Abstract
Opioids activate the descending antinociceptive pathway from the ventrolateral periaqueductal gray (vlPAG) by both pre- and postsynaptic inhibition of tonically active GABAergic neurons (i.e., disinhibition). Previous research has shown that short-term desensitization of postsynaptic μ-opioid receptors (MOPrs) in the vlPAG is increased with the development of opioid tolerance. Given that pre- and postsynaptic MOPrs are coupled to different signaling mechanisms, the present study tested the hypothesis that short-term desensitization of presynaptic MOPrs also contributes to opioid tolerance. Twice-daily injections of morphine (5 mg/kg s.c.) for 2 days caused a rightward shift in the morphine dose-response curve on the hot plate test (D50 = 9.9 mg/kg) compared with saline-pretreated (5.3 mg/kg) male Sprague-Dawley rats. In vitro whole-cell patch-clamp recordings from vlPAG slices revealed that inhibition of evoked inhibitory postsynaptic currents (eIPSCs) by the MOPr-selective agonist [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin was decreased in morphine-tolerant (EC50 = 708 nM) compared with saline-pretreated rats (EC50 = 163 nM). However, short-term desensitization of MOPr inhibition of eIPSCs was not observed in either saline- or morphine-pretreated rats. Reducing the number of available MOPrs with the irreversible opioid receptor antagonist, β-chlornaltrexamine decreased maximal MOPr inhibition with no evidence of desensitization, indicating that the lack of observed desensitization is not caused by receptor reserve. These results demonstrate that tolerance to the antinociceptive effect of morphine is associated with a decrease in presynaptic MOPr sensitivity or coupling to effectors, but this change is independent of short-term MOPr desensitization.
Introduction
Opioids are potent and effective analgesics. Their clinical use, however, is notable for the rapid development of tolerance to their analgesic effects, so that treatment often requires increasingly larger doses (McQuay, 1999). The periaqueductal gray (PAG) is a brain stem area important for the development of opioid tolerance. Direct microinjection of opioids into the PAG produces antinociception (Jacquet and Lajtha, 1976; Bodnar, 2000), and repeated microinjections of morphine into the vlPAG induce tolerance to the antinociceptive effects of morphine (Jacquet and Lajtha, 1976; Siuciak and Advokat, 1987; Morgan et al., 2006). Numerous cellular and molecular adaptations associated with repeated and long-term administration of opioids have been identified in the vlPAG (Ingram et al., 1998, 2007; Connor et al., 1999a; Bagley et al., 2005a,b), suggesting that changes in μ-opioid receptor (MOPr) signaling pathways are crucial for the expression of morphine tolerance.
Opioids activate the descending antinociceptive pathway via inhibition of tonically active GABAergic inputs within the vlPAG (Basbaum and Fields, 1984; Moreau and Fields, 1986). MOPrs are located at both pre- and postsynaptic sites on GABAergic neurons (Vaughan et al., 1997; Kalyuzhny and Wessendorf, 1998; Commons et al., 2000), and the signaling pathways associated with each site are distinct (Christie, 2008). Postsynaptic MOPrs in the vlPAG activate G-protein-coupled inwardly rectifying potassium (GIRK) channels and inhibit voltage-gated calcium channels (Chieng and Christie, 1994; Ingram et al., 1998; Connor et al., 1999b). Presynaptic MOPrs do not couple to either GIRK or calcium channels but to the phospholipase A2 signaling pathway that activates a voltage-sensitive potassium (Kv) channel (Wimpey and Chavkin, 1991; Vaughan et al., 1997). Activation of the Kv channel inhibits GABA release from the presynaptic terminals (Vaughan et al., 1997).
Short-term desensitization of the postsynaptic effects of opioids (i.e., activation of GIRK channels and inhibition of calcium channels) occurs rapidly upon agonist superfusion and reaches a plateau within 15 min of continued agonist administration. The rate and extent of desensitization is typically greater in animals pretreated with long-term morphine (Dang and Williams, 2005; Ingram et al., 2008). The adaptations underlying short-term desensitization are thought to contribute to the development of tolerance (Ingram et al., 1998, 2007; Connor et al., 1999a; Bagley et al., 2005a,b). Although presynaptic MOPr coupling to the Kv channel is disrupted after morphine pretreatment (Hack et al., 2003), it is not known whether this loss of Kv signaling is caused by short-term MOPr desensitization or through another mechanism, such as a decrease in agonist activation of MOPrs or modulation of proteins downstream of receptor activation. The objective of the present study is to test the hypothesis that short-term desensitization of the presynaptic actions of MOPrs in the vlPAG contributes to the tolerance observed after repeated administration of morphine.
Materials and Methods
Subjects.
