Abstract
Triple reuptake inhibitors (TRIs) that block the dopamine transporter (DAT), norepinephrine transporter, and serotonin transporter are being developed as a new class of antidepressant that may have better efficacy and fewer side effects compared with traditional antidepressants. We describe a novel TRI, 2-[4-(4-chlorophenyl)-1-methylpiperidin-3-ylmethylsulfanyl]-1-(3-methylpiperidin-1-yl)-ethanone (JZAD-IV-22), that inhibits all three monoamine transporters with approximately equal potency in vitro. (+/−)-1-(3,4-dichlorophenyl)-3-azabicyclo-[3.1.0]hexane hydrochloride (DOV 216,303), a TRI shown to be an effective antidepressant in a clinical trial, shows reuptake inhibition similar to that of JZAD-IV-22 in vitro. Furthermore, both JZAD-IV-22 and DOV 216,303 increase levels of dopamine, norepinephrine, and serotonin in the mouse prefrontal cortex when administered by peripheral injection. JZAD-IV-22 and DOV 216,303 exhibited antidepressant-like efficacy in the mouse forced-swim and tail-suspension tests at doses that increased neurotransmitter levels. Because development of DAT inhibitors could be hindered by abuse liability, both JZAD-IV-22 and DOV 216,303 were compared in two assays that are markers of abuse potential. Both JZAD-IV-22 and DOV 216,303 partially substituted for cocaine in a drug discrimination assay in rats, and high doses of DOV 216,303 produced locomotor sensitization in mice. JZAD-IV-22 showed no evidence of sensitization at any dose tested. These results demonstrate that JZAD-IV-22 is a TRI with antidepressant-like activity similar to that of DOV 216,303. The striking feature that distinguishes the two TRIs is that locomotor sensitization, a common underlying feature of drugs of abuse, is seen with DOV 216,303 but is completely lacking in JZAD-IV-22. These findings may have implications for the potential for abuse liability in humans.
Introduction
Depression affects approximately 121 million people worldwide and is one of the leading causes of disability, according to the World Health Organization (http://www.who.int/mental_health/management/depression/definition/en/). Currently available antidepressants do not provide full symptom relief; up to 70% of depressed patients respond only partially to treatment (Rush et al., 2006). Marketed antidepressants inhibit the reuptake of serotonin (5-HT; e.g., citalopram, paroxetine, sertraline, fluoxetine), norepinephrine (NE; e.g., desipramine), both 5-HT and NE (e.g., venlafaxine, duloxetine), or NE and dopamine (DA; e.g., bupropion), but none of the currently marketed treatments elevates all three monoamines.
Putative antidepressants that can inhibit dopamine, norepinephrine, and serotonin transporters (DAT, NET, and SERT, respectively) are referred to as triple reuptake inhibitors (TRIs) and are thought to offer advantages over currently available antidepressants. The addition of the DA component to 5-HT and NE reuptake inhibitors is hypothesized to produce better efficacy and fewer side effects (Skolnick et al., 2003). Based on preclinical (Wise, 2002) and clinical (Zisook et al., 2006) literature, addition of a dopaminergic component would be expected to decrease apathy and anhedonia and increase goal-directed/reward-based behaviors, because apathy and anhedonia are associated with deficits in dopaminergic neurotransmission in rodent models (Willner, 1997; Salamone and Correa, 2009) and in patients with depression (D'Aquila et al., 2000). Based on evidence that the DAT/NET inhibitor bupropion, combined with selective serotonin-reuptake inhibitors, reduced the sexual side effects associated with this class of drugs (Zisook et al., 2006), addition of DAT inhibition to a SERT/NET inhibitor may reduce sexual side effects caused by the SERT inhibition. Given these findings, the TRI strategy represents a promising approach to developing a superior antidepressant with increased efficacy and reduced sexual side effects.
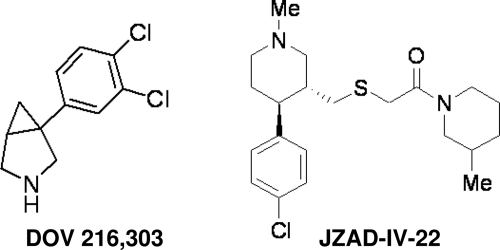
The current studies identified a novel triple uptake inhibitor, 2-[4-(4-chlorophenyl)-1-methylpiperidin-3-ylmethylsulfanyl]-1-(3-methylpiperidin-1-yl)-ethanone (JZAD-IV-22) (see Fig. 1 for chemical structure), from a library of piperidine-based nocaine/modafinil hybrid molecules (Zhou et al., 2004). To identify JZAD-IV-22, we first screened molecules from the nocaine/modafinil library in an in vitro transporter inhibition assay. Compounds that were equally potent in inhibiting DAT, NET, and SERT were then screened in the mouse forced-swim assay for antidepressant efficacy and a locomotor activity assay to eliminate compounds that have potential to be psychostimulant. Locomotor psychostimulants were eliminated because they would be potentially confounding in the antidepressant efficacy tests (forced-swim assay and tail suspension) and could increase the likelihood of abuse liability (Wise and Bozarth, 1987). Leads were then tested with in vivo microdialysis to confirm the increase in extracellular monoamine levels in the mouse prefrontal cortex (PFC), an area of the brain innervated by 5-HT, NE, and DA fibers that plays a role in depressive disorders (Koenigs and Grafman, 2009). The behavioral and neurochemical properties of JZAD-IV-22 were compared with those of (+/−)-1-(3,4-dichlorophenyl)-3-azabicyclo-[3.1.0]hexane hydrochloride (DOV 216,303) (see Fig. 1 for chemical structure). DOV 216,303 was chosen as a comparator because it is a TRI with an in vitro profile similar to that of JZAD-IV-22 (Skolnick et al., 2006), was active in preclinical tests predictive of antidepressant efficacy [including the forced-swim test (Skolnick et al., 2006), the rat olfactory bulbectomy model (Breuer et al., 2008), and the differential reinforcement of low-rate 72-s test (Paterson et al., 2010a)], and has shown antidepressant efficacy in a clinical trial (Skolnick et al., 2006).
Fig. 1.
Chemical structures of DOV 216,303 and JZAD-IV-22.
