Abstract
Accumulating evidence suggests that acetylcholine nicotinic systems may contribute importantly to the abuse-related effects of d-methamphetamine (d-MA). The present study was conducted to compare the effects of indirect dopamine (DA) agonists (d-amphetamine, d-MA, and l-methamphetamine), full [(−)-nicotine, anabaseine, (+)-epibatidine, (−)-epibatidine, isoarecolone] and partial (varenicline) nicotinic agonists, and other cholinergic compounds (mecamylamine, dihydro-β-erythroidine hydrobromide, methyllycaconitine, atropine, scopolamine, rivastigmine, and donepezil) in rats trained to discriminate 0.3 mg/kg i.p. d-MA from saline. All indirect DA agonists fully substituted for d-MA in a dose-related manner. Among nicotinic agonists, only (−)-nicotine fully substituted for d-MA in a dose-dependent manner, whereas all other nicotinic agonists and, to a limited extent, muscarinic antagonists produced partial d-MA-like responding. Other cholinergic compounds failed to produce d-MA-like discriminative stimulus effects. In drug interaction studies, varenicline served to dose-dependently attenuate the d-MA-like effects of (−)-nicotine, whereas mecamylamine, but not varenicline, reduced the discriminative stimulus effects of the training dose of d-MA. Differences between (−)-nicotine and other nicotinic agonists may be related to their ability to activate the DA system. These results provide further evidence that nicotinic mechanisms may be useful neurochemical targets for the development of therapeutics for the management of monoaminergic stimulant abuse and addiction.
Introduction
The ability of monoaminergic psychomotor stimulant drugs, including d-methamphetamine (d-MA) and cocaine, to increase extracellular levels of synaptic dopamine (DA) in the mesocorticolimbic DA system and indirectly serve as DA agonists is widely believed to play a critical role in their abuse-related behavioral effects in laboratory animals and humans (Howell and Kimmel, 2008). Accumulating evidence suggests that cholinergic and, in particular, nicotinic mechanisms also may contribute importantly to these abuse-related behavioral effects of psychomotor stimulant drugs (e.g., Schoffelmeer et al., 2002; Hiranita et al., 2004; Zanetti et al., 2007), perhaps through modulation of DA neurotransmission. For example, nicotine has been shown to increase DA levels in the nucleus accumbens (Di Chiara, 2000) in a manner that is additive or synergistic with the effects of psychomotor stimulants (e.g., Gerasimov et al., 2000). These findings are consistent with studies documenting the involvement of dopaminergic activity in maintaining intravenous self-administration of nicotine itself (Di Chiara, 2000) and with nicotine's ability to dose-dependently increase or decrease intravenous cocaine self-administration behavior (e.g., Bechtholt and Mark, 2002). Together, these results suggest that nicotinic mechanisms can influence the behavioral and neurochemical effects of psychomotor stimulant drugs and vice versa.
Drug discrimination procedures have been extensively used to characterize the behavioral pharmacology of psychomotor stimulant drugs that may be related to their subjective effects in humans and their abuse liability. For example, directly and indirectly acting DA agonists have been shown to mimic, and DA receptor antagonists to attenuate, the discriminative stimulus effects of d-MA and cocaine (Callahan et al., 1997; Tidey and Bergman, 1998; Munzar and Goldberg, 2000). Moreover, a close correspondence has been reported between the discriminative stimulus effects of d-MA and cocaine and their ability to increase extracellular concentrations of DA in the nucleus accumbens shell of rats or the caudate nucleus of squirrel monkeys, findings that further contribute to our current understanding of DA's role in their subjective effects (Czoty et al., 2004; Desai et al., 2010).
To date, the contribution of nicotinic activity to the discriminative stimulus effects of d-MA and cocaine has received little attention, and the few studies of nicotine–monoaminergic stimulant interactions in drug discrimination experiments have yielded mixed data (for review see Smith and Stolerman, 2009). DA receptor agonists and antagonists have been shown to partially mimic and attenuate, respectively, the discriminative stimulus effects of (−)-nicotine, suggesting that overlap in the effects of (−)-nicotine and drugs such as d-MA or cocaine involves their DA receptor-mediated effects (for review see Smith and Stolerman, 2009). However, experiments with psychomotor stimulants such as d-MA or cocaine in (−)-nicotine-trained rats or with nicotinic drugs in rats trained with cocaine, d-MA, or d-amphetamine (d-AMP) have yielded a range of data including full, partial, or no substitution for the training drug (for review see Smith and Stolerman, 2009). Although the reasons for such widely varying results are not clear, it seems reasonable to conclude that the discriminative stimulus effects of psychomotor stimulant drugs overlap incompletely with those of (−)-nicotine.
The present study was conducted to further characterize nicotinic and monoaminergic stimulant interactions by evaluating the effects of different types of cholinergic compounds in rats trained to discriminate 0.3 mg/kg d-MA from saline under a 20-response fixed ratio (FR 20) schedule of food reinforcement. First, the effects of several monoamine releasers (d-MA, l-MA, d-AMP) were studied to confirm the expected effects of monoaminergic drugs in this group of d-MA-trained subjects. Subsequently, the ability of nicotinic agonists [(−)-nicotine, anabaseine, (+)-epibatidine, (−)-epibatidine, isoarecolone, varenicline] and antagonists [mecamylamine, dihydro-β-erythroidine hydrobromide (DHβE), and methyllycaconitine (MLA)], muscarinic antagonists (atropine, scopolamine), and acetylcholine esterase (AChE) inhibitors (rivastigmine, donepezil) to mimic d-MA's discriminative stimulus effects were determined. Finally, drug interaction studies were conducted to determine whether drugs that block nicotine's actions, including the partial agonist varenicline and the noncompetitive nicotinic antagonist mecamylamine, also might attenuate the discriminative stimulus effects of d-MA.
Materials and Methods
Subjects.
Seven experimentally naive male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington MA) weighing approximately 290 to 350 g served as subjects. All rats were group-housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle (lights on 7:00 AM). All behavioral experiments were conducted 5 days/week during the light phase of the light/dark cycle between 11:00 AM and 6:00 PM. All subjects were maintained at 85% of free-feeding weight and were fed approximately 15 g of standard laboratory chow approximately 30 min after the session. Water was freely available at all times except during experimental sessions. Experimental protocols were approved by the Institutional Animal Care and Use Committee at McLean Hospital. Rats were maintained in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, National Institutes of Health.
Apparatus.
Subjects were tested in two-lever operant conditioning chambers that were housed in light- and sound-attenuating enclosures (Med Associates, St. Albans, VT). Each chamber was provided with continuous white noise and a fan to mask extraneous sounds. The operant chamber was equipped with a feeder and a tray for food delivery that was centered on the front wall beneath and equidistant from the two response levers. In each chamber, the two response levers were set 17 cm apart and required a downward force of 0.4 N to produce a click that was audible in the chamber and recorded as a response. Light-emitting diodes located directly above the response levers served as stimulus lights and a lamp (house light) located on the back panel of the chamber provided ambient illumination. Experimental variables and data collection were controlled by computers with Med Associates' interfacing equipment and operating software.
d-MA Discrimination.
