furthermore, exogenous ATP activates P2Y1 receptors on the enterocyte brush border, augmenting DBS (Mizumori et al., 2009). We hence studied the sequential effect of exogenous luminal ATP on DBS. Luminal perfusion of ATP (0.1 mM) increased HCO3− secretion (Fig. 7) as reported previously (Mizumori et al., 2009). ATP-induced HCO3− secretion was partially reduced by the addition of a highly potent P2Y1 receptor antagonist MRS2500 (1 μM). A highly selective A2B receptor antagonist PSB603 (10 μM) also reduced ATP-induced HCO3− secretion. Coperfusion of MRS2500 and PSB603 abolished ATP-induced HCO3− secretion (Fig. 7). These data suggest that luminal ATP stimulates HCO3− secretion partially via the P2Y1 receptor; simultaneously sequential degradation of ATP by brush border phosphohydrolases supplies luminal surface ADO in situ to activate the A2B receptors. ATP release thus seems to be the sole source of extracellular purines, which sequentially activate brush border ATP and ADO receptors.
Fig. 7.
Effect of P2Y1 or A2B receptor antagonist on ATP-induced HCO3− secretion in rat duodenum. The potent P2Y1 receptor antagonist MRS2500 (1 μM) or selective A2B receptor antagonist PSB603 (10 μM) was coperfused with ATP (0.1 mM). ATP-induced HCO3− secretion was partially via P2Y1 or A2B receptor. Data represent mean ± S.E.M. (n = 6 rats). *, p < 0.05 versus pH 7.0 Krebs group; †, p < 0.05 versus ATP group; ‡, p < 0.05 versus ATP + MRS2500 group; §, p < 0.05 versus ATP + PSB603 group.
Discussion
Exogenous luminal AMP and ADO, not INO, augmented DBS, consistent with the activation of brush border P1 receptors. Studies with selective P1 agonists and antagonists were consistent with brush border A2B receptors mediating ADO-induced DBS. The presence of A2B receptors, CFTR, and ADA at the enterocyte brush border was confirmed by immunohistochemistry. Additive augmentation of DBS by the ADA inhibitor DCF and the CNT inhibitor ForB implicated these proteins in the regulation of luminal surface ADO abundance, whereas the ENT does not seem to affect luminal ADO concentrations. Finally, DBS augmented by perfusion of exogenous ATP was abolished by coperfusion of P1 and P2 receptor antagonists, but partially by perfusion of either antagonist singly, providing the novel observation that luminal ADO is derived from ATP in the intestine. This is the first study demonstrating that luminal ADO stimulates DBS measured in vivo in an intact preparation, ADO-induced DBS is mediated in intestine by brush border A2B receptors and CFTR, and ADO is endogenously generated in the duodenal lumen.
Despite its considerable importance to neuromuscular signaling, differentiation, and inflammatory modulation, few in vivo descriptions of ADO effects on epithelial secretory function exist. In the 1970s and 1980s, ATP but not ADO was reported to increase intestinal ion secretion (Kohn et al., 1970; Gerencser and Armstrong, 1972; Korman et al., 1982). The earliest descriptions of the prosecretory effect of ADO in intestine were reported in 1984, in which basolateral addition of ∼0.5 to 1.0 mM ADO to chambered rabbit ileum or colon in vitro elicited an increase in electrogenic net Cl− secretion (Dobbins et al., 1984; Grasl and Turnheim, 1984). More recently, numerous groups have reported the prosecretory effects of ADO in cultured epithelial cells or mounted intestinal tissues through the activation of A2 or A1 receptors, respectively (Ghanem et al., 2005; Novak et al., 2008; Wang et al., 2008; Rajagopal and Pao, 2010).
The ready availability of potent and selective adenosine receptor agonists and antagonists enabled us to pharmacologically characterize ADO-induced DBS. CPA, CGS21680, and IB-MECA (A1, A2A, and A3 selective agonists, respectively) did not affect DBS. Nevertheless, NECA increased DBS, indicating that either receptor A1 or A2 is involved in ADO-mediated DBS. Given the nonselective nature of NECA, and the lack of specific A2B agonists, we used specific A1-, A2A-, A2B-, and A3-selective antagonists, DPCPX, CSC, MRS1754, and MRS1523, respectively. Of these antagonists, only MRS1754, the A2B antagonist inhibited ADO-induced DBS. Immunostaining confirmed the presence of A2B receptors on the villous brush border, consistent with the pharmacological observations. In contrast, A2A receptors predominantly localized on the endothelial cells, consistent with the role of ADO in the regulation of blood flow via A2A receptors (Pennanen et al., 1994; Belardinelli et al., 1998). A1 or A3 receptor-like immunoreactivity was not observed on the villous brush border, whereas A1 receptors were located in the myenteric plexus, as reported previously in human jejunum (Christofi et al., 2001). No staining for A3 receptor-like immunoreactivity was observed in the duodenum, although the antibody used recognized the A3 receptor in the esophageal mucosa (data not shown).