A concern for developing DAT inhibitors is their potential to produce positive reinforcing effects, increasing the risk of abuse in humans (Kuhar et al., 1991; Woolverton and Johnson, 1992). DAT inhibition, however, cannot fully account for abuse liability because several drugs used clinically inhibit DAT and show no or minimal abuse liability in humans (e.g., bupropion). Because both DOV 216,303 and JZAD-IV-22 are potent DAT inhibitors, we compared both compounds in two assays to assess potential abuse liability, locomotor sensitization in mice, and drug discrimination in rats trained to discriminate saline from cocaine. We have demonstrated that under our treatment conditions, both assays are fairly good predictors of abuse liability in humans (Paterson et al., 2010b).
The present studies compared JZAD-IV-22 and DOV 216,303 as follows: 1) in an in vitro assay to measure potency to inhibit DAT, NET, and SERT, 2) using in vivo microdialysis to measure increases of DA, NE, and 5-HT in the PFC, 3) for antidepressant-like efficacy and potency in the mouse forced-swim and tail-suspension assays, and 4) for potential abuse liability in rat drug discrimination and mouse locomotor sensitization assays. We determined whether doses of JZAD-IV-22 and DOV 216,303 that were active in tests of antidepressant efficacy would show evidence of abuse liability.
Materials and Methods
In Vitro DAT/NET/SERT Inhibition.
DAT/NET/SERT inhibition studies were performed by Cerep (Poitiers, France). Methods for DA uptake in rat striatum synaptosomes (Janowsky et al., 1986), NE uptake in rat hypothalamus synaptosomes (Perovic and Müller, 1995), and 5-HT uptake in rat brain synaptosomes (Perovic and Müller, 1995) were based on published results.
Subjects.
Male Sprague-Dawley rats from Harlan (Indianapolis, IN) and male C57BL/6J (C57), BALB/cJ (BALB), and A/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Maastricht, The Netherlands). Rats were single-housed in OptiRAT ventilated cages (Animal Care Systems, Centennial, CO), and mice were group-housed in OptiMICE ventilated cages (Animal Care Systems) (behavioral studies) or Makrolon type II cages (37 × 21 × 14 cm) (Tecniplast, Philadelphia, PA) (microdialysis studies) and maintained on a 12-h light/dark cycle. The room temperature was maintained at 20 to 24°C with relative humidity at approximately 40 to 60%. For rats, chow was restricted to maintain rats at 85% of body weight of free-feeding age-matched control rats. Water was provided ad libitum for the duration of the study except during testing. For mice, food and water were available ad libitum for the duration of the study, except during testing. Mice arrived at 5 to 7 weeks of age, and behavioral testing began at 8 to 11 weeks of age. Testing was conducted during the light phase according to established protocols approved by the Institutional Animal Care and Use Committee of PsychoGenics, Inc. in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and in accordance with the Guide to the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and the Ethical Committee for Animal Research of the Faculties of Veterinary Medicine, Pharmaceutical Sciences, Chemistry and Biology at Utrecht University, The Netherlands.
Experimental Procedures
In Vivo Microdialysis.
BALB mice were placed in a stereotaxic frame using a mouse adaptor (Kopf) with modified ear bars. During surgery, mice were placed on a heating pad. Microdialysis probes were implanted in the PFC under Isoflurane/N2O/O2 anesthesia (400 mg/kg) and lidocaine (2%) was applied on the skull. The prefrontal coordinates were anteroposterior, +2.0 mm; mediolateral, +0.7 mm (under an 8° angle) from bregma; dorsoventral −3.3 mm from the dura, with the tooth-bar also set at 0 mm. The active dialysis surface length of the cuprophane membrane was 2 mm. The probe was secured in place with dental cement on the skull. After surgery, mice were injected with a carprofen solution (5 mg/kg s.c.) for postsurgical pain relief and with saline (until a total volume of 0.5 ml s.c. was reached) to prevent dehydration. The mice were then housed individually. Microdialysis experiments started 48 h after surgery. Ringer's solution (147 mM NaCl, 4 mM KCl, 2.3 mM CaCl2, and 1.0 mM MgCl2) was perfused through the microdialysis probe at a flow at 1.166 μl/min using a high-precision pump (KD Scientific 220; KD Scientific, Holliston, MA). Mouse dual-channel swivels (type 375/D/22QM; Instech Laboratories, Plymouth Meeting, PA) connected to PEEK tubing (i.d., 0.005 inches; o.d., 0.020 inches) were used to allow unrestrained movements of the mice. Samples were collected by hand 2.5 h after the start of the dialysis probe perfusion to obtain stable baseline values for monoamines and metabolites. The average of the first four samples was calculated and represents the baseline level. The experiments were performed during the light period and the mice were tested in their home cage. Samples were collected every 30 min in vials containing 11.7 μl of acetic acid (0.1 M) and stored at −80°C until high-performance liquid chromatography (HPLC) analysis. On each dialysis day, six mice were tested with treatment groups in a balanced design. Monoamine levels and metabolites were analyzed by HPLC with electrochemical detection.
After 2 h of baseline sample collection, JZAD-IV-22 (15, 30, or 60 mg/kg), DOV 216,303 (5, 15, 30, or 60 mg/kg), or saline vehicle was administered. Samples were collected for 3 h for DOV 216,303 and 2 h for JZAD-IV-22. Mice were euthanized at the end of the study for verification of probe placement.
HPLC-Electrochemical Detector Determination of Monoamines in Microdialysate.
NE, DA, and 5-HT were detected simultaneously by HPLC with electrochemical detection using an Alexys 100 LC-EC system (Leyden, The Netherlands). The system consisted of two pumps, one autosampler with a 10-port injection valve, two columns, and two detector cells. Column 1 (ALF 105; C18 1 × 50 mm, 3-μm particle size), in combination with detector cell 1, separated and detected DA and 5-HT. Column 2 (ALF 115; C18 1 × 150 mm, 3-μm particle size), in combination with detector cell 2, separated and detected NE. The mobile phase for column 1 consisted of 50 mM phosphoric acid, 8 mM KCl, 0.1 mM EDTA, pH 6.0, 12% methanol, and 500 mg/l 1-octanesulfonic acid sodium salt. The mobile phase for column 2 consisted of 50 mM phosphoric acid, 50 mM citric acid, 8 mM KCl, 0.1 mM EDTA, pH 3.2, 10% methanol, and 500 mg/l 1-octanesulfonic acid sodium salt. Both mobile phases were pumped at 50 μl/min. Samples were kept at 8°C during analysis. From each microdialysis sample, 5 μl was injected simultaneously onto each column. The neurotransmitters were detected electrochemically using μVT-03 flow cells (Antec) with glassy carbon working electrodes. Potential settings were +0.30 V versus Ag/AgCl for DA and 5-HT and +0.59 V versus Ag/AgCl for NA and metabolites. The columns and detector cells were kept at 35°C in a column oven. The chromatogram was recorded and analyzed using the Alexys data system (Antec). The limit of detection was 0.05 nM (signal-to-noise ratio 3:1).