Subjects were first trained to press both response levers under a FR 20 schedule of food reinforcement. Completion of the FR 20 response requirement resulted in the delivery of one 45-mg pellet (BioServe, Frenchtown, NJ) into the food tray. Subsequently, subjects were trained to discriminate intraperitoneal injections of 0.3 mg/kg d-MA from saline. After d-MA injection, responses only on one lever were reinforced; after saline injection, responses only on the other lever were reinforced. The assignment of d-MA-associated and saline-associated levers was counterbalanced across rats. Training sessions with d-MA and saline were conducted 5 days per week and occurred in a double alternation sequence (e.g., saline, d-MA, d-MA, saline). Each session began by placing the subject inside the experimental chamber 5 min after intraperitoneal injection. After a 5-min timeout during which all lights were extinguished and responding had no scheduled consequences, the house light and light-emitting diodes were illuminated. Only completion of the FR 20 on the injection-associated (correct) lever was reinforced; responses on the other lever (incorrect) reset the FR response requirement for food delivery. Presentation of each food pellet was followed by a 20-s timeout period during which all lights were off and lever responses had no scheduled consequences. Sessions ended after each rat obtained 20 food pellets or after the passage of 15 min, whichever occurred first.
Testing was initiated when the performance criteria of at least 80% appropriate responding overall and during the first FR 20 of the session were met over four consecutive training sessions. Thereafter, test sessions were conducted whenever subjects met the above criteria for 2 consecutive training days in the sequence saline/d-MA or d-MA/saline. Test sessions consisted of four cycles of 25 min (10-min timeout plus 15-min test) during which the FR 20 schedule was in effect. All schedule parameters and contingencies during test sessions were identical to those in training sessions, with the exception that 20 consecutive responses on either lever resulted in the delivery of a food reinforcer. During the test session, incremental doses of the test drug were administered at the start of consecutive 25-min cycles (cumulative dosing). This procedure allowed determination of the effects of up to four cumulative doses during a single test session. To determine the effects of five or more doses of a drug, overlapping ranges of cumulative doses were studied in separate sessions.
Drug interaction studies were conducted by using pretreatment times based on published reports (Stolerman et al., 1995, 1997; Rollema et al., 2007) and data from preliminary experiments. In interaction studies involving d-MA, initial experiments were conducted to establish the effects of 0.3 mg/kg d-MA. Subsequent experiments were conducted by administering vehicle or doses of varenicline (0.03–0.3 mg/kg) or mecamylamine (3.0–5.6 mg/kg) 10 min before the injection of 0.3 mg/kg d-MA, i.e., 20 min before the start of the first session component. Injections of saline were administered before subsequent session components. Interaction studies involving (−)-nicotine, which has a relatively short duration of action, were conducted by injecting doses of varenicline before redetermination of the cumulative dose-effect function for (−)-nicotine.
Drugs.
d-MA hydrochloride, d-AMP sulfate, pentobarbital, atropine sulfate, scopolamine hydrobromide, and (−)-nicotine bitartrate were obtained from Sigma-Aldrich (St. Louis, MO). Mecamylamine hydrochloride, anabaseine dihydrochloride (3,4,5,6-tetrahydro-2,3′-bipyridine dihydrochloride), (+)-epibatidine [(2R)-2-(6-chloro-3-pyridinyl)-7-azabicyclo[2.2.1]heptane monohydrochloride], (−)-epibatidine [(2R)-2-(6-chloro-3-pyridinyl)-7-azabicyclo[2.2.1]heptane monohydrochloride], isoarecolone hydrochloride (1-methyl-4-acetyl-1,2,3,6-tetrahydropyridine hydrochloride), DHβE, and MLA citrate were obtained from the National Institute of Drug Abuse (Bethesda, MD). Varenicline [6,7,8,9-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine] was generously donated by Dr. Hans Rollema (Pfizer Global Research and Development, Groton, CT). l-MA hydrochloride and rivastigmine were generously provided by CoLucid Pharmaceuticals, Inc.(Research Triangle Park, NC), and donepezil was obtained from the National Institute of Mental Health Chemical Synthesis Program (Bethesda, MD). All drugs were dissolved in 0.9% saline, and the pH of nicotine was adjusted to 7.0 with 0.1 N sodium hydroxide. Doses of each drug are expressed in terms of the free base. Anabaseine, (+)-epibatidine, (−)-epibatidine, isoarecolone, mecamylamine, DHβE, and MLA were administered subcutaneously; all other injections were given intraperitoneally.
Data Analysis.
The overall response rate and the percentage of responses occurring on the d-MA-associated lever during all test cycles were calculated for each rat. Data for each dose of a drug from individual rats were averaged to provide mean values (± S.E.M.) for the group of subjects. Only data from test cycles in which ≥20 responses were emitted were included in the calculation of mean values for the percentage of responding on the d-MA-associated lever. If fewer than three subjects met this criterion for a particular dose of drug, no mean value was calculated for percentage d-MA-associated responding at that dose. Data were initially analyzed by comparison to the effects of vehicle injection and 0.3 mg/kg of d-MA. Doses of drugs that produced, on average, <50% responding on the d-MA-associated lever were considered to be significantly different from the training dose of d-MA, whereas doses of drugs that produced a mean of 50 to 80% responding on the d-MA-associated lever were considered to substitute partially for the training dose of 0.3 mg/kg of d-MA. Doses of drugs that produced a mean of >80% responding on the d-MA-associated lever were considered to substitute fully for the training dose of 0.3 mg/kg d-MA. Averaged dose-effect data also were analyzed by using standard linear regression and analysis of variance. Whenever possible, ED50 values and their 95% confidence limits were determined from points on the linear part of the ascending portions of the dose-effect curves (Snedecor and Cochran, 1967).