Male Sprague-Dawley rats between the ages of 21 and 40 days were used in both electrophysiological and behavioral experiments. Food and water were available ad libitum, and lights were maintained on a reversed 12-h cycle so that testing was conducted during the active dark phase. Experiments were conducted in accordance with the animal care and use guidelines outlined in the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Washington State University.
Behavioral Assessment of Tolerance.
Rats were injected with saline or morphine (5 mg/kg s.c.) twice a day for 2 days (trials 1–4). Rats were returned to their cages immediately after each injection. On the third day, baseline nociception was assessed by measuring the latency to lick a hind paw when placed on a hot plate (52.5°C). The rat was immediately removed from the plate and returned to the home cage after a response or if no response was observed within 60 s. The development of tolerance was assessed by examining shifts in the dose-response curve after microinjection of cumulative quarter log doses of morphine into the vlPAG (Morgan et al., 2006). Injections were spaced every 20 min and rats were tested on the hot plate 15 min after each injection. Data were fit to a standard dose-response curve with the maximum latency equal to 60 s as the upper constraint and mean baseline hotplate latency as the lower constraint (GraphPad Software Inc., San Diego, CA).
Electrophysiological Recordings.
Previous studies have demonstrated that changes in MOPr sensitivity are observed with the above morphine administration paradigm for up to 5 days after the last morphine injection (Ingram et al., 2007, 2008), suggesting that long-term changes are induced by the repeated intermittent morphine injection paradigm. To study these long-term changes in the absence of morphine, brain slices were cut 1 to 3 days after the last injection of morphine for in vitro patch-clamp recordings from the vlPAG (Ingram et al., 2008). Behavioral testing was performed on a total of 17 animals before electrophysiological recording (see Fig. 1), and the rest of the recordings were from animals pretreated with the same paradigm except for the cumulative dose-response on day 3.
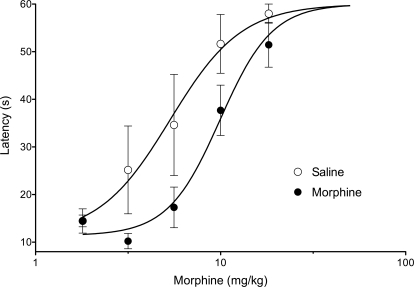
Fig. 1.
Repeated morphine administration induces antinociceptive tolerance. Rats were pretreated with either saline (n = 5) or morphine (5 mg/kg s.c., n = 12) twice a day for 2 days. On day 3, all rats received cumulative doses of morphine and were tested on the hot plate test 15 min after each injection. Hot plate latencies for morphine-pretreated rats were significantly shifted to the right (F1,80 = 10.93, p < 0.01).
Rats were anesthetized with halothane and decapitated. Brains were removed quickly and immersed in ice-cold artificial cerebrospinal fluid (aCSF) containing 126 mM NaCl, 21.4 mM NaHCO3, 11.1 mM dextrose, 2.5 mM KCl, 2.4 mM CaCl2, 1.2 mM MgCl2, and 1.2 mM NaH2PO4, pH 7.35, and equilibrated with 95% O2/5% CO2. Brain slices containing the ventral PAG were cut with a Vibratome (250–300 μm thick; Leica Microsystems, Inc., Deerfield, IL) and placed in a holding chamber with aCSF bubbled with 95% O2/5% CO2 maintained at approximately 32°C until needed for recording.
Brain slices were placed into a recording chamber mounted on an Olympus BX51 upright microscope and superfused with warmed aCSF (32°C). Cells were viewed with a water immersion 40× objective (Olympus America Inc., Center Valley, PA) and Nomarski infrared optics. Recordings were made with electrodes pulled to 2- to 7-MΩ resistance with an internal solution consisting of 148 mM cesium chloride, 10 mM HEPES, 1 mM MgCl2, 1 mM EGTA, 0.3 mM CaCl2, 4 mM MgATP, and 3 mM NaGTP, pH 7.4. Junction potentials were corrected at the beginning of the experiments. Capacitance and series resistance compensation (>70–80%) were corrected, and access resistance was monitored throughout the experiments. Data were collected with a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA) at 5 to 10 kHz and low-pass-filtered at 2 to 5 kHz. Currents were digitized with Digidata 1322A (Molecular Devices), collected, and analyzed with AxoGraph X Scientific software (http://axographx.com). Experiments were monitored with Chart software (MacLab; ADInstruments Pty Ltd., Castle Hill, Australia). Neurons were voltage-clamped at −70 mV, and postsynaptic currents were evoked every 20 s using a bipolar tungsten-stimulating electrode placed immediately adjacent to the outer boundary of the vlPAG. The stimulation strength was adjusted until consistent postsynaptic currents were obtained (between 1 and 10 mA). Drugs were applied by bath superfusion, where full bath exchange occurs within 3 min. Each response is an average of synaptics elicited over four to six trials. Experiments were carried out in the presence of the glutamate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (5 μM), and the glycine receptor antagonist strychnine (1–10 μM), to ensure isolation of GABAA-mediated evoked inhibitory postsynaptic currents (eIPSCs) (Vaughan and Christie, 1997). The eIPSCs are inward currents in these studies because of the approximately equimolar intracellular/extracellular chloride concentrations when using CsCl-based internal solution.