Mouse Forced-Swim Test.
BALB mice were used in the forced-swim test because the BALB strain has been shown to be responsive to a wide range of antidepressants (Lucki et al., 2001 and data not shown). BALB mice were individually placed into clear glass cylinders (i.e., 15 cm tall × 10 cm wide, 1-l beakers) containing 23 ± 1°C water 12 cm deep (approximately 800 ml). Mice were administered water vehicle, DOV 216,303 (2.5, 5, 15, or 30 mg/kg), JZAD-IV-22 (15, 30, or 60 mg/kg), or sertraline (10 mg/kg); 30 min later, the time the animal spent immobile was recorded over a 6-min trial. Immobility was defined as the postural position of floating in the water. Doses for the forced-swim test were selected to find the minimally efficacious dose, with higher doses producing minimal effects on acute locomotor activity. The smaller cylinder (10 cm diameter) was used rather than larger cylinders reported by other groups (Lucki et al., 2001; Zhang et al., 2002) because the smaller cylinder is more sensitive to detecting novel antidepressant mechanisms. The antimobility effect of both the anticholinergic atropine and the adenosine antagonist caffeine was detected in a 10-cm but not a 30-cm cylinder (Sunal et al., 1994). Both anticholinergics (Drevets and Furey, 2010) and adenosine antagonists (El Yacoubi et al., 2003) show promise as novel antidepressant mechanisms for treating depression.
Mouse Tail-Suspension Test.
A/J mice were used in the tail-suspension test because we have found this strain to be responsive to a wide range of antidepressants (data not shown). A/J mice were pretreated with vehicle, DOV 216,303 (2.5, 5, 15, or 30 mg/kg), JZAD-IV-22 (15, 30, or 60 mg/kg), or desipramine (20 mg/kg) as a positive control 30 min before testing in the tail-suspension assay. The tail-suspension chambers consisted of white polyvinylchloride cubicles (33 × 33 × 31.75 cm; MED Associates, St. Albans, VT). A piece of transparent tape was attached to the tail of each mouse from approximately mid-tail, with approximately 2 cm past the end of the tail. The mice were then suspended via the tape from the tail-suspension force transducer. The force transducer transmitted the movements of the mouse to a recording device connected to a computer. Immobility time was automatically recorded during the 10-min test period.
Rat Drug Discrimination
Apparatus.
All drug discrimination testing took place in operant chambers (30.5 × 24.1 × 21.0 cm) located in sound-attenuating cubicles equipped with an exhaust fan (MED Associates). Each chamber contained two response levers situated on one wall of the chamber. A stimulus light was located above each lever, and a house light was located at the top of the opposite wall. A pellet receptacle was situated between the two levers for delivery of food pellets (45 mg). Data were collected and test session functions were controlled by Med PC IV software (MED Associates).
Experimental Procedures.
Rats were tested according to a double-alternation 2-week schedule [drug (D), vehicle (V), D, D, V; V, D, V, V, D]. During training sessions, rats were administered either cocaine (10 mg/kg) or vehicle 5 min before session initiation; appropriate lever responding was reinforced by delivery of food pellets under a fixed-ratio 20 schedule. After consistent responding on the appropriate drug- or vehicle-associated lever for at least 80% of total lever presses, compound testing was initiated. During test sessions, responding on both levers was reinforced via food pellet delivery (Paterson et al., 2010b). All test compounds were dissolved in saline and tested in the following sequence: cocaine, DOV 216,303, JZAD-IV-22. All compounds were administered according to a randomized-order, counter-balanced within-subjects design for each test compound. DOV 216,303 and JZAD-IV-22 were administered 15 or 20 min before testing, respectively. Higher doses of both DOV 216,303 (10 mg/kg) and JZAD-IV-22 (3 mg/kg) suppressed response rates in rats, which limited dose selection and did not allow for direct dose comparisons between mouse and rat.
Mouse Locomotor Sensitization
Apparatus.
Locomotor activity was measured in square Plexiglas chambers (27.3 × 27.3 × 20.3 cm; MED Associates) surrounded by 16-beam infrared photobeam sources and detectors. Mice were tested under ambient light and data were collected by Med Associates software.
Experimental Procedures.
C57 mice were used for locomotor sensitization studies, because we have previously shown that C57, but not BALB mice, show locomotor sensitization to cocaine (Paterson et al., 2010b; data not shown). Locomotor sensitization was assessed over a 20-day testing period, and under four testing phases: baseline locomotor activity (3 consecutive days), drug-induced locomotion (5 consecutive days), washout (10 days), and challenge (2 days testing of vehicle and test compound according to a crossover design: i.e., on challenge day 1, half of subjects received drug and half received vehicle; conditions were reversed on challenge day 2). C57 mice were administered test compounds immediately before being placed in the open field for a 30-min test session.
Drugs.
Cocaine and desipramine were purchased from Sigma-Aldrich (St. Louis, MO), sertraline was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada), DOV 216,303 was synthesized by AsisChem (Cambridge, MA), and JZAD-IV-22 was synthesized in house according to the reported procedures (Zhou et al., 2004). All compounds were administered by intraperitoneal injection (except where noted) in a 10 ml/kg injection volume for mice and a 1 ml/kg injection volume for rats.
Statistical Analyses.
All studies were analyzed using analysis of variance (ANOVA) followed by the Newman-Keuls post hoc comparisons (Statview software, SAS Institute, Cary, NC). All data are expressed as mean ± S.E.M. and the level of significance was set a priori at α <0.05.
Microdialysis data, expressed as percentage change from baseline in levels of DA, NE, and 5-HT, were analyzed. Forced swim data and tail-suspension data (time immobile) were analyzed with a one-way ANOVA with drug as the between-group factor.