Data were further analyzed to compare potency and maximum effects among drugs, evaluate drug interactions, and examine correspondence between the effects of nicotinic drugs in the present experiments and their affinities for different types of nicotinic receptor. First, differences in potency (ED50) and the maximal effects of selected pairs of drugs were assessed with Student's t tests. When appropriate, analysis of variance followed by Dunnett's t test or a paired t test was used to evaluate statistical significance of selected data points (defined at the 95% level of confidence; p < 0.05). The effects of d-MA or (−)-nicotine alone and after pretreatment with varenicline or mecamylamine in drug interaction experiments were evaluated by using data only from the first component of sessions in which drug combinations were evaluated. This was done to minimize the influence of pharmacokinetic factors in experimental results, i.e., decreasing concentrations of the pretreatment drug over the 100-min session. In experiments to evaluate varenicline–nicotine interactions, ED50 values were determined for nicotine alone and in the presence of varenicline; pairs of ED50 values were considered to be significantly different if their 95% confidence limits did not overlap. When significant differences in ED50 values were obtained, relative potency estimates were calculated by standard parallel-line bioassay techniques as described by Finney (1964). Correspondence between the ED50 values of nicotinic drugs that substituted for d-MA in the present experiments and their in vitro binding affinities for α4β2 and α7 nicotinic receptors, obtained from previously published radioligand binding experiments, was evaluated by comparing potencies relative to nicotine in both types of experiments. Affinity values from multiple studies in rat brain were averaged and, whenever possible, were taken from experiments using [3H]nicotine binding for the α4β2 receptor subtype and 125I-α-bungarotoxin binding for the α7 nicotinic receptor subtype (see Table 2).
TABLE 2.
ED50 values (with 95% confidence intervals; 95% CL) and potencies of nicotinic agonists relative to the d-MA-like effects produced by (−)-nicotine in rats trained to discriminate intraperitoneal injections of 0.3 mg/kg d-MA from saline
The in vitro affinity of nicotinic agonists in inhibiting (−)-[3H]nicotine binding at the (α4β2) nicotinic ACh receptors and inhibiting 125I-α- bungarotoxin binding for the α7 nicotinic receptors represents the average of values taken from the references cited. In vitro binding affinity values at nicotinic receptor subtypes represent the average of values taken from experiments conducted in rat brain using [3H]nicotine binding for the α4β2 subtype and 125I-α-bungarotoxin binding for the α7 nicotinic receptor subtype.
| Drug | Doses | ED50 (95% CL) | Relative Behavior Potency | In Vitro Affinity (Ki Values) at α4β2 | Relative Affinity at α4β2 Receptors | In Vitro Affinity (Ki Values) at α7 | Relative Affinity at α7 Receptors |
|---|---|---|---|---|---|---|---|
| mg/kg (μmol/kg) | mg/kg (μmol/kg) | nM | nM | ||||
| (−)-Nicotine | 0.01–0.1 (0.06–0.62) | 0.03 (0.02–0.04) | 1 | 3.4a–e | 1 | 4895c | 1 |
| 0.18 (0.12–0.25) | |||||||
| (+) Epibatidine | 0.0001–0.001 (0.00048–0.0048) | 0.00024 (0.00002–0.00077) | 0.007 | 0.05b,d | 0.015 | 255f | 0.052 |
| 0.0012 (0.000096–0.0037) | |||||||
| (−) Epibatidine | 0.0001–0.001 (0.00048–0.0048) | 0.00032 (0.00006–0.0017) | 0.008 | 0.06b,d | 0.018 | 109f | 0.022 |
| 0.0015 (0.00029–0.0081) | |||||||
| Isoarecolone | 1.0–10 (6.88–68.75) | 2.49 (0.18–9.48) | 95.1 | 611c | 179.7 | >100,000c | 20.43 |
| 17.12 (1.24–65.18) | |||||||
| Anabaseine | 0.3–3.0 (1.87–18.72) | 0.54 (2.10–75.15)g | 18.7 | 32h | 9.41 | 58h | 0.012 |
| 3.37 (13.11–469.0) | |||||||
| Varenicline | 0.01–3.0 (0.05–14.20) | N.C. | 0.17e,i | 0.05 | 620i | 0.127 |
N.C., not conducted.
Jensen et al., 2003; Anderson and Arenric, 1994; Xiao and Kellar, 2004; Marks et al., 1986, 1996; Decker et al., 1995.
Estimate because of a nonsignificant regression.
Results
d-MA Discrimination.
Subjects achieved criterion levels of d-MA discrimination over the course of 63.4 (SD = 14.7) training sessions. Subsequently, the training dose of d-MA (0.3 mg/kg) maintained reliable discriminative stimulus control throughout the study (>18 months). The position and slope of the dose-response function for d-MA did not differ significantly from first to last determinations, and the ED50 of d-MA remained relatively constant (see Table 1). In addition, no significant changes in response rates were observed throughout the experiment. Consequently, dose-effect data for d-MA at the beginning and end of the present studies were averaged for further analyses and data presentation. Averaged data are given in the d-MA row in Table 1 and are the basis for calculation of the relative potency for l-MA and d-AMP.
TABLE 1.
Effective doses and their 95% confidence intervals (95% CL) and relative potencies of d-MA and other indirect DA agonists in rats trained to discriminate intraperitoneal injections of 0.3 mg/kg (2.01 μmol/kg) d-MA from saline
| Drug | Doses | ED50 (95% CL) | Potency Relative to d-MA |
|---|---|---|---|
| mg/kg (μmol/kg) | mg/kg (μmol/kg) | ||
| d-MA: beginning | 0.01–0.3 (0.07–2.01) | 0.07 (0.04–0.14) | |
| 0.47 (0.27–0.94) | |||
| d-MA: end of study | 0.01–0.3 (0.07–2.01) | 0.06 (0.04–0.11) | 1.10 (0.47–2.68) |
| 0.40 (0.27–0.74) | |||
| d-MA | 0.01–0.3 (0.07–2.01) | 0.08 (0.05–0.11) | |
| 0.54 (0.34–0.74) | |||
| l-MA | 0.03–1.0 (0.20–6.70) | 0.41 (0.20–1.63) | 0.20 (0.09–0.42) |
| 2.75 (1.34–10.9) | |||
| d-AMP | 0.03–1.0 (0.22–7.40) | 0.05 (0.02–0.11) | 1.49 (0.71–3.42) |
| 0.37 (0.15–0.81) |
Indirect Monoamine Agonists and Pentobarbital.
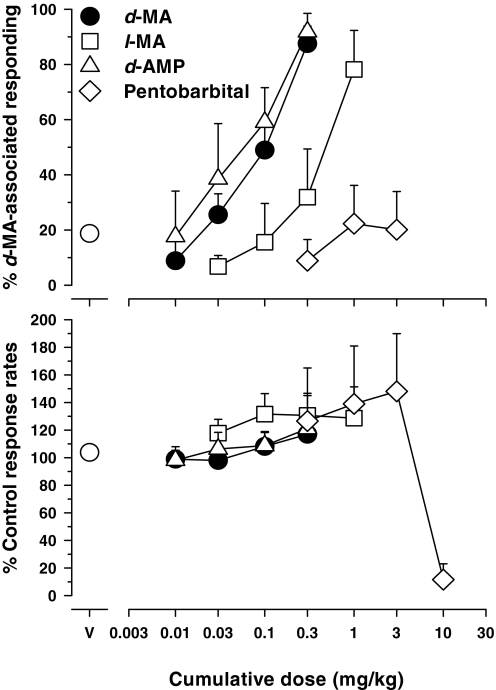
Both the levorotatory isomer of d-MA, l-MA, and d-AMP produced dose-dependent and nearly full or full substitution for 0.3 mg/kg d-MA, with maxima of 78 and 92% responding on the d-MA-associated lever after cumulative doses of, respectively, 1.0 mg/kg of l-MA and 0.3 mg/kg of d-AMP (Fig. 1, top, □ and ▵, respectively). The rank order of potency with which these monoamine-releasing agents substituted for the training dose of d-MA was d-AMP ≈ d-MA > l-MA. Based on relative potency estimates, d-MA and d-AMP were equipotent and approximately five to eight times more potent than l-MA (Table 1). Overall response rates were not substantively changed from vehicle values by any of the indirect monoamine agonists studied in these experiments (Fig. 1, bottom).