DCF, a potent ADA inhibitor, and ForB, a CNT inhibitor, increased DBS. These data demonstrate the presence of endogenous ADO generation in the lumen. Furthermore, ForB, not NBTI, enhanced ADO-induced DBS, suggesting that brush border CNT, unlike ENT, is related to nucleoside transport from the lumen to the enterocytes as predicted (Pastor-Anglada et al., 2007). Present on the apical membrane of epithelial cells, CNTs may absorb luminal nucleosides into the enterocytes. Of the three known paralogs belonging to the SLC28A family, CNT1–3, CNT2 is the most likely duodenal brush border ADO transporter. Because no selective CNT inhibitor is available, data supporting this hypothesis include the high relative duodenal expression of CNT2 and the relative affinity of CNT2 for purines compared with CNT1 or CNT3 (Gray et al., 2004; Larráyoz et al., 2004; Lu et al., 2004; Kim et al., 2007). Similar to our data, an “adenosine scavenging” mechanism affects basolateral extracellular ADO concentrations in cultured T84 cell monolayers (Tally et al., 1996). Pharmacological inhibition of the proteins implicated in adenosine scavenging increases extracellular ADO concentration, increasing Isc via the basolateral A2B receptor. Since that report was published many of the proteins involved in the adenosine scavenging mechanism have been identified; the phenomenon described probably was caused by ENTs localized on the T84 cell basolateral membrane, which transport in either direction in a concentration-dependent fashion and may regulate intracellular nucleoside concentrations (Pastor-Anglada et al., 2007). Furthermore, our findings are consistent with the previous report that luminal ADA and CNT, not ENT, regulate luminal ADO concentrations in the human airway epithelia (Hirsh et al., 2007).
MRS2500, a P2Y1 antagonist, and PSB603, an A2B antagonist, each decreased DBS augmented by luminal perfusion of ATP, and coperfusion of both antagonists completely suppressed ATP-induced DBS. Therefore, luminal ATP and its metabolite ADO may simultaneously activate P2Y and P1 receptors, respectively. We have reported previously that IAP inhibition increases DBS as a probable consequence of increasing ATP output into the lumen because of reduced ATP hydrolysis. This ATP release is partially reduced by CFTR inhibition (Mizumori et al., 2009), whereas CFTR inhibition abolished ADO-induced DBS, suggesting that the CFTR-dependent effect of ATP on DBS may be explained by ADO-A2B signaling. Nevertheless, CFTR inhibition abolished the secretory effect of ADO, which is fully consistent with ADO-induced CFTR activation via A2B Gs receptors that increases intracellular cAMP (Dobbins et al., 1984).
The source of endogenous ADO is controversial. The nonlytic release of ATP from many epithelia has been reported in numerous publications (Woo et al., 2008; Seminario-Vidal et al., 2009). The released ATP is enzymatically hydrolyzed by ENTPDase to AMP, which is then converted by ecto-5′-nucleotidase to ADO (Zimmermann, 2000). Alternatively, ATP is sequentially dephosphorylated to ADO by alkaline phosphatase (Yegutkin, 2008). ADO can then be further degraded to INO by ADA. We have previously reported nonlytic ATP release and brush border IAP and CD39 activity in rat duodenum (Mizumori et al., 2009), suggesting that luminal surface ADO is derived from released ATP, as predicted earlier in T84 cell monolayers (Stutts et al., 1995). Our data in the present study support this hypothesis, because luminal ATP-induced DBS was inhibited by the A2B receptor antagonist. Another possible source of extracellular ADO is the extracellular cAMP–ADO pathway, consisting of cAMP transporter, ecto-phosphodiesterase, and ecto-5′-nucleotidase (Gödecke, 2008). This pathway is observed in the skeletal muscle (Chiavegatti et al., 2008) and ileal muscle strip (Giron et al., 2008). Perfusion of the rat duodenum with cAMP, however, did not increase the rate of DBS, inconsistent with the presence of the cAMP–ADO pathway in rat duodenum (unpublished observations).
In summary, we have reported for the first time that endogenously produced luminal surface ADO increases HCO3− secretion in an intact epithelium in vivo through the activation of A2B receptors. Our data complement the “adenosine scavenging” hypothesis wherein extracellular ADO concentrations are regulated by apical nucleoside transporters and ADA, which in turn regulate intestinal anion secretion.