Drug discrimination data were expressed as the percentage of cocaine-appropriate lever responding and as the rate of responding during the test session. Percentage of cocaine-appropriate lever responding was calculated by dividing the number of responses on the drug-appropriate lever by the total responses on both levers. Response rates were calculated by dividing the total number of responses by the total duration of the session in seconds. If the response rate was less than 0.02 responses per second at a specific dose, the cocaine-appropriate lever responding data were excluded for that dose. Response rate data were analyzed by ANOVA followed by post hoc tests when appropriate. Linear regression analyses and ED50 values were performed using Prism 5.0 software (GraphPad Software Inc., San Diego, CA). Full substitution, partial, and no substitution were defined as more than 80% drug-appropriate responding, 20 to 80%, and less than 20% drug-appropriate responding, respectively.
Mouse locomotor sensitization data were measured as total distance traveled (centimeters per 30 min). Induction of sensitization was assessed by a repeated-measures ANOVA with drug dose as the between-group variable and drug treatment day (1–5) as the within-subjects variable. Expression of sensitization was analyzed with ANOVA with drug treatment during the induction phase and drug treatment challenge phase as the between-group variables.
Results
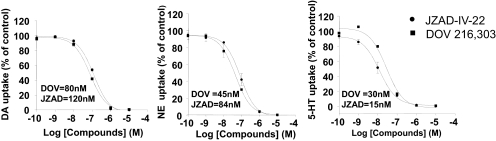
Inhibition of [3H]Neurotransmitter Uptake by DOV 216,303 and JZAD-IV-22
The IC50 values for DOV 216,303 and JZAD-IV-22 at inhibiting uptake of [3H]DA, [3H]NE, and [3H]5-HT into rat striatum synaptosomes, rat hypothalamus synaptosomes, rat brain synaptosomes, respectively, were 80, 45, and 30 nM for DOV 216,303 and 120, 84, and 15 nM for JZAD-IV-22 (Fig. 2). The potency of DOV 216,303 to inhibit dopamine, norepinephrine, and serotonin uptake was well within the range of previous report with this compound (Skolnick et al., 2006).
Fig. 2.
DOV 216,303 and JZAD-IV-22 show similar reuptake profiles for DA, NE, and 5-HT. Saturation curves of the specific DA uptake in rat striatal synaptosomes, NE uptake in rat hypothalamic synaptosomes, and 5-HT in rat whole brain synaptosomes for DOV 216,303 and JZAD-IV-22 are shown. Inserts are IC50 values for DOV 216,303 and JZAD-IV-22.
Microdialysis: Effects of JZAD-IV-22 and DOV 216,303 on Monoamine Levels in the PFC
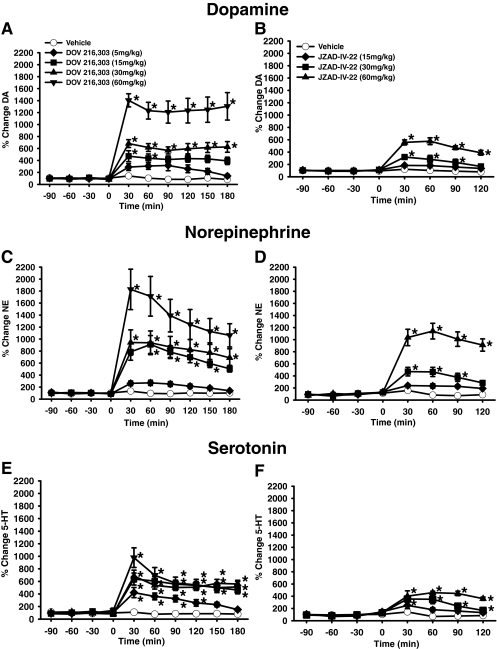
Dopamine Levels in Prefrontal Cortex.
DOV 216,303 treatment produced a significant increase in DA in the PFC that varied across dose and time [main effect of drug treatment, F(4,24) = 24.0, p < 0.0001; main effect of time, F(9,216) = 79.70 p < 0.0001; time × treatment interaction, F(36,216) = 18.21, p < 0.0001]. Post hoc tests showed that the 30 and 60 mg/kg doses of DOV 216,303 increased DA at all time points. The 15 mg/kg dose of DOV 216,303 increased DA only at the 30- and 60-min time points. The 5 mg/kg dose of DOV 216,303 was not different from vehicle at any time point (Fig. 3A).
Fig. 3.
Effects of DOV 216,303 and JZAD-IV-22 on extracellular DA, NE, and 5-HT levels in the PFC in mice. Shown are the percentage increase above baseline levels of DA for DOV 216,303 (A); DA for JZAD-IV-22 (B); NE for DOV 216,303 (C); NE for JZAD-IV-22 (D); 5-HT for DOV 216,303 (E); 5-HT for JZAD-IV-22 (F). Data are expressed as mean ± S.E.M. (DOV 216,303, n = 5–6/group; JZAD-IV-22, n = 4–5/group). Asterisks (*, p < 0.05) indicate significant differences compared with saline.
JZAD-IV-22 treatment produced a significant increase in DA in the PFC that varied across dose and time [main effect of drug treatment, F(3,15) = 45.83, p < 0.0001; main effect of time, F(7,105) = 80.68, p < 0.0001; and time × drug treatment interaction, F(21,105) = 25.84, p < 0.001]. Post hoc tests showed that the 60 mg/kg dose of JZAD-IV-22 increased levels of DA compared with vehicle at all time points. The 30 mg/kg dose of JZAD-IV-22 increased DA at all time points except 120 min. The 15 mg/kg dose of JZAD-IV-22 was not different from vehicle at any time point (Fig. 3B).
Norepinephrine Levels in Prefrontal Cortex.
DOV 216,303 treatment produced a significant increase in NE in the PFC that varied across dose and time [main effect of treatment, F(4,24) = 12.16, p < 0.0001; main effect of time, F(9,216) = 60.89, p < 0.0001; and a time × treatment interaction: F(36,216) = 11.31, p < 0.0001]. Post hoc tests revealed that the 30 and 60 mg/kg doses of DOV 216,303 increased NE at all time points, and the 15 mg/kg dose of DOV 216,303 increased NE at all time points except 30 and 180 min. The 5 mg/kg dose of DOV 216,303 did not differ from vehicle at any time point (Fig. 3C).
JZAD-IV-22 treatment produced a significant increase in NE in the PFC that varied across dose and time [main effect of drug treatment, F(3,15) = 38.23; p < 0.0001; main effect of time, F(7,105) = 74.58; p < 0.0001; and time × treatment interaction, F(21,105) = 28.88, p < 0.0001]. Post hoc tests showed that the 60 mg/kg dose of JZAD-IV-22 increased NE at all time points. The 30 mg/kg dose of JZAD-IV-22 increased NE at all time points except 120 min. The 15 mg/kg dose of JZAD-IV-22 was not different from vehicle at any time point (Fig. 3D).