Fig. 1.
Effects of cumulative administration of the indirect monoamine agonists d-MA, l-MA, and d-AMP and the barbiturate pentobarbital in rats trained to discriminate 0.3 mg/kg d-MA from saline. Ordinates: percentage d-MA-associated responding (top), response rates as a percentage of response rate after saline administration (bottom). Abscissae: drug dose in mg/kg (log scale). Each data point represents all subjects tested (at least six rats) at each dose. The percentage of responses emitted on the d-MA-associated lever was not plotted if fewer than half of the subjects responded at that dose. During control sessions, mean rates of responding were 1.27 ± 0.09 responses per second after injection of MA and 1.08 ± 0.03 responses per second after injection of saline. Note the variability for vehicle is small and has been included in the symbols.
In contrast to the indirect monoamine agonists, the barbiturate pentobarbital did not substitute for the training dose of d-MA (maximum: 22.3% after 1.0 mg/kg; Fig. 1, top, ♢). Decreases in response rate precluded the testing of higher doses of pentobarbital (Fig. 1, bottom, ♢).
Nicotinic Agonists.
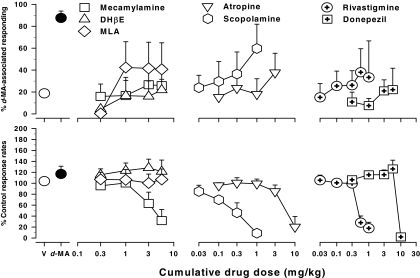
Like the indirect monoamine agonists studied in these experiments, (−)-nicotine fully substituted for 0.3 mg/kg d-MA in a dose-dependent manner. As shown in Fig. 2, top left, ■, full substitution (93%) was achieved after the cumulative dose of 0.1 mg/kg (−)-nicotine. The ED50 value for substitution by (−)-nicotine for d-MA was 0.03 mg/kg (0.18 μmol/kg, Table 2). (−)-Nicotine did not change response rates significantly from control values (Fig. 2, bottom left, ■).
Fig. 2.
Effects of the cumulatively administered nicotinic agonists, (−)-nicotine, (+) epibatidine, (−) epibatidine, isoarecolone, anabaseine, and varenicline in rats trained to discriminate 0.3 mg/kg d-MA from saline. Ordinates and abscissae are as in Fig. 1. Each data point represents all subjects tested (at least five to seven rats) at each dose.
Like (−)-nicotine, cumulative administration of anabaseine, isoarecolone, and the (+)- and (−)-isomers of epibatidine produced dose-dependent increases in responding on the d-MA-associated lever (Fig. 2, top, ▵, ▿, ♢, and □, respectively). Unlike (−)-nicotine, however, substitution was incomplete at the highest doses of these agonists that could be studied and ranged from approximately 55 to 70% responding on the d-MA-associated lever. Higher doses of each drug completely eliminated responding in all rats, precluding further testing (Fig. 2, bottom). No indication of a plateau in maximal effect was apparent with either enantiomer of epibatidine or isoarecolone. However, the d-MA-like effects of anabaseine did appear to level off, and doses of anabaseine ranging from 1.0 to 5.6 mg/kg produced comparable, approximately 40 to 55%, levels of responding on the d-MA-associated lever. With regard to potency, relative potency estimates indicate that (+)-epibatidine and (−)-epibatidine were approximately 75- and 50-fold more potent than (−)-nicotine, whereas isoarecolone and anabaseine were approximately 175- and 20-fold less potent than (−)-nicotine in producing d-MA-like discriminative stimulus effects (Table 2). Thus, the rank order of potency with which nicotinic agonists produced d-MA-like effects was: (+)-epibatidine ≈ (−)-epibatidine > (−)-nicotine > anabaseine > isoarecolone (Table 2).
Like other nicotinic agonists, the nicotinic partial agonist, varenicline (0.01–3.0 mg/kg) produced responding on the d-MA-associated lever (Fig. 2, top right). However, these effects generally were not dose-related and, as with anabaseine, resulted in intermediate, approximately 30 to 60%, levels of responding on the d-MA-associated lever. The highest dose of varenicline markedly decreased responding in all subjects, precluding further testing (Fig. 2, bottom right).
Cholinergic Antagonists and Acetylcholinesterase Inhibitors.
Cholinergic antagonists and AChE inhibitors failed to substitute for the training dose of 0.3 mg/kg d-MA in the present studies (Fig. 3). The effects of the nonselective nicotinic antagonist mecamylamine and the selective α4β2 and α7 nicotinic antagonists DHβE and MLA, respectively, did not differ significantly from those of vehicle and, at the highest doses of these drugs, <50% responding on the d-MA-associated lever was observed [Dunnett's t test (F values (4,21-29) ≤ 1.01; p values > 0.05; Fig. 3, top)]. The highest dose of mecamylamine (5.6 mg/kg) also significantly reduced rates of responding to approximately 30% of vehicle values (Fig. 3, bottom left, □). Neither DHβE or MLA significantly altered response rates, although doses of DHβE up to 2-fold higher than those previously effective as nicotinic antagonists in rats were evaluated in the present experiments (Stolerman et al., 1997; Shoaib et al., 2000; Fig. 3, bottom left).
Fig. 3.
Effects of the nicotinic antagonists (mecamylamine, DHβE, MLA), muscarinic antagonists (atropine, scopolamine), and AChE inhibitors (rivastigmine, donepezil) after cumulative injections in rats trained to discriminate 0.3 mg/kg d-MA from saline. Ordinates and abscissae are as in Fig. 1. Each data point represents all subjects tested (at least five to seven rats) at each dose.
Like nicotinic antagonists, the muscarinic antagonists atropine and scopolamine failed to substitute for the training dose of 0.3 mg/kg d-MA over a wide range of behaviorally active doses. Although the effects of scopolamine and, to a lesser extent, atropine appear dose-related (Fig. 3, center), the highest cumulative doses of atropine (3.2 mg/kg) and scopolamine (1.0 mg/kg) produced only approximately 35 and 60% responding on the d-MA-associated lever responding, respectively. These doses of the muscarinic antagonists reduced rates of responding to approximately 20% or less of control values, precluding studies with yet higher doses (Fig. 3, bottom center).
