Abstract
The N-methyl-d-aspartate (NMDA) receptor family regulates various central nervous system functions, such as synaptic plasticity. However, hypo- or hyperactivation of NMDA receptors is critically involved in many neurological and psychiatric conditions, such as pain, stroke, epilepsy, neurodegeneration, schizophrenia, and depression. Consequently, subtype-selective positive and negative modulators of NMDA receptor function have many potential therapeutic applications not addressed by currently available compounds. We have identified allosteric modulators with several novel patterns of NMDA receptor subtype selectivity that have a novel mechanism of action. In a series of carboxylated naphthalene and phenanthrene derivatives, compounds were identified that selectively potentiate responses at GluN1/GluN2A [e.g., 9-iodophenanthrene-3-carboxylic acid (UBP512)]; GluN1/GluN2A and GluN1/GluN2B [9-cyclopropylphenanthrene-3-carboxylic acid (UBP710)]; GluN1/GluN2D [3,5-dihydroxynaphthalene-2-carboxylic acid (UBP551)]; or GluN1/GluN2C and GluN1/GluN2D receptors [6-, 7-, 8-, and 9-nitro isomers of naphth[1,2-c][1,2,5]oxadiazole-5-sulfonic acid (NSC339614)] and have no effect or inhibit responses at the other NMDA receptors. Selective inhibition was also observed; UBP512 inhibits only GluN1/GluN2C and GluN1/GluN2D receptors, whereas 6-bromo-2-oxo-2H-chromene-3-carboxylic acid (UBP608) inhibits GluN1/GluN2A receptors with a 23-fold selectivity compared with GluN1/GluN2D receptors. The actions of these compounds were not competitive with the agonists l-glutamate or glycine and were not voltage-dependent. Whereas the N-terminal regulatory domain was not necessary for activity of either potentiators or inhibitors, segment 2 of the agonist ligand-binding domain was important for potentiating activity, whereas subtype-specific inhibitory activity was dependent upon segment 1. In terms of chemical structure, activity profile, and mechanism of action, these modulators represent a new class of pharmacological agents for the study of NMDA receptor subtype function and provide novel lead compounds for a variety of neurological disorders.
Introduction
l-Glutamate, the primary excitatory neurotransmitter in the vertebrate central nervous system (CNS), activates three distinct families of ligand-gated ion channels that are named for agonists by which they are selectively activated. These families are N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (Watkins et al., 1981; Monaghan et al., 1989; Dingledine et al., 1999). Whereas AMPA and kainate receptors underlie fast excitatory synaptic transmission in the CNS, NMDA receptor activation triggers diverse calcium-dependent intracellular responses that regulate distinct forms of synaptic plasticity, such as long-term potentiation, long-term depression, and experience-dependent synaptic refinement (Monaghan et al., 1989; Dingledine et al., 1999). Such NMDA receptor-mediated mechanisms are thought to play key roles in learning and memory but also contribute to the expression of epilepsy, schizophrenia, drug addiction, mood disorders, post-traumatic stress disorder, and neuropathic pain (Kalia et al., 2008; Sanacora et al., 2008). Excessive NMDA receptor activation may also be a common mechanism causing neuronal cell death in stroke, traumatic brain injury, and various neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's, amyotrophic lateral sclerosis, and Creutzfeldt-Jakob disease (Villmann and Becker, 2007; Kalia et al., 2008). These findings have led to high expectations for clinical studies of NMDA receptor-based therapeutic agents. Unfortunately, the results from these studies have been largely disappointing because of adverse effects and limited therapeutic efficacy (O'Collins et al., 2006; Kalia et al., 2008). To date, most NMDA receptor pharmacological agents tested in the clinic have been nonselective agents that cannot distinguish between NMDA receptor subtypes. This lack of subtype selectivity has probably contributed to the inability to optimize the therapeutic effect of NMDA receptor pharmacological agents, while minimizing their adverse effects (see Discussion).
NMDA receptor complexes consist of subunits from seven genes: GluN1, GluN2A–GluN2D, and GluN3A–GluN3B (Dingledine et al., 1999). The majority of NMDA receptors are believed to consist of two GluN1 subunits and two GluN2 subunits (Laube et al., 1998). l-Glutamate and a necessary coagonist (either glycine or d-serine) bind to homologous binding sites on GluN2 and GluN1 subunits, respectively, to cause the opening of the receptor's Na+/K+/Ca2+-permeable ion channel (Dingledine et al., 1999). It is noteworthy that the GluN2 subunits have varied developmental profiles and anatomical distributions and confer distinct physiological, biochemical, and pharmacological properties to the NMDA receptor complex (Buller et al., 1994; Monyer et al., 1994; Cull-Candy et al., 2001). Evidence suggests that specific NMDA receptor subunits have distinct, and sometimes opposing, roles in various physiological and pathophysiological actions (Hrabetova et al., 2000; DeRidder et al., 2006; Chen et al., 2008). However, their specific roles have been difficult to study in the absence of highly selective antagonists.
Currently, four functionally distinct classes of compounds are therapeutic candidates for the inhibition of NMDA receptor function: those that inhibit glutamate or glycine binding, those that block the ion channel, and those that inhibit the receptor by binding to an N-terminal regulatory domain (NTD) (Jane et al., 2000). Of these four drug targets, the first three drug binding sites are highly conserved in different NMDA receptor subtypes, and only for the NTD drug-binding site are there compounds that fully distinguish GluN2 subunits. These latter compounds are limited to those that are selective for GluN2B-containing receptors. Hence, the only subtype-selective agents that have been tested in the clinic are antagonists that selectively block GluN1/GluN2B receptors. Although there are several potential therapeutic applications for positive modulators of NMDA receptor function, there are no such compounds available for clinical studies.
In this study, we have identified a series of naphthalene