Serotonin Levels in Prefrontal Cortex.
DOV 216,303 treatment produced a significant increase in 5-HT in the PFC that varied according to dose and time [main effect of treatment, F(4,24) = 13.09, p < 0.0001; main effect of time, F(9,216) = 98.75, p < 0.0001; time × treatment interaction, F(36,216) = 11.0, p < 0.0001]. Post hoc tests revealed that the 15, 30, and 60 mg/kg DOV 216,303 increased 5-HT at all time points. The 5 mg/kg dose of DOV 216,303 increased 5-HT at all time points except 150 and 180 min (Fig. 3E).
JZAD-IV-22 treatment produced a significant increase in 5-HT in the PFC that varied according to dose and time [main effect of drug treatment, F(3,15) = 29.46; p < 0.0001; main effect of time, F(7,105) = 34.80, p < 0.0001; and a time × treatment interaction, F(21,105) = 8.68, p < 0.0001]. Post hoc tests showed that the 30 and 60 mg/kg doses of JZAD-IV-22 increased 5-HT at all time points. The 15 mg/kg dose of JZAD-IV-22 was not different from vehicle at any time point (Fig. 3F).
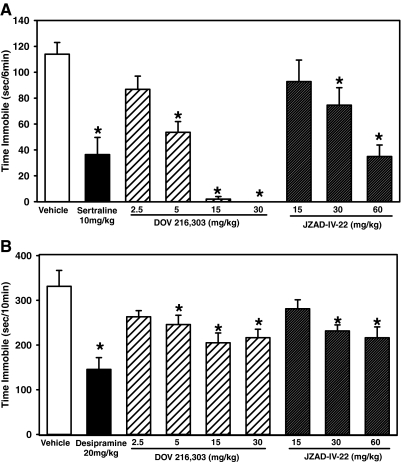
DOV 216,303 and JZAD-IV-22 in the Mouse Forced-Swim Test
Immobility time in the forced-swim test varied according to drug treatment [F(8,94) = 16.3, p < 0.0001]. Post hoc tests showed DOV 216,303 at 5, 15, and 30 mg/kg significantly reduced immobility but the 2.5 mg/kg dose was not different from vehicle. The two highest doses of JZAD-IV-22 (30 and 60 mg/kg) reduced immobility, but the lowest dose (15 mg/kg) was indistinguishable from vehicle. Sertraline (10 mg/kg) produced the expected reduction in immobility (Fig. 4A). These results show that both DOV 216,303 and JZAD-IV-22 show antidepressant-like effects in the forced-swim test, but DOV 216,303 is approximately six times more potent than JZAD-IV-22.
Fig. 4.
Effects of DOV 216,303 and JZAD-IV-22 in the mouse forced-swim and tail-suspension tests. Shown is time immobile (seconds) in the forced swim (A) and tail-suspension (B) tests. Both DOV 216,303 and JZAD-IV-22 showed an antidepressant-like reduction in immobility. Data are expressed as mean ± S.E.M. Asterisks (*, p < 0.05) indicate significant differences compared with vehicle (n = 10–18/group for forced swim; n = 9–10 for tail suspension).
DOV 216,303 and JZAD-IV-22 in the Mouse Tail-Suspension Test
Immobility time in the tail-suspension test varied according to drug treatment [F(8,80) = 5.4, p < 0.001]. Post hoc tests showed DOV 216,303 at 5, 15, and 30 mg/kg significantly reduced immobility, but the 2.5 mg/kg dose was not different from vehicle. The two highest doses of JZAD-IV-22 (30 and 60 mg/kg) reduced immobility, but the lowest dose (15 mg/kg) was indistinguishable from vehicle. Desipramine (20 mg/kg) produced the expected reduction in immobility (Fig. 4B). These results confirm the antidepressant-like effects of both DOV 216,303 and JZAD-IV-22 and demonstrate that the potency of both compounds in the tail-suspension test corresponds to the potency observed in the forced-swim assay.
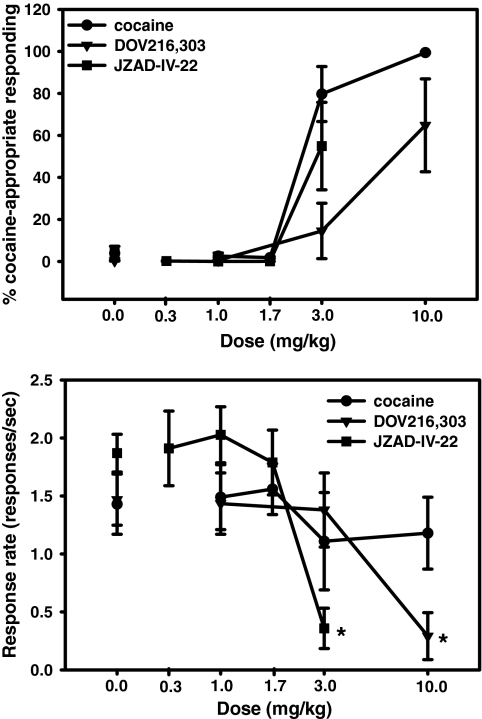
Assessment of the Discriminative Stimulus Properties of DOV 216,303 and JZAD-IV-22 in Cocaine-Trained Rats
Administration of cocaine (0, 1.0, 1.7, 3.0, and 10.0 mg/kg) engendered a dose-dependent increase in percentage of cocaine-appropriate responding, with full substitution at 10 mg/kg, and an ED50 value of 1.79 mg/kg [95% confidence limits, 0.38–8.42]. Administration of DOV 216,303 (0, 1.0, 3.0, and 10.0 mg/kg) engendered a dose-dependent increase in percentage cocaine-appropriate responding, with partial substitution (64.8%) at 10 mg/kg. Likewise, administration of JZAD-IV-22 (0, 0.3, 1.0, 1.7, and 3.0 mg/kg) partially substituted at the 3 mg/kg dose (54.9%). At the 3 mg/kg dose of JZAD-IV-22, one rat exhibited full substitution for cocaine and two rats showed partial substitution [two rats were excluded because of decreased response rates (<0.02/s)]. At the 10 mg/kg dose of DOV 216,303, two rats exhibited full substitution for cocaine, and one rat partial substitution (three subjects were excluded because of locomotor suppressive effects of the compound). Administration of both DOV 216,303 [F(3,18) = 10.4, p < 0.001] and JZAD-IV-22 [F(4,20) = 19.98, p < 0.0001] resulted in decreased response rates. Post hoc tests indicated that response rates were significantly decreased after administration of 10 mg/kg DOV 216,303 and 3.0 mg/kg JZAD-IV-22. Administration of cocaine did not alter response rates [F(4,24) = 1.05, not significant; Fig. 5].