The AChE inhibitors rivastigmine (0.03–1.0 mg/kg) and donepezil (0.3–10 mg/kg) also did not substitute for d-MA and, over the range of doses that could be studied, failed to engender d-MA-associated lever responding significantly above vehicle values (Fig. 3, top right). The highest doses of each drug (1.0 mg/kg rivastigmine and 10 mg/kg donepezil) greatly reduced or eliminated responding. Thus, additional experiments with higher doses of the AChE inhibitors were not conducted.
Effects of Pretreatment with Varenicline or Mecamylamine.
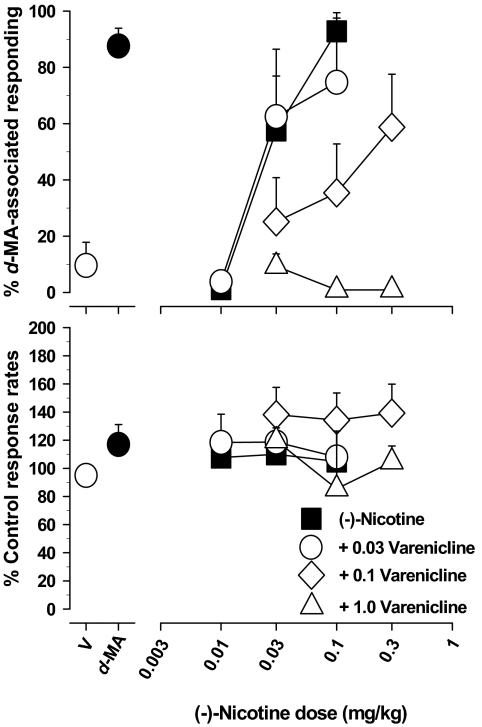
Pretreatment with varenicline (0.03–1.0 mg/kg) attenuated the d-MA-like effects of (−)-nicotine in a dose-related manner. The lowest dose of varenicline, 0.03 mg/kg, did not significantly alter the ability of (−)-nicotine to substitute for d-MA. However, a 3-fold higher dose of varenicline (0.1 mg/kg) produced an approximately 10-fold rightward shift in the (−)-nicotine dose-response curve (Fig. 4), and the highest dose of varenicline (1.0 mg/kg) completely eliminated the d-MA-like effects of 0.03 to 0.3 mg/kg (−)-nicotine. Throughout these experiments, response rates were not significantly altered by either (−)-nicotine or (−)-nicotine in combination with varenicline (Fig. 4, bottom).
Fig. 4.
Effects of pretreatment with the partial nicotinic agonist varenicline on d-MA-like responding produced by (−)-nicotine in rats trained to discriminate 0.3 mg/kg d-MA from saline. Ordinates are as in Fig. 1. Abscissae show dose of (−)-nicotine (mg/kg; log scale). See Fig. 1 for other details. Each data point represents all subjects tested (at least three to seven rats) at each dose.
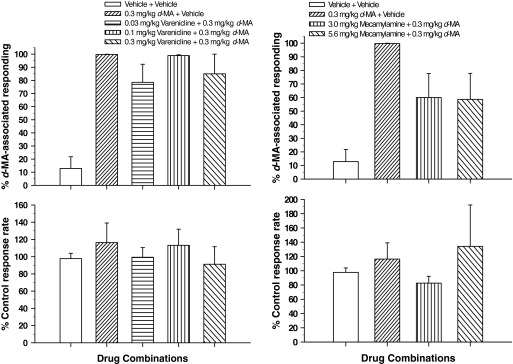
Despite its clear attenuation of the d-MA-like effects of (−)-nicotine, varenicline (0.03–0.32 mg/kg) failed to significantly alter the effects of d-MA itself (t values ≤ 1.53; p values ≥ 0.186). Although the highest dose of 1.0 mg/kg was not studied, lower doses of varenicline that dose-dependently attenuated the effects of (−)-nicotine had no consistent effect on the discriminative stimulus effects of the training dose of d-MA (e.g., 0.3 mg/kg; Fig. 5, left). As in experiments with (−)-nicotine, response rates were not altered by the combination of varenicline and d-MA (t values ≤ 0.944; p values ≥ 0.388; Fig. 5, left bottom).
Fig. 5.
Changes in the d-MA dose-effect curve for discriminative stimulus effects produced by pretreatments with the varenicline and the nicotinic antagonist mecamylamine. Ordinates are as in Fig. 1. Abscissae show drug combinations. See Fig. 1 for other details. Each data point represents all subjects tested (at least five to seven rats) at each dose.
In contrast to varenicline, pretreatment with the nonselective nicotinic antagonist mecamylamine (3.0 and 5.6 mg/kg) somewhat attenuated the discriminative stimulus effects of the training dose of 0.32 mg/kg d-MA, with a reduction in the percentage of responding on the d-MA-associated lever that approached statistical significance (t = 2.38, 2.28; p = 0.072, 0.063, respectively; Fig. 5, right). Pretreatment doses of 3.0 and 5.6 mg/kg produced a comparable 40% reduction in the percentage responding on the d-MA-associated lever, with no consistent effects on response rate (Fig. 5, bottom right). Response rates were reduced to approximately 80% of control values after treatment with 3.0 mg/kg mecamylamine and increased to approximately 135% of control values after treatment with 5.6 mg/kg mecamylamine. However, considerable variability in the rate-increasing effects of the latter pretreatment dose among subjects precluded statistical significance (t values ≤ 1.94; p values ≥ 0.111).
Discussion
The present studies were conducted to compare the effects of dopaminergic stimulants and cholinergic ligands in subjects trained to discriminate 0.3 mg/kg d-MA from vehicle. The dopaminergic drugs d-AMP and the enantiomers of MA engendered dose-dependent and full or nearly full substitution for d-MA. These results are consistent with previous findings in d-MA-trained rats, pigeons, and squirrel monkeys (Sasaki et al., 1995; Tidey and Bergman, 1998; Munzar and Goldberg, 2000; Czoty et al., 2004; Desai et al., 2010). The two enantiomers of MA had similar discriminative stimulus effects; however, the levorotatory enantiomer, l-MA, was approximately 5-fold less potent than d-MA, presumably reflecting its greater potency in stimulating presynaptic DA release. These findings agree with previous comparisons showing l-MA to be moderately less potent than d-MA in stimulating DA release in rat brain (Rothman and Baumann, 2003) and producing stimulant-like subjective effects in humans (Mendelson et al., 2008). The present results also are comparable with previous findings with AMP showing greater potency of d-AMP compared with l-AMP in studies of their discriminative stimulus and other behavioral effects (e.g., Glennon and Young, 1984; Yasar et al., 1993). Of interest, the enantiomers of AMP have been reported to increase brain levels of norepinenphrine with comparable potency (e.g., Heikkila et al., 1975; Kuczenski and Segal, 1989). In conjunction, the greater potency of the d-enantiomer of MA or AMP in both neurochemical and behavioral studies further supports the idea that dopaminergic mechanisms play a prominent role in the discriminative stimulus and subjective effects of monoaminergic psychomotor stimulant drugs.