Drugs.
Morphine, [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), met5-enkephalin (ME), β-chlornaltrexamine (β-CNA), strychnine, bicuculline, and naloxone were obtained from Sigma-Aldrich (St. Louis, MO). 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione was obtained from Tocris Bioscience (Ellisville, MO). The Kv channel inhibitor α-dendrotoxin (Benishin et al., 1988) was obtained from Alomone Labs (Jerusalem, Israel). All drugs were diluted in appropriate buffers as concentrated stocks for further dilution in aCSF solution.
Statistical Analyses.
All data are expressed as mean ± S.E., unless otherwise noted. The dose of half-maximal antinociception (D50) was estimated with nonlinear regression (GraphPad Software Inc.) using the mean hot plate latency at each dose of morphine. Differences in morphine potency between groups were determined with analysis of variance. In electrophysiological experiments, differences between groups were assessed using Student's t test and analysis of variance when appropriate.
Results
Baseline hot plate latencies were not significantly different between saline- (17.2 ± 3.3 s; n = 5) and morphine- (13.1 ± 1.1 s; n = 12) pretreated rats immediately before the assessment of tolerance (t15 = 1.526, p > 0.05). The cumulative morphine dose-response curve was shifted significantly to the right for morphine (D50 = 9.9 mg/kg; 95% CI, 8.0–12.3 mg/kg), compared with saline-pretreated rats (D50 = 5.3 mg/kg; 95% CI, 3.8–7.6 mg/kg; F1,80 = 10.93, p < 0.05; Fig. 1). This rightward shift indicates that the morphine pretreatment induced tolerance to the antinociceptive effect of morphine.
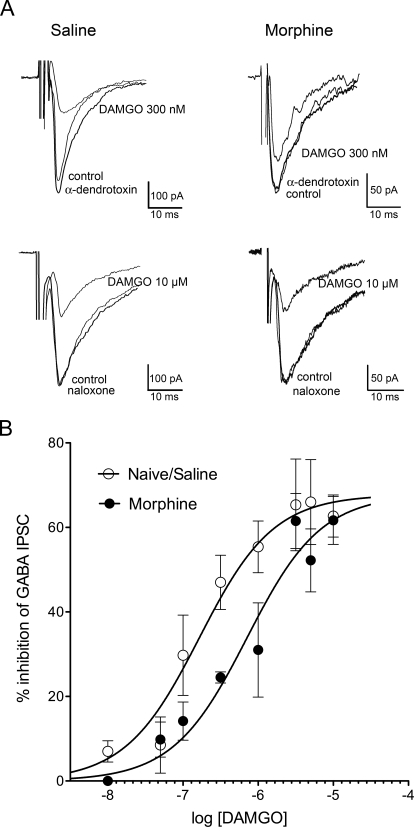
Evoked inhibitory postsynaptic current (eIPSCs) amplitudes from vlPAG neurons were decreased in the presence of the MOPr-selective agonist DAMGO. Inhibition of eIPSCs by DAMGO was concentration-dependent in saline- and morphine-pretreated rats. As expected, DAMGO responses were significantly shifted to the right in slices from morphine (EC50 = 708 nM; 95% CI, 376 nM–1.3 μM) compared with saline-pretreated rats (EC50 = 163 nM; 95% CI, 85–312 nM; F1,12 = 23.88; p < 0.01; Fig. 2). This demonstration of cellular tolerance is consistent with the antinociceptive tolerance observed when following the repeated administration paradigm (Fig. 1).
Fig. 2.
Repeated morphine pretreatment decreases DAMGO-mediated inhibition of GABA release. A, representative eIPSC traces showing control, DAMGO (300 nM or 10 μM) or return to control amplitude after naloxone (10 μM) or the Kv channel inhibitor, α-dendrotoxin (100 nM), superfusion in saline/naïve or morphine-pretreated rats. DAMGO (300 nM)-mediated inhibition was diminished in rats pretreated with morphine. B, concentration–response curve for DAMGO-mediated inhibition of eIPSCs in saline/naive slices compared with morphine-pretreated rats. Responses were measured at the 5-min time point and only two to three increasing concentrations of agonist were applied to each slice. There was a significant shift in the concentration-response curve for morphine (EC50 = 708 nM) compared with saline (EC50 = 163 nM; F1,12 = 23.88, p < 0.01; n = 4–16 cells per concentration).