Fig. 5.
Effects of DOV 216,303 and JZAD-IV-22 in rats trained to discriminate cocaine (10 mg/kg) from saline. Shown are the effects of cocaine (n = 7), DOV 216,303 (n = 7), and JZAD-IV-22 (n = 6) on percentage cocaine-appropriate responding (A) and response rate (B). Data are expressed as mean ± S.E.M. Asterisks (*, p < 0.05) indicate significant differences compared with saline.
Mouse Locomotor Sensitization
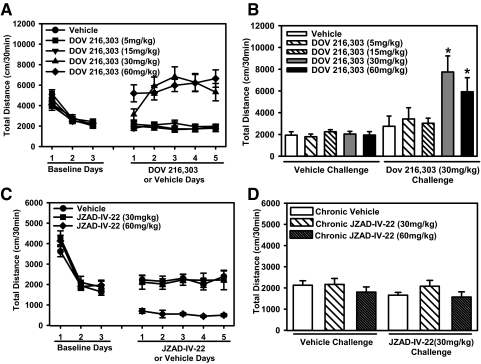
Repeated administration of DOV 216,303 resulted in the induction of a sensitized locomotor response that varied according to treatment dose and day [main effect of drug, F(4,45) = 20.2, p < 0.0001; main effect of day, F(4,180) = 4.9, p < 0.001; drug × day interaction, F(16,180) = 4.6, p < 0.0001]. Post hoc tests confirmed that administration of 30 mg/kg DOV 216,303 induced locomotor sensitization as measured by increased locomotor activity on days 3 and 4 compared with day 1 (a trend toward an increase in locomotion was noted between days 1 and 2) (Fig. 6A). Although the 60 mg/kg dose of DOV 216,303 produced an overall increase in locomotor activity, the activity did not increase progressively from day 1 to day 5 of testing, possibly because of a ceiling effect, the onset of stereotypical behavior as seen with high doses of psychostimulants, or the high level of locomotor activity observed after acute exposure on day 1. The lower doses of DOV 216,303 (5 and 15 mg/kg) produced no short-term increases in activity or induction of locomotor sensitization. A challenge dose of DOV 216,303 (30 mg/kg) after a 10-day washout period induced locomotor sensitization in mice treated with 30 or 60 mg/kg, but not 5 or 15 mg/kg during the induction phase [significant induction treatment × challenge treatment interaction effect, F(4,86) = 3.8, p < 0.01]. Post hoc tests confirmed mice given repeated exposure to 30 or 60 mg/kg DOV 216,303 during the induction phase showed a higher locomotor response to a 30 mg/kg challenge dose of DOV 216,303 compared with mice treated with vehicle during the induction phase. Locomotor response to a 30 mg/kg dose of DOV 216,303 during the challenge phase did not differ between mice treated with 5 or 15 mg/kg DOV 216,303 or vehicle during the induction phase (Fig. 6B).
Fig. 6.
The effects of repeated administration of DOV 216,303 and JZAD-IV-22 on locomotor activity. A and C, the line graphs show total locomotor activity measured after 3 days of vehicle administration (baseline days) and 5 days of drug or vehicle administration: A, 5, 15, 30, or 60 mg/kg DOV 216,303 or vehicle; C, 30 or 60 mg/kg JZAD-IV-22 or vehicle. B and D, bar graphs show total locomotor activity measured after a challenge dose of 30 mg/kg DOV 216,303 or vehicle (B) or 30 mg/kg JZAD-IV-22 or vehicle (D) after a 10-day washout of repeated drug administration. Data are expressed as mean ± S.E.M. Asterisks (*, p < 0.05) indicate significant differences (n = 9–10/group).
Daily administration of JZAD-IV 22 (30 or 60 mg/kg) did not produce an increase in activity across days during the induction phase (Fig. 6C). ANOVA showed that JZAD-IV-22 significantly affected locomotor activity [F(2,108) = 26.5, p < 0.0001], with post hoc tests showing that the high dose of JZAD-IV-22 (60 mg/kg) produced an overall decrease in locomotor activity during the induction phase. The low dose of JZAD-IV-22 (30 mg/kg) had no effect on locomotor activity (Fig. 6C) during the induction phase. A challenge dose of JZAD-IV-22 (30 mg/kg) after a 10-day washout period did not increase locomotor activity in mice that received repeated treatment of JZAD-IV-22 during the induction phase, confirming JZAD-IV-22 did not induce locomotor sensitization (Fig. 6D).
Discussion
The optimum preclinical profile for a TRI, defined as maximum antidepressant efficacy and minimum abuse liability, cannot be predicted based on in vitro or in vivo neurochemical profiles alone. Therefore, we used a behavioral screening approach to supplement the neurochemical data for identifying TRIs with favorable in vivo profiles. JZAD-IV-22 was identified among a subset of nocaine/modafinil hybrid analogs as a promising new TRI for treatment of depression based on its in vitro inhibition profile of DAT, NET, and SERT, its antidepressant-like activity in the forced-swim and tail-suspension tests, and lack of short-term locomotor activation in mouse. The behavioral and neurochemical properties of JZAD-IV-22 were compared with DOV 216,303, because DOV 216,303 has demonstrated antidepressant efficacy in a clinical trial and preclinical tests (Skolnick et al., 2006). The antidepressant-like efficacy of both JZAD-IV-22 and DOV 216,303 in the mouse forced-swim and tail-suspension tests corresponded with enhanced monoamine levels in the PFC. Assessment of potential abuse liability showed that although both DOV 216,303 and JZAD-IV-22 partially substituted for cocaine in a drug discrimination assay in rats, only DOV 216,303, at doses that are at least six times higher than the minimally efficacious dose in the forced-swim and tail-suspension tests, induced locomotor sensitization in mice. It is noteworthy that even high doses of JZAD-IV-22 that increased all three monoamine levels in the PFC to the same extent as DOV 216,303 did not induce either locomotor stimulation or sensitization in mice. These findings suggest that JZAD-IV-22 is dissimilar to abused psychostimulants such as cocaine and that JZAD-IV-22 may have a superior antidepressant/abuse profile compared with DOV 216,303.