Acknowledgments
We thank Coleen Palileo for assistance with manuscript preparation.
This work was supported by a Department of Veterans Affairs Merit Review Award (to J.D.K.); the National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK54221] (to J.D.K.); and the animal core of the National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant P30-DK0413] (to J. E. Rozengurt).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171520.
- ADA
- adenosine deaminase
- DBS
- duodenal bicarbonate secretion
- ADO
- adenosine
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CNT
- concentrative nucleoside transporter
- CPA
- N6-cyclopentyladenosine
- DPCPX
- 8-cyclopentyl-1,3-dipropylxanthine
- CSC
- 8-(3-chlorostyryl)caffeine
- DCF
- 2′-deoxycoformycin
- ENT
- equilibrative nucleoside transporter
- ForB
- formycin B
- IAP
- intestinal alkaline phosphatase
- IB-MECA
- N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide
- INO
- inosine
- NBTI
- S-(4-nitrobenzyl)-6-thioinosine
- NECA
- 5′-(N-ethylcarboxamido)-adenosine
- NT
- nucleoside transporter
- Isc
- short-circuit current
- ENTPDase
- ecto-nucleoside triphosphate diphosphohydrolase
- MRS1754
- N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide
- MRS2500
- (1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt
- PSB603
- 8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine
- MRS1523
- 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate
- CGS21680
- 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine.
References
- Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. (2006) Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology 131:142–152 [DOI] [PubMed] [Google Scholar]
- Akiba Y, Jung M, Ouk S, Kaunitz JD. (2005) A novel small molecule CFTR inhibitor attenuates HCO3− secretion and duodenal ulcer formation in rats. Am J Physiol Gastrointest Liver Physiol 289:G753–G759 [DOI] [PubMed] [Google Scholar]
- Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. (2007) Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293:G1223–G1233 [DOI] [PubMed] [Google Scholar]
- Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. (2008) Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther 120:233–253 [DOI] [PubMed] [Google Scholar]
- Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. (1989) Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol Cell Physiol 256:C197–C203 [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. (1998) The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther 284:1066–1073 [PubMed] [Google Scholar]
- Chiavegatti T, Costa VL, Jr, Araújo MS, Godinho RO. (2008) Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol 153:1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. (2001) Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol 439:46–64 [DOI] [PubMed] [Google Scholar]
- Dobbins JW, Laurenson JP, Forrest JN., Jr (1984) Adenosine and adenosine analogues stimulate adenosine cyclic 3′,5′-monophosphate-dependent chloride secretion in the mammalian ileum. J Clin Invest 74:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemström G, Kivilaakso E. (1983) Demonstration of a pH gradient at the luminal surface of rat duodenum in vivo and its dependence on mucosal alkaline secretion. Gastroenterology 84:787–794 [PubMed] [Google Scholar]
- Flemström G, Garner A, Nylander O, Hurst BC, Heylings JR. (1982) Surface epithelial HCO3− transport by mammalian duodenum in vivo. Am J Physiol Gastrointest Liver Physiol 243:G348–G358 [DOI] [PubMed] [Google Scholar]
- Gerencser GA, Armstrong WM. (1972) Sodium transfer in bullfrog small intestine. Stimulation by exogenous ATP. Biochim Biophys Acta 255:663–674 [DOI] [PubMed] [Google Scholar]
- Ghanem E, Lövdahl C, Daré E, Ledent C, Fredholm BB, Boeynaems JM, Van Driessche W, Beauwens R. (2005) Luminal adenosine stimulates chloride secretion through A1 receptor in mouse jejunum. Am J Physiol Gastrointest Liver Physiol 288:G972–G977 [DOI] [PubMed] [Google Scholar]
- Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. (2008) Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134:1116–1126 [DOI] [PubMed] [Google Scholar]
- Gödecke A. (2008) cAMP: fuel for extracellular adenosine formation? Br J Pharmacol 153:1087–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasl M, Turnheim K. (1984) Stimulation of electrolyte secretion in rabbit colon by adenosine. J Physiol 346:93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. (2004) The concentrative nucleoside transporter family, SLC28. Pflugers Arch 447:728–734 [DOI] [PubMed] [Google Scholar]
- Hirokawa M, Takeuchi T, Chu S, Akiba Y, Wu V, Guth PH, Engel E, Montrose MH, Kaunitz JD. (2004) Cystic fibrosis gene mutation reduces epithelial cell acidification and injury in acid-perfused mouse duodenum. Gastroenterology 127:1162–1173 [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. (2007) Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry 46:10373–10383 [DOI] [PubMed] [Google Scholar]
- Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. (1997) Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology 113:533–541 [DOI] [PubMed] [Google Scholar]
- Kim HR, Park SW, Cho HJ, Chae KA, Sung JM, Kim JS, Landowski CP, Sun D, Abd El-Aty AM, Amidon GL, et al. (2007) Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol Res 56:224–236 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Turner JR, Braasch DA, Buddington RK. (2005) Lumenal adenosine and AMP rapidly increase glucose transport by intact small intestine. Am J Physiol Gastrointest Liver Physiol 289:G1007–G1014 [DOI] [PubMed] [Google Scholar]
- Kohn PG, Newey H, Smyth DH. (1970) The effect of adenosine triphosphate on the transmural potential in rat small intestine. J Physiol 208:203–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman LY, Lemp GF, Jackson MJ, Gardner JD. (1982) Mechanism of action of ATP on intestinal epithelial cells. Cyclic AMP-mediated stimulation of active ion transport. Biochim Biophys Acta 721:47–54 [DOI] [PubMed] [Google Scholar]
- Larráyoz IM, Casado FJ, Pastor-Anglada M, Lostao MP. (2004) Electrophysiological characterization of the human Na+/nucleoside cotransporter 1 (hCNT1) and role of adenosine on hCNT1 function. J Biol Chem 279:8999–9007 [DOI] [PubMed] [Google Scholar]
- Lu H, Chen C, Klaassen C. (2004) Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug Metab Dispos 32:1455–1461 [DOI] [PubMed] [Google Scholar]
- Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. (2009) Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol 587:3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. (2006) Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573:827–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamedali KA, Guicherit OM, Kellems RE, Rudolph FB. (1993) The highest levels of purine catabolic enzymes in mice are present in the proximal small intestine. J Biol Chem 268:23728–23733 [PubMed] [Google Scholar]
- Novak I, Hede SE, Hansen MR. (2008) Adenosine receptors in rat and human pancreatic ducts stimulate chloride transport. Pflugers Arch 456:437–447 [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Errasti-Murugarren E, Aymerich I, Casado FJ. (2007) Concentrative nucleoside transporters (CNTs) in epithelia: from absorption to cell signaling. J Physiol Biochem 63:97–110 [DOI] [PubMed] [Google Scholar]
- Pennanen MF, Bass BL, Dziki AJ, Harmon JW. (1994) Adenosine: differential effect on blood flow to subregions of the upper gastrointestinal tract. J Surg Res 56:461–465 [DOI] [PubMed] [Google Scholar]
- Rajagopal M, Pao AC. (2010) Adenosine activates a2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension 55:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel MW, Yao SY, Ng AM, Mackey JR, Cass CE, Young JD. (1998) Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol Membr Biol 15:203–211 [DOI] [PubMed] [Google Scholar]
- Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. (1997) A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505:411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L, Lazarowski ER, Okada SF. (2009) Assessment of extracellular ATP concentrations. Methods Mol Biol 574:25–36 [DOI] [PubMed] [Google Scholar]
- Strohmeier GR, Reppert SM, Lencer WI, Madara JL. (1995) The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270:2387–2394 [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Lazarowski ER, Paradiso AM, Boucher RC. (1995) Activation of CFTR Cl− conductance in polarized T84 cells by luminal extracellular ATP. Am J Physiol Cell Physiol 268:C425–C433 [DOI] [PubMed] [Google Scholar]
- Tally KJ, Hrnjez BJ, Smith JA, Mun EC, Matthews JB. (1996) Adenosine scavenging: a novel mechanism of chloride secretory control in intestinal epithelial cells. Surgery 120:248–254 [DOI] [PubMed] [Google Scholar]
- Wang D, Sun Y, Zhang W, Huang P. (2008) Apical adenosine regulates basolateral Ca2+-activated potassium channels in human airway Calu-3 epithelial cells. Am J Physiol Cell Physiol 294:C1443–C1453 [DOI] [PubMed] [Google Scholar]
- Witte DP, Wiginton DA, Hutton JJ, Aronow BJ. (1991) Coordinate developmental regulation of purine catabolic enzyme expression in gastrointestinal and postimplantation reproductive tracts. J Cell Biol 115:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. (2008) Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCζ-dependent pathway. J Physiol 586:2779–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Rajendran VM. (2009) Adenosine: an immune modulator of inflammatory bowel diseases. World J Gastroenterol 15:4491–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694 [DOI] [PubMed] [Google Scholar]
- Zimmermann H. (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309 [DOI] [PubMed] [Google Scholar]