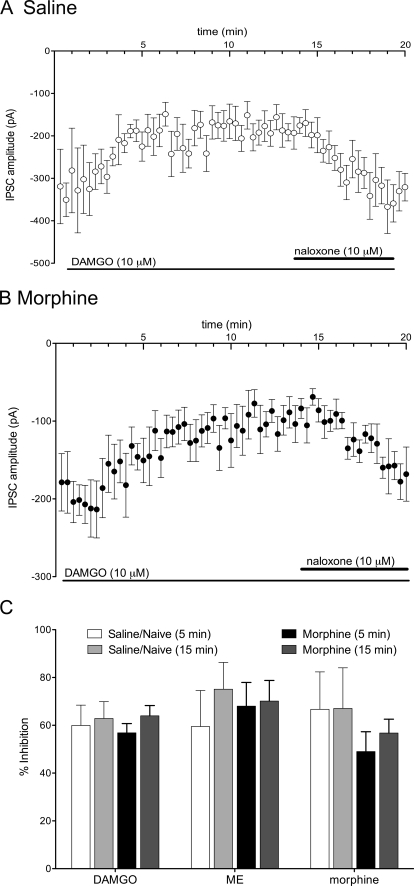
Previous research has shown that MOPr desensitization is concentration-dependent and occurs more readily when MOPrs are exposed to maximal concentrations of opioid agonists. To assess whether agonist-specific desensitization of eIPSCs occurred in the vlPAG, maximal concentrations of DAMGO (10 μM), ME (20 μM), and morphine (30 μM) were superfused for 15 min over slices taken from saline- (Fig. 3, A and C) and morphine- (Fig. 3, B and C) pretreated rats. All three opioid receptor agonists inhibited the GABAergic eIPSCs in saline-pretreated rats by 60 ± 9% (DAMGO, n = 9), 59 ± 15% (ME, n = 4), and 67 ± 16% (morphine, n = 4) after 5 min of superfusion (Fig. 3C). The inhibition was maintained after 15 min of agonist superfusion (DAMGO, 63 ± 7%; ME, 75 ± 11%; and morphine, 67 ± 17%). In morphine-pretreated rats, superfusion of agonists for 5 min induced comparable inhibition of the eIPSCs, as observed with saline-pretreated rats [57 ± 4% (DAMGO, n = 10); 68 ± 10% (ME, n = 6); 49 ± 8% (morphine, n = 8)], and this inhibition was also maintained over the 15-min superfusion period (DAMGO, 64 ± 4%; ME, 70 ± 9%; morphine, 57 ± 6%). There were no significant differences in inhibition in morphine compared with saline-pretreated rats (F2,64 = 0.9033, p > 0.05).
Fig. 3.
Presynaptic opioid inhibition of GABAergic eIPSCs does not desensitize. A, mean eIPSC amplitudes during superfusion with DAMGO (10 μM) in saline-pretreated slices (n = 8). After the 3-min bath equilibration period, the inhibition by DAMGO was consistent during the superfusion period and reversed with superfusion of naloxone (10 μM). Note the lack of desensitization during the DAMGO superfusion period. B, mean eIPSC amplitudes in experiments during superfusion of DAMGO (10 μM) from morphine-pretreated slices (n = 9). Note the lack of desensitization during the DAMGO superfusion period. C, bar graph showing compiled data for percentage inhibition after 5- and 15-min superfusion of maximal concentrations of DAMGO (10 μM), ME (20 μM), and morphine (30 μM). Data were derived from four to eight experiments per bar.
Although previous studies showed that postsynaptic MOPr desensitization in the vlPAG occurs maximally within 15 min (Ingram et al., 2008), we applied DAMGO (10 μM) for 30 min in naive- (n = 7) and morphine- (n = 8) pretreated rats to determine whether desensitization of presynaptic eIPSCs takes longer than 15 min to develop. Desensitization of DAMGO-mediated inhibition of eIPSCs was not observed with this prolonged superfusion (Fig. 4). The eIPSCs were blocked by superfusion of bicuculline (10 μM; n = 10), demonstrating that the eIPSCs were GABAergic in these studies (Fig. 4, A and B).