(−)-Nicotine also produced dose-dependent increases in responding on the 0.3 mg/kg d-MA-associated lever and, at the highest dose, fully substituted for d-MA without significantly altering response rates. In previous studies using 1.0 mg/kg d-MA as the discriminative stimulus, (−)-nicotine also increased drug-associated responding, but only partially substituted for d-MA (Gatch et al., 2008). Based on ED50 values in the two studies, (−)-nicotine was approximately 4-fold less potent in subjects trained with the higher dose of d-MA. Such differences in the maximal effects and potency of (−)-nicotine are not surprising and probably can be attributed to dose-related differences in the stimulus intensity of the training dose of d-MA. In this regard, previous studies of nondopaminergic drugs (e.g., noradrenergic transport blockers, N-methyl-d-aspartate antagonists, and adenosine antagonists) have yielded similar findings in subjects trained with differing doses of monoaminergic stimulants, i.e., greater potency and substitution at lower compared with higher training doses (e.g., Spealman, 1995; Kantak et al., 1995; Bergman et al., 2006). Together, these findings suggest that the generalization gradient for drug discrimination narrows as training dose increases and probably becomes more dependent on similar neurochemical properties of test and training drugs.
Previous drug discrimination studies have yielded mixed results regarding common properties of (−)-nicotine and monoaminergic stimulants (for review, Smith and Stolerman, 2009). For example, DA receptor agonists and antagonists have been shown to partially mimic and attenuate, respectively, the discriminative stimulus effects of (−)-nicotine, suggesting that overlap in the effects of (−)-nicotine and monoaminergic stimulants involve common DA receptor-mediated effects. However, studies with (−)-nicotine in subjects trained to discriminate monoaminergic stimulants or with monoaminergic stimulants in (−)-nicotine-trained subjects have yielded differing results including full, partial, and no substitution. These varying findings may be related to differences in species or methodology, especially in training dose, across studies. The absence of consistent patterns of substitution in these studies supports the idea that (−)-nicotine's discriminative stimulus effects overlap, but are not identical to, those of monoaminergic stimulants.
Like (−)-nicotine, (+)-epibatidine, (−)-epibatidine, and isoarecolone engendered dose-related increases in responding on the d-MA-associated lever. Unlike (−)-nicotine, however, each of these agonists failed to fully substitute for d-MA and, at the highest doses, dramatically decreased response rates. In previous (−)-nicotine discrimination studies, isoarecolone and the two enantiomers of epibatidine substituted fully for (−)-nicotine (Reavill et al., 1987; Damaj et al., 1994). In view of the present findings with (−)-nicotine, the inability of isoarecolone and epibatidine enantiomers to similarly substitute for d-MA at doses below those that markedly decreased response rates was somewhat unexpected. It is unclear whether such differences among ligands with comparable behavioral profiles can be ascribed to pharmacokinetic considerations (e.g., differences in intensity of stimulus effects related to rapidity of onset) or reflects yet unidentified differences in mechanism of action.
The nicotinic agonists anabaseine and varenicline also produced responding on the d-MA-associated lever but, in contrast to the ligands discussed above, their effects plateaued at an intermediate level across a 10- to 30-fold range of doses. Varenicline, currently prescribed for smoking cessation (e.g., Cahill et al., 2007), previously has been shown to fully substitute for (−)-nicotine in nicotine-trained rats (Rollema et al., 2007). To our knowledge, the effects of anabaseine, a minor tobacco alkaloid, have not been characterized previously in drug discrimination studies. The plateau in the d-MA-like effects of anabaseine and varenicline in the present experiments suggests that they are incapable of producing (−)-nicotine-like full substitution for d-MA. To the extent that the limited effects of varenicline in the present experiments are consistent with its nicotinic partial agonist actions, these data raise the possibility that, like varenicline, anabaseine also may serve as a nicotinic partial agonist. However, this suggestion remains speculative in the absence of interaction studies to determine whether anabaseine, like varenicline, also might antagonize the d-MA-like effects of (−)-nicotine.
In view of the ability of nicotinic direct agonists to produce d-MA-like discriminative stimulus effects, it might be reasonable to expect similar findings with AChE inhibitors that indirectly increase cholinergic activity at nicotinic receptors. However, unlike nicotinic receptor agonists, the AChE inhibitors rivastigmine and donepezil failed to produce appreciable levels of d-MA-like effects in the present experiments. These data are consistent with a previous study indicating a lack of cocaine-like discriminative stimulus effects of AChE inhibitors (Järbe, 1978) and may reflect interference by increased muscarinic activity that also is consequent to cholinesterase inhibition.
Of interest, the muscarinic antagonists atropine and scopolamine produced some evidence of d-MA-like effects at doses that did not greatly decrease response rates. These latter results agree with previous findings that muscarinic antagonists may substitute for or enhance cocaine's discriminative stimulus effects in rats (e.g., Katz et al., 1999) and add to a increasing literature documenting the ability of muscarinic antagonists to augment the behavioral effects of psychomotor stimulant drugs (Williams and Adinoff, 2008). Tanda et al. (2007) showed that selective antagonism at muscarinic M1 receptors does not increase DA efflux, but may accentuate cocaine-induced increases in DA levels in rat brain. Such neurochemical actions may provide a mechanistic basis for the ability of muscarinic antagonists to either enhance or partially reproduce monoaminergic stimulant effects.
Unlike nicotinic agonists or muscarinic antagonists, the noncompetitive (mecamylamine), α4β2-selective (DHβE), and α7-selective (MLA) nicotinic receptor antagonists failed to engender appreciable levels of d-MA-associated lever responding. These results are consistent with data from previous studies in cocaine-trained rats (Desai et al., 2003) and are pharmacologically coherent, i.e., they probably reflect the inability of nicotinic receptor antagonists to activate nicotinic receptors.