JZAD-IV-22 and DOV 216,303 exhibited antidepressant-like properties at the same doses across the forced-swim and tail-suspension tests, although JZAD-IV-22 was less potent than DOV 216,303. It is noteworthy that both TRI compounds in this report exhibited efficacy similar to that of the SERT inhibitor sertraline in the forced-swim test, but were slightly less efficacious than the preferential NET inhibitor desipramine in the tail-suspension test. In vivo microdialysis studies showed that administration of JZAD-IV-22 significantly increased levels of DA, NE, and 5-HT in the PFC only at doses (30 and 60 mg/kg) that were effective in the mouse forced-swim and tail-suspension tests. In contrast, the minimally efficacious dose of DOV 216,303 in the forced-swim and tail-suspension tests (5 mg/kg) significantly increased only 5-HT (not NE or DA) in the PFC, although higher doses of DOV 216,303 (15, 30, and 60 mg/kg) increased levels of all three monoamines. The increases in monoamine levels were observed at the 30-min time point that corresponded to the time of forced-swim and tail-suspension testing. These results suggest that JZAD-IV-22 is a true TRI at all doses that are behaviorally active in the tail-suspension and forced-swim assays, whereas DOV 216,303 is a true TRI only at higher doses.
Drug discrimination in rats (Silverman and Ho, 1976; Holtzman, 1985; Carter and Griffiths, 2009) and locomotor sensitization in mice (Short and Shuster, 1976; Robinson and Berridge, 1993; Paterson et al., 2010b) provide measures of potential abuse liability. Drug discrimination studies revealed that both DOV 216,303 and JZAD-IV-22 partially substituted for cocaine to approximately the same extent, consistent with the similar monoamine transporter reuptake properties of both compounds. Rate-lowering effects of JZAD-IV-22 and DOV 216,303 precluded testing at higher doses. Although the TRIs and cocaine exhibit similar potencies at DA, NE, and 5-HT transporters (Zhou et al., 2004), neither of the TRIs fully substituted for cocaine. A failure to fully substitute for cocaine in the drug discrimination assays may be attributable either to a difference in pharmacological mechanisms of action between the TRI compounds and cocaine or to pharmacokinetic or pharmacodynamic differences (Desai et al., 2005a,b; Samaha and Robinson, 2005; Beuming et al., 2008; Loland et al., 2008). The behavioral and neurochemical data in the present studies make it unlikely that a lack of brain penetration resulted in the failure of DOV 216,303 and JZAD-IV-22 to substitute for cocaine, although the rate of brain penetration may be a critical factor (Weikop et al., 2004). A full receptor screen to assess additional off-target activity of the TRI compounds used in this report would be useful. For example, it has been shown that some benztropine analogs that have high affinity for DAT do not show cocaine-like discriminative stimulus properties, an effect likely resulting from muscarinic M1 receptor antagonism (Katz et al., 2004).
Despite the similar effects of DOV 216,303 and JZAD-IV-22 in rats trained to discriminate cocaine from saline, these compounds were dramatically different in their capacity to induce a short-term locomotor activation and long-term sensitized locomotor response. In contrast to DOV 216,303, which produced a short-term locomotor activation in mice as has been reported previously (Skolnick et al., 2006), JZAD-IV-22 did not increase locomotor activity after short-term administration, and the highest dose tested, 60 mg/kg, produced a small decrease in locomotion. Furthermore, JZAD-IV-22 showed no evidence of locomotor sensitization after long-term administration at either of the doses that were efficacious in forced-swim and tail-suspension assays (30 and 60 mg/kg). In contrast, repeated administration of both high doses of DOV 216,303 (30 and 60 mg/kg) produced locomotor sensitization as measured by an increase in locomotor activity in response to a 30 mg/kg challenge dose of DOV 216,303 after a 10-day washout.
Several hypotheses could explain why DOV 216,303 and JZAD-IV-22 both show partial substitution in the drug discrimination assay but differ in their locomotor-sensitized response. First, species differences, in which DOV 216,303 produced hyperactivity in mice but not rats, have been previously reported (Skolnick et al., 2006). The short-term locomotor-activating effects of DOV 216,303 observed in mice are likely to play a role in the locomotor sensitized response, because short-term locomotor responses to psychostimulants are often good predictors of locomotor sensitized responses (Wise and Bozarth, 1987). JZAD-IV-22, which was chosen in our behavioral screen to be devoid of short-term locomotor stimulant effects, did not produce a sensitized locomotor response. Second, it is possible that subtle differences in monoamine reuptake inhibition in the PFC induced by the two compounds could explain the differential sensitized response. For example, DOV 216,303 produced a more sustained increase in DA in the PFC compared with JZAD-IV-22. Differential changes in other neurotransmitters such as glutamate, which are known to mediate sensitized responses to cocaine (Steketee, 2005) or differences in neurotransmitters in other brain areas such as the nucleus accumbens (Pierce and Kalivas, 1997), could also explain the different locomotor sensitization properties of DOV 216,303 and JZAD-IV-22. Finally, altered neurochemical effects during repeated administration of DOV 216,303 and JZAD-IV-22 could underlie the different locomotor sensitization properties of the two compounds. In support of this hypothesis, differences in monoamine response have been reported in rats after short- and long-term treatment with DOV 216,303 (Prins et al., 2010).
Although DOV 216,303 and JZAD-IV-22 show differences in locomotor sensitization, a locomotor-sensitized response may not be completely predictive of abuse liability of these compounds in humans. Our previous data, however, support locomotor sensitization as a good predictor of abuse potential, because we have previously shown that bupropion, an antidepressant that shows no evidence of abuse liability in humans, substitutes for cocaine in the drug discrimination test but does not produce locomotor sensitization in mice (Paterson et al., 2010b). Furthermore, the rat drug discrimination studies show only partial cocaine-like discriminative stimulus properties for either DOV 216,303 or JZAD-IV-22, similar to nonabused compounds such as the tricyclic antidepressant desipramine and suggestive of low abuse potential for both TRIs. Finally, the differences between the mouse and rat studies may be a result of species differences, as the rats displayed dose-limiting effects of both DOV 216,303 and JZAD-IV-22 that were not observed in the mouse studies.