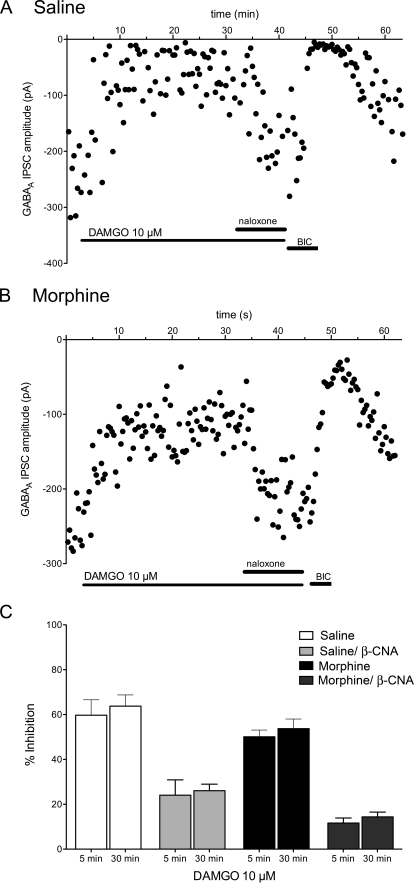
Fig. 4.
Long agonist superfusion (30 min) does not induce desensitization. A, a single experiment is plotted showing the amplitudes of GABAergic eIPSCs every 20 s in a naive rat. Superfusion of DAMGO (10 μM) for 30 min inhibits the eIPSCs, and the inhibition is reversed in the presence of naloxone (10 μM). The eIPSCs are abolished by the GABAA inhibitor bicuculline (10 μM). The bars denote drug applications. Note change in time scale compared with Fig. 3. B, a single experiment from a slice from a morphine-pretreated rat also shows no evidence of short-term desensitization. C, bar graph showing compiled mean percentage inhibition from eIPSCs averaged over four to six trials at 5- and 30-min agonist superfusion. In additional experiments, β-CNA (5 μM) was superfused for 3 min to reduce receptor reserve and allowed to wash out for 10 min before DAMGO superfusion. There was no desensitization of the DAMGO-mediated inhibition in either saline- or morphine-pretreated rats (n = 5–8 cells/group).
The lack of desensitization of presynaptic MOPr inhibition of GABAergic eIPSCs could be caused by more efficient coupling of MOPrs to effectors or greater receptor reserve in presynaptic terminals. This hypothesis was tested by superfusing the irreversible opioid receptor antagonist, β-CNA (5 μM) over the slices for 3 min to reduce receptor reserve. After a 10-min washout period to remove unbound β-CNA from the slice, DAMGO (10 μM) was superfused for 30 min in slices from saline/naive- and morphine-pretreated rats. Although β-CNA significantly decreased DAMGO-mediated inhibition from 60 ± 7% (n = 7) to 24 ± 7% (n = 5) in saline/naive rats and from 50 ± 3% (n = 8) to 12 ± 2% (n = 6) in morphine-pretreated rats (F3,43 = 46.10, p < 0.01), there was no evidence of short-term desensitization after 30 min of DAMGO administration in either saline- or morphine-pretreated rats after β-CNA superfusion (F1,43 = 0.8565, p > 0.05; Fig. 4C).
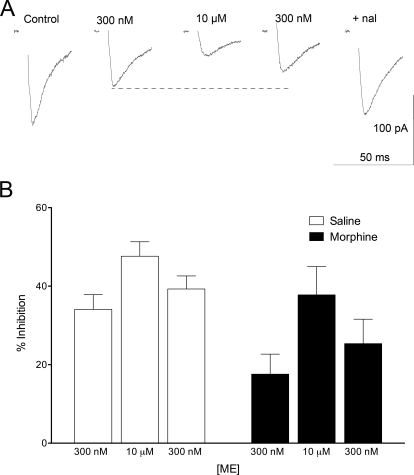
The results of experiments with high concentrations of the opioid agonists were unexpected in that there was no apparent short-term desensitization during the 15-min drug application. Previous studies in our lab and other labs have demonstrated that postsynaptic MOPr coupling decreases significantly within 15 min of continuous agonist exposure because of desensitization of MOPrs, especially with higher-efficacy opioid agonists such as ME and DAMGO (Bagley et al., 2005a; Christie, 2008; Ingram et al., 2008). To assess desensitization more carefully, a submaximal concentration of ME (300 nM) was administered to establish baseline inhibition of eIPSCs. After this baseline, a high concentration of ME (10 μM) was superfused to induce MOPr desensitization that would then be more evident with a subsequent return to the low concentration of ME (Dang and Williams, 2004, 2005). In these experiments, ME was the agonist of choice because it easily washes into and out of the slice. Representative eIPSCs for this experiment are shown in Fig. 5A. As expected, the inhibition of eIPSCs by 300 nM ME was decreased in morphine (18 ± 5%; n = 6) compared with saline-pretreated rats (34 ± 4%, n = 11; t15 = 2.613, p < 0.05; Fig. 5B). However, short-term desensitization of MOPr-mediated inhibition of GABAergic eIPSCs was not observed in either saline- or morphine-pretreated rats. That is, the 300 nM ME response was always equal to or greater than the initial response to 300 nM ME after the desensitizing concentration of ME. Because it was possible that the high dose of ME takes longer to wash out, we also prolonged the second ME (300 nM) superfusion to 15 min (n = 4) with no indication of desensitization. Decreased inhibition in the presence of continuous opioid agonist superfusion was not observed in any of the experiments.