In drug interaction experiments with d-MA, the nicotinic partial agonist varenicline was without effect, whereas doses of mecamylamine that did not greatly disrupt FR response rates partly attenuated the discriminative stimulus effects of d-MA. The inability of varenicline, which readily antagonized the d-MA-like effects of (−)-nicotine, to attenuate the effects of d-MA itself provides strong evidence that monoaminergic and nicotinic actions, although dissimilar, have overlapping or convergent discriminative stimulus properties. The effects of mecamylamine, which occurred at doses that are comparable with or greater than those required to block (−)-nicotine's discriminative stimulus effects (Stolerman et al., 1997; Shoaib et al., 2000), were somewhat surprising because mecamylamine previously has been reported not to attenuate the effects of psychomotor stimulants in drug discrimination or locomotor activity studies (Karler et al., 1996; Desai et al., 2003; Gatch et al., 2008). Although it is difficult to reconcile the present findings with those observations, it is interesting that, in studies of intravenous cocaine self-administration behavior, Levin et al. (2000) have reported that mecamylamine attenuated the reinforcing effects of cocaine in rats without greatly interfering with food-maintained behavior. Thus, under some conditions, noncompetitive antagonism by mecamylamine may overshadow or mask stimulus effects of monoaminergic stimulants.
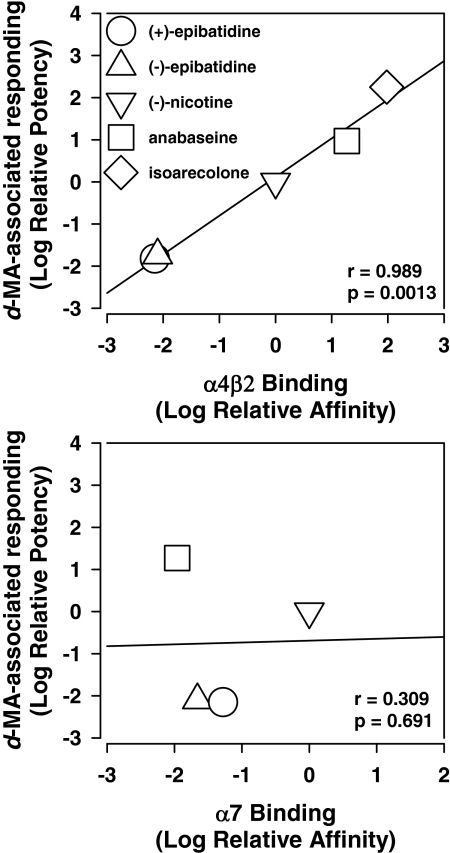
Previous studies have proposed a close association between the binding affinity of several nicotinic agonists at the α4β2 nicotinic receptor subtype and their (−)-nicotine-like discriminative stimulus effects or their stimulant effects on motor behavior (Reavill et al., 1987, 1988; Stolerman et al., 1995). However, it is unknown whether the discriminative stimulus effects of nicotinic receptor agonists are mediated specifically by actions at the α4β2 subtype of nicotinic receptor or they also involve actions at α7 receptors. In this regard, comparison of the potencies with which nicotinic agonists in the present study substituted for d-MA with their reported binding affinities at α4β2 and α7 nicotinic receptor subtypes (Table 2) reveals a close correspondence between relative behavioral potency and relative potency for inhibiting [3H]nicotine binding at the α4β2 receptors in rat brain tissue (r = 0.989; Fig. 6, top; Table 2). In contrast, no correspondence is apparent between relative behavioral potency and relative potency for inhibiting 125I-α-bungarotoxin binding at α7 receptors in rodent brain (r = 0.309; Fig. 6, bottom; Table 2). Although a role for α7-mediated actions cannot be excluded on the basis of such limited data, these findings are nevertheless consistent with the previously reported failure of the α7 nicotinic antagonist, MLA, to block (−)-nicotine's discriminative stimulus effects (Brioni et al., 1996) and suggest that actions at the α4β2 nicotinic receptor subtype mediate the d-MA-like discriminative stimulus effects of nicotinic agonists.
Fig. 6.
Relationship between the relative potencies of nicotinic drugs in the present d-MA-discrimination studies and their relative affinities at α4β2 and α7nicotinic receptors in radioligand binding studies (see Materials and Methods). Abscissae show affinity relative to (−)-nicotine for inhibiting binding of radioligand to α4β2 (top) and α7 (bottom) nicotinic receptors; ordinates show potency of nicotinic drugs relative to (−)-nicotine, based on ED50 values, for engendering d-MA-associated lever responding (from Table 2). Numbers refer to the drugs as given in Table 2. Isoarecolone was excluded from this correlation analysis at the α7 nicotinic receptor subtypes because affinity values obtained at this site are not clearly defined (see Table 2).
The involvement of nicotinic receptors in the d-MA-like effects of nicotinic agonists is further supported by the dose-dependent antagonism of the d-MA-like effects of (−)-nicotine by the nicotinic partial agonist varenicline. Nicotinic receptor activation probably triggers other neurochemical action leading to psychomotor stimulant and, in particular, d-MA-like effects. In this regard, previous studies in rats have shown that nicotinic receptor activation can increase levels of DA in selected brain regions (Grady et al., 1992; Dwoskin et al., 1993). For example, microdialysis studies have shown that (−)-nicotine, (±)-epibatidine, and varenicline produce reliable increases in DA efflux in nucleus accumbens (Bednar et al., 2004; Rollema et al., 2007). It seems plausible, then, that the d-MA-like effects of these nicotinic agonists may be attributed to their ability to stimulate DA release. This suggestion must be tempered with caution, however, because isoarecolone, which also produced dose-related increases in responding on the d-MA lever in the present experiments, seems not to significantly elevate DA levels in rat nucleus accumbens (Mirza et al., 1996). Although these latter findings need to be replicated or further elaborated, they raise the possibility that the d-MA-like effects of nicotinic agonists are not invariantly linked to DA release, and other neurochemical mechanisms also may play a prominent role in the overlapping behavioral effects of nicotinic and monoaminergic stimulants.
Acknowledgments
We thank Jared Martin for technical support and Dr. Hans Rollema (Pfizer Inc.) for providing varenicline.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants RO1-DA03774, RO1-DA10566] and a Ruth L. Kirschstein National Service Award.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.173773.
- MA
- methamphetamine
- AChE
- acetylcholine esterase
- AMP
- amphetamine
- DA
- dopamine
- DHβE
- dihydro-β-erythroidine hydrobromide
- FR
- fixed ratio
- MLA
- methyllycaconitine.