The results of the present study confirm the preclinical antidepressant-like efficacy of DOV 216,303 and describe JZAD-IV-22, a novel piperidine-based TRI that shows a comparable neurochemical and behavioral profile to DOV 216,303. The notable difference between the two TRIs is that DOV 216,303 increased acute locomotor activity and showed a sensitized locomotor response after repeated administration, whereas JZAD-IV-22 did not induce either short-term locomotor stimulation or a sensitized locomotor response after repeated administration. The absence of locomotor sensitization with JZAD-IV-22 may offer a clinical advantage in reducing the risk of abuse, although the drug discrimination assays would predict that both JZAD-IV-22 and DOV 216,303 would possess equal risk of abuse potential in humans. In conclusion, the piperidine-based TRIs are an attractive series for the development of novel antidepressants that could have advantages over currently available medications in terms of improved efficacy and reduced side effects. They could also serve as starting points for investigating additional clinical indications, such as Parkinson's-related depression, obesity, and pain.
Acknowledgments
We thank Michael Manzano, Christina Ruiz, Katie Cavino, and Wenzhong Min for excellent technical support for the behavioral studies.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH078433].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.174011.
- TRI
- triple reuptake inhibitor
- JZAD-IV-22
- 2-[4-(4-chlorophenyl)-1-methylpiperidin-3-ylmethylsulfanyl]-1-(3-methylpiperidin-1-yl)-ethanone
- DOV 216,303
- (+/−)-1-(3,4-dichlorophenyl)-3-azabicyclo-[3.1.0]hexane hydrochloride
- DAT
- dopamine transporter
- NET
- norepinephrine transporter
- SERT
- serotonin transporter
- DA
- dopamine
- NE
- norepinephrine
- 5-HT
- serotonin
- PFC
- prefrontal cortex
- C57
- C57BL/6J
- BALB
- BALB/cJ
- D
- drug
- V
- vehicle
- HPLC
- high-performance liquid chromatography
- ANOVA
- analysis of variance.
References
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, et al. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11:780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer ME, Chan JS, Oosting RS, Groenink L, Korte SM, Campbell U, Schreiber R, Hanania T, Snoeren EM, Waldinger M, et al. (2008) The triple monoaminergic reuptake inhibitor DOV 216,303 has antidepressant effects in the rat olfactory bulbectomy model and lacks sexual side effects. Eur Neuropsychopharmacol 18:908–916 [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105 (Suppl 1):S14–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila PS, Collu M, Gessa GL, Serra G. (2000) The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 405:365–373 [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. (2005a) Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther 315:397–404 [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. (2005b) Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25:1889–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. (2010) Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry 67:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Costentin J, Vaugeois JM. (2003) Adenosine A2A receptors and depression. Neurology 61:S82–S87 [DOI] [PubMed] [Google Scholar]
- Holtzman SG. (1985) Drug discrimination studies. Drug Alcohol Depend 14:263–282 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Janowsky A, Berger P, Vocci F, Labarca R, Skolnick P, Paul SM. (1986) Characterization of sodium-dependent [3H]GBR-12935 binding in brain: a radioligand for selective labeling of the dopamine transport complex. J Neurochem 46:1272–1276 [DOI] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. (2004) Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309:650–660 [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. (2009) The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299–302 [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823 [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. (2001) Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 155:315–322 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balci F, Campbell U, Olivier BE, Hanania T. (2010a) The triple reuptake inhibitor DOV216,303 exhibits limited antidepressant-like properties in the differential reinforcement of low-rate 72-second responding assay, likely due to dopamine reuptake inhibition. J Psychopharmacol doi:10.1177/0269881110364272 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Fedolak A, Olivier B, Hanania T, Ghavami A, Caldarone B. (2010b) Psychostimulant-like discriminative stimulus and locomotor sensitization properties of the wake-promoting agent modafinil in rodents. Pharmacol Biochem Behav 95:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perovic S, Müller WE. (1995) Pharmacological profile of hypericum extract. Effect on serotonin uptake by postsynaptic receptors. Arzneimittelforschung 45:1145–1148 [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216 [DOI] [PubMed] [Google Scholar]
- Prins J, Denys DA, Westphal KG, Korte-Bouws GA, Quinton MS, Schreiber R, Groenink L, Olivier B, Korte SM. (2010) The putative antidepressant DOV 216,303, a triple reuptake inhibitor, increases monoamine release in the prefrontal cortex of olfactory bulbectomized rats. Eur J Pharmacol 633:55–61 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. (2009) Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite 53:422–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. (2005) Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci 26:82–87 [DOI] [PubMed] [Google Scholar]
- Short PH, Shuster L. (1976) Changes in brain norepinephrine associated with sensitization to d-amphetamine. Psychopharmacology (Berl) 48:59–67 [DOI] [PubMed] [Google Scholar]
- Silverman PB, Ho BT. (1976) Discriminative response control by psychomotor stimulants. Psychopharmacol Commun 2:331–337 [PubMed] [Google Scholar]
- Skolnick P, Krieter P, Tizzano J, Basile A, Popik P, Czobor P, Lippa A. (2006) Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev 12:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. (2003) “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci 73:3175–3179 [DOI] [PubMed] [Google Scholar]
- Steketee JD. (2005) Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol 17:69–86 [DOI] [PubMed] [Google Scholar]
- Sunal R, Gümüşel B, Kayaalp SO. (1994) Effect of changes in swimming area on results of “behavioral despair test”. Pharmacol Biochem Behav 49:891–896 [DOI] [PubMed] [Google Scholar]
- Weikop P, Egestad B, Kehr J. (2004) Application of triple-probe microdialysis for fast pharmacokinetic/pharmacodynamic evaluation of dopamimetic activity of drug candidates in the rat brain. J Neurosci Methods 140:59–65 [DOI] [PubMed] [Google Scholar]
- Willner P. (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134:319–329 [DOI] [PubMed] [Google Scholar]
- Wise RA. (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240 [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492 [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. (1992) Neurobiology of cocaine abuse. Trends Pharmacol Sci 13:193–200 [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O'Donnell JM. (2002) Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology 27:587–595 [DOI] [PubMed] [Google Scholar]
- Zhou J, He R, Johnson KM, Ye Y, Kozikowski AP. (2004) Piperidine-based nocaine/modafinil hybrid ligands as highly potent monoamine transporter inhibitors: efficient drug discovery by rational lead hybridization. J Med Chem 47:5821–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Haight BR, Clines DC, Rockett CB. (2006) Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry 59:203–210 [DOI] [PubMed] [Google Scholar]