Fig. 5.
Desensitization to ME (10 μM) inhibition in presynaptic terminals is not observed at lower agonist concentrations. A, representative traces from a morphine-pretreated rat showing the desensitization paradigm used in these studies. An approximate EC50 concentration of ME (300 nM) was superfused to determine a baseline inhibition of the GABAergic eIPSCs, followed by superfusion of a high dose (10 μM ME) for 15 min and a second superfusion of ME (300 nM) to determine whether any desensitization of MOPrs occurred. Desensitization of MOPrs would be expected to return eIPSCs to control amplitude. B, bar graph plotting percentage inhibition of eIPSCs in saline- (n = 11) and morphine- (n = 6) pretreated rats. Although the responses in morphine-pretreated rats were diminished compared with control (F1,46 = 10.33, p < 0.01), the second ME (300 nM) response was never significantly smaller than the first response, indicating a lack of desensitized MOPrs.
Discussion
The results show a decrease in opioid-mediated presynaptic signaling in morphine-tolerant rats that is independent of agonist-induced MOPr desensitization. In stark contrast to postsynaptic MOPrs in the vlPAG, MOPrs in presynaptic GABAergic terminals did not desensitize during prolonged superfusions of opioid agonists in either saline- or morphine-pretreated rats. This lack of observed desensitization is not a function of receptor reserve, because blocking MOPrs with the irreversible opioid antagonist, β-CNA, did not reveal desensitization of MOPr inhibition of GABA release. These results indicate that receptor processes thought to diminish MOPr coupling to effectors, such as desensitization, are not the same for MOPrs localized to presynaptic and postsynaptic sites.
MOPrs are coupled to multiple effectors in vlPAG neurons, depending on their location within the cell. Postsynaptic MOPrs have been shown to couple to GIRK channels, calcium channels, adenylyl cyclase, and the ERK1/2 pathway (Chieng and Christie, 1994; Bagley et al., 2005a; Ingram et al., 2007, 2008; Macey et al., 2009). Presynaptic MOPrs in the vlPAG do not couple significantly to either GIRK or calcium channels but to voltage-sensitive potassium (Kv) channels (Vaughan et al., 1997). Long-term administration of opioids alters coupling of MOPrs to each of these effectors (Bagley et al., 2005a,b). Short-term MOPr desensitization at postsynaptic sites has been thoroughly described in many cell types in addition to PAG neurons (Christie et al., 1987; Osborne and Williams, 1995; Blanchet and Lüscher, 2002; Borgland et al., 2003; Dang and Williams, 2004; Arttamangkul et al., 2006). This desensitization consistently occurs within 5 to 10 min of opioid administration and is greater in morphine-pretreated rats compared with naive- or saline-pretreated rats (Dang and Williams, 2005; Ingram et al., 2008). The extent of desensitization of GIRK currents in PAG neurons is less than observed in locus ceruleus neurons (Osborne and Williams, 1995; Blanchet and Lüscher, 2002; Dang and Williams, 2005; Arttamangkul et al., 2006) or cell lines expressing MOPrs (Alvarez et al., 2002; Borgland et al., 2003). This may be caused by a lower density of MOPrs (Law et al., 2000), significantly smaller GIRK currents in the PAG (Bagley et al., 2005a), or tissue specificity. It is possible that MOPr desensitization is inefficient in PAG neurons compared with locus ceruleus neurons in both pre- and postsynaptic locations but the fact that postsynaptic MOPr desensitization is increased significantly after long-term morphine treatment without evidence for presynaptic desensitization argues against this interpretation. Furthermore, the time course of desensitization varies significantly depending on the effector studied (reviewed in Connor et al., 2004), suggesting that differences in pre- and postsynaptic MOPr effectors (GIRK versus Kv channels) may be an important factor in the observed rates of desensitization. MOPr activation of Kv channels in the presynaptic terminals could desensitize faster or slower than activation of GIRK channels, so we examined changes in opioid inhibition of GABA release continuously for up to 30 min in some experiments and still saw no evidence of desensitization. Alternatively, the relatively slow onset of drug in slices may “mask” desensitization that occurs before the 5-min maximal inhibition. If this were the case, presynaptic desensitization would occur on a faster time scale than postsynaptic desensitization in PAG slices (Ingram et al., 2008), and the desensitization would reach a maximum or plateau in less than 5 min with no further desensitization during prolonged drug applications. Given that the t1/2 for MOPr desensitization of GIRK channels is on the order of 3 min in locus ceruleus neurons (Dang and Williams, 2005) and that there is a 3-min lag time in the superfusion system for the current studies, it is not likely that desensitization would be complete at our 5-min time point. Furthermore, if slow slice diffusion contributed to “masking” desensitization, some decrease in inhibition with the prolonged 30-min opioid superfusion would be expected, which was not the case, further supporting the interpretation that short-term desensitization is not a factor in presynaptic terminals.