References
- Anderson DJ, Arneric SP. (1994) Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur J Pharmacol 253:261–267 [DOI] [PubMed] [Google Scholar]
- Badio B, Daly JW. (1994) Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol 45:563–569 [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. (2002) Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 162:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar I, Friberg L, Nordberg A. (2004) Modulation of dopamine release by the nicotinic agonist epibatidine in the frontal cortex and the nucleus accumbens of naive and chronic nicotine treated rats. Neurochem Int 45:1049–1055 [DOI] [PubMed] [Google Scholar]
- Bergman J, Frasca J, Zakarian A, Paronis CA. (2006) Discriminative stimulus effects of adenosine antagonists in methamphetamine-trained monkeys, in Annual Meeting College on Problems of Drug Dependence; 2006 June 17–22; Scottsdale, AZ Abstract 69, College on Problems of Drug Dependence, Philadelphia, PA [Google Scholar]
- Brioni JD, Kim DJ, O'Neill AB. (1996) Nicotine cue: lack of effect of the α7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol 301:1–5 [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. (2007) Nicotine receptor partial agonists for smoking cessation. Cochrane Database System Review, CD006103, Cochrane Collaboration, London, UK: [DOI] [PubMed] [Google Scholar]
- Callahan PM, De La Garza R, 2nd, Cunningham KA. (1997) Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 57:601–607 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. (2004) Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 175:170–178 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Creasy KR, Grove AD, Rosecrans JA, Martin BR. (1994) Pharmacological effects of epibatidine optical enantiomers. Brain Res 664:34–40 [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. (1995) Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci 56:545–570 [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. (2003) Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 167:335–343 [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. (2010) Monoaminergic psychomotor stimulants: discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther 333:834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. (2000) Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393:295–314 [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. (1993) S(−)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem 60:2167–2174 [DOI] [PubMed] [Google Scholar]
- Finney DJ. (1964) Statistical Method in Biological Assay, 2nd ed, Hafner, New York [Google Scholar]
- Gatch MB, Flores E, Forster MJ. (2008) Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend 93:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. (2000) Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse 38:432–437 [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. (1984) Further investigation of the discriminative stimulus properties of MDA. Pharmacol Biochem Behav 20:501–505 [DOI] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. (1992) Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem 59:848–856 [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. (2003) Attentional effects of nicotinic agonists in rats. Neuropharmacology 44:1054–1067 [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Mytilineou C, Cohen G. (1975) Amphetamine: evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther 194:47–56 [PubMed] [Google Scholar]
- Hiranita T, Anggadiredja K, Fujisaki C, Watanabe S, Yamamoto T. (2004) Nicotine attenuates relapse to methamphetamine-seeking behavior (craving) in rats. Ann NY Acad Sci 1025:504–507 [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. (2008) Monoamine transporters and psychostimulant addiction. Biochem Pharmacol 75:196–217 [DOI] [PubMed] [Google Scholar]
- Järbe TU. (1978) Cocaine as a discriminative cue in rats: interactions with neuroleptics and other drugs. Psychopharmacology (Berl) 59:183–187 [DOI] [PubMed] [Google Scholar]
- Jensen AA, Mikkelsen I, Frølund B, Bräuner-Osborne H, Falch E, Krogsgaard-Larsen P. (2003) Carbamoylcholine homologs: novel and potent agonists at neuronal nicotinic acetylcholine receptors. Mol Pharmacol 64:865–875 [DOI] [PubMed] [Google Scholar]
- Kantak KM, Edwards MA, Spealman RD. (1995) Effects of N-methyl-d-aspartate antagonists in rats discriminating different doses of cocaine: comparison with direct and indirect dopamine agonists. J Pharmacol Exp Ther 274:657–665 [PubMed] [Google Scholar]
- Karler R, Calder LD, Bedingfield JB. (1996) A novel nicotinic-cholinergic role in behavioral sensitization to amphetamine-induced stereotypy in mice. Brain Res 725:192–198 [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. (1999) Novel 3α-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288:302–315 [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Papke RL, Lingle CJ. (1997) Anabaseine is a potent agonist on muscle and neuronal α-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther 283:979–992 [PubMed] [Google Scholar]
- Kuczenski R, Segal D. (1989) Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci 9:2051–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. (2000) The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav 71:565–570 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Robinson SF, Collins AC. (1996) Nicotinic agonists differ in activation and desensitization of 86Rb+ efflux from mouse thalamic synaptosomes. J Pharmacol Exp Ther 277:1383–1396 [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. (1986) Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and α-bungarotoxin. Mol Pharmacol 30:427–436 [PubMed] [Google Scholar]
- Mendelson JE, McGlothlin D, Harris DS, Foster E, Everhart T, Jacob P, 3rd, Jones RT. (2008) The clinical pharmacology of intranasal l-methamphetamine. BMC Clin Pharmacol 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Pei Q, Stolerman IP, Zetterström TS. (1996) The nicotinic receptor agonists (−)-nicotine and isoarecolone differ in their effects on dopamine release in the nucleus accumbens. Eur J Pharmacol 295:207–210 [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. (2000) Dopaminergic involvement in the discriminative stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 148:209–216 [DOI] [PubMed] [Google Scholar]
- Reavill C, Jenner P, Kumar R, Stolerman IP. (1988) High affinity binding of [3H] (−)-nicotine to rat brain membranes and its inhibition by analogues of nicotine. Neuropharmacology 27:235–241 [DOI] [PubMed] [Google Scholar]
- Reavill C, Spivak CE, Stolerman IP, Waters JA. (1987) Isoarecolone can inhibit nicotine binding and produce nicotine-like discriminative stimulus effects in rats. Neuropharmacology 26:789–792 [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. (2007) Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994 [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, et al. (2010) Preclinical properties of the α4β2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol 160:334–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40 [DOI] [PubMed] [Google Scholar]
- Sasaki JE, Tatham TA, Barrett JE. (1995) The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology (Berl) 120:303–310 [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. (2002) Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci 22:3269–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Zubaran C, Stolerman IP. (2000) Antagonism of stimulus properties of nicotine by dihydro-β-erythroidine (DHβE) in rats. Psychopharmacology 149:140–146 [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. (2009) Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol 192:295–333 [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. (1967) Statistical Methods, 6th ed, Iowa State University Press, Ames, IA [Google Scholar]
- Spealman RD. (1995) Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 275:53–62 [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. (1997) Selective antagonism of behavioural effects of nicotine by dihydro-β-erythroidine in rats. Psychopharmacology (Berl) 129:390–397 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. (1995) Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology (Berl) 117:430–437 [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Decker MW, Brioni JD, Donnelly-Roberts D, Anderson DJ, Bannon AW, Kang CH, Adams P, Piattoni-Kaplan M, Buckley MJ. (1994) (+/−)-Epibatidine elicits a diversity of in vitro and in vivo effects mediated by nicotinic acetylcholine receptors. J Pharmacol Exp Ther 271:624–631 [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321:334–344 [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. (1998) Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther 285:1163–1174 [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. (2008) The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 33:1779–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107 [DOI] [PubMed] [Google Scholar]
- Yasar S, Schindler CW, Thorndike EB, Szelenyi I, Goldberg SR. (1993) Evaluation of the stereoisomers of deprenyl for amphetamine-like discriminative stimulus effects in rats. J Pharmacol Exp Ther 265:1–6 [PubMed] [Google Scholar]
- Zanetti L, Picciotto MR, Zoli M. (2007) Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology (Berl) 190:189–199 [DOI] [PubMed] [Google Scholar]