Surprisingly few studies have specifically addressed MOPr desensitization at presynaptic sites. Differential desensitization has been shown for adenosine A1 receptors in hippocampal neurons where postsynaptic A1 desensitization occurs much faster (hours) than presynaptic desensitization (>24 h) (Wetherington and Lambert, 2002). In addition, postsynaptic MOPrs in locus ceruleus neurons of opioid-naive rats desensitize rapidly in response to prolonged opioid exposure (Williams et al., 2001; Dang and Williams, 2005), but presynaptic MOPr desensitization is not observed (Blanchet and Lüscher, 2002). Likewise, MOPr inhibition of presynaptic GABA release from vlPAG neurons does not desensitize after continuous morphine administration (Ingram et al., 1998), although coupling to Kv channels is abolished (Ingram et al., 1998; Hack et al., 2003). These results demonstrate that differential regulation can occur in subcellular locations and possibly even within the same cell.
The lack of observed short-term desensitization of presynaptic MOPrs in opioid-naive rats might be expected if there were sufficient spare receptors in presynaptic terminals to maintain signaling fidelity. However, inactivating a subpopulation of MOPrs with the irreversible opioid receptor antagonist β-CNA (Virk and Williams, 2008) did not reveal an underlying desensitization of MOPrs. Inhibition of GABAergic eIPSCs was consistent throughout the duration of superfusion of a high concentration of DAMGO (10 μM), even after maximal inhibition was diminished in the presence of β-CNA. These results further indicate that presynaptic MOPrs maintain their signaling in conditions that induce enhanced short-term MOPr desensitization at postsynaptic sites (Ingram et al., 2008).
G-protein-coupled receptors are widely accepted to undergo regulation by a cascade involving G-protein receptor kinase phosphorylation and β-arrestin binding that induce desensitization of receptors (Lin et al., 1997). These regulatory steps have been shown to be important in mediating aspects of MOPr desensitization and morphine tolerance in various knockout models, including G-protein receptor kinase and β-arrestin knockout mice (Zhang et al., 1998; Bohn et al., 2000; Li and Wang, 2001). However, these models primarily address MOPr regulation in postsynaptic sites. The fact that MOPrs do not seem to undergo short-term desensitization in presynaptic terminals is not consistent with the idea that G-protein receptor kinase and β-arrestin-mediated desensitization are important for regulating MOPr activity on GABAergic terminals in the vlPAG. On the other hand, several molecules involved in desensitization, including β-arrestins, have also been implicated as scaffolding molecules for signaling cascades (Lefkowitz et al., 2006). Thus, it is possible that these knockout models disrupt signaling pathways that are crucial for MOPr actions in presynaptic terminals, even though MOPrs do not undergo short-term desensitization. Few studies have directly addressed MOPr regulation and trafficking in presynaptic sites, and it is clear that further studies are necessary to explore the differences in pre and postsynaptic MOPr regulation.
It is well accepted that opioids activate the descending antinociceptive pathway via inhibition of tonically active GABAergic inputs within the vlPAG (Basbaum and Fields, 1984; Moreau and Fields, 1986). To date, opioid antinociceptive tolerance has been largely explained as a decrease in opioid responses at the cellular level by processes involving desensitization and internalization of receptors or by changes in opioid-sensitive networks (reviewed in Bailey and Connor, 2005; Christie, 2008). Given that direct microinjection of opioids into the PAG produces antinociception (Jacquet and Lajtha, 1976; Bodnar, 2000) and repeated microinjections of morphine into the vlPAG induce antinociceptive tolerance (Morgan et al., 2006), it is likely that cellular processes in the vlPAG are sufficient to cause changes in antinociceptive behaviors. The results of this study show that although presynaptic MOPrs do not undergo short-term desensitization in either saline- or morphine-pretreated rats, the potency of opioid agonists to inhibit GABA release is reduced, as would be expected in the development of tolerance. The decrease in MOPr agonist potency for coupling to the presynaptic Kv channel in this study is different from results in an earlier study demonstrating an increase in agonist potency after continuous morphine exposure for 5 days (Ingram et al., 1998). The increased potency after continuous morphine administration was driven by enhanced MOPr coupling to adenylyl cyclase in presynaptic terminals and observed upon withdrawal of the continuous morphine exposure. Coupling to the presynaptic Kv channel was completely abolished after continuous morphine administration in the previous study, consistent with the decrease in MOPr coupling observed with the repeated morphine administration paradigm. Furthermore, naloxone-precipitated withdrawal behaviors and currents are not elicited after the repeated injection paradigm used in this study (Ingram et al., 2008). Thus, adaptations in agonist potency with repeated or long-term morphine exposure may be specific to the effector. Further experiments focused specifically on these presynaptic adaptations and on the potential role of β-arrestins and other molecules involved in scaffolding complexes associated with presynaptic MOPrs will be necessary to fully understand the mechanisms underlying changes in presynaptic MOPr regulation during the development of tolerance.
Acknowledgments
We thank Dr. John T. Williams and Dr. Mark Connor for critical reading of this manuscript.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA015498].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172643.
- PAG
- periaqueductal gray
- MOPr
- μ-opioid receptor
- vlPAG
- ventrolateral periaqueductal gray
- GIRK
- G-protein-coupled inwardly rectifying potassium channel
- aCSF
- artificial cerebrospinal fluid
- eIPSC
- evoked inhibitory postsynaptic current
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- ME
- met5-enkephalin
- β-CNA
- β-chlornaltrexamine
- 95% CI
- 95% confidence interval.
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) mu-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. (2006) Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci 26:4118–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Chieng BC, Christie MJ, Connor M. (2005a) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Gerke MB, Vaughan CW, Hack SP, Christie MJ. (2005b) GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron 45:433–445 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338 [DOI] [PubMed] [Google Scholar]
- Benishin CG, Sorensen RG, Brown WE, Krueger BK, Blaustein MP. (1988) Four polypeptide components of green mamba venom selectively block certain potassium channels in rat brain synaptosomes. Mol Pharmacol 34:152–159 [PubMed] [Google Scholar]
- Blanchet C, Lüscher C. (2002) Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector. Proc Natl Acad Sci USA 99:4674–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. (2000) Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci 7:181–194 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278:18776–18784 [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. (1994) Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol 113:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ. (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154:384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. (1987) Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol 32:633–638 [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. (2000) Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol 419:532–542 [DOI] [PubMed] [Google Scholar]
- Connor M, Borgland SL, Christie MJ. (1999a) Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. Br J Pharmacol 128:1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. (2004) Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol 143:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Schuller A, Pintar JE, Christie MJ. (1999b) Mu-opioid receptor modulation of calcium channel current in periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br J Pharmacol 126:1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2004) Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci 24:7699–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2005) Morphine-induced mu-opioid receptor desensitization. Mol Pharmacol 68:1127–1132 [DOI] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. (2003) Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology 45:575–584 [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. (2007) Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology 32:600–606 [DOI] [PubMed] [Google Scholar]
- Ingram SL, Macey TA, Fossum EN, Morgan MM. (2008) Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology 33:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. (1998) Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18:10269–10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. (1976) The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res 103:501–513 [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. (1998) Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 392:528–547 [PubMed] [Google Scholar]
- Law PY, Erickson LJ, El-Kouhen R, Dicker L, Solberg J, Wang W, Miller E, Burd AL, Loh HH. (2000) Receptor density and recycling affect the rate of agonist-induced desensitization of mu-opioid receptor. Mol Pharmacol 58:388–398 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. (2006) New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24:643–652 [DOI] [PubMed] [Google Scholar]
- Li AH, Wang HL. (2001) G protein-coupled receptor kinase 2 mediates mu-opioid receptor desensitization in GABAergic neurons of the nucleus raphe magnus. J Neurochem 77:435–444 [DOI] [PubMed] [Google Scholar]
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. (1997) Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem 272:31051–31057 [DOI] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. (2009) Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther 331:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuay H. (1999) Opioids in pain management. Lancet 353:2229–2232 [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. (1986) Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397:37–46 [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. (2006) Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav 85:214–219 [DOI] [PubMed] [Google Scholar]
- Osborne PB, Williams JT. (1995) Characterization of acute homologous desensitization of mu-opioid receptor-induced currents in locus coeruleus neurones. Br J Pharmacol 115:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. (1987) Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res 424:311–319 [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390:611–614 [DOI] [PubMed] [Google Scholar]
- Virk MS, Williams JT. (2008) Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol 73:1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. (2002) Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. J Neurosci 22:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343 [DOI] [PubMed] [Google Scholar]
- Wimpey TL, Chavkin C. (1991) Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron 6:281–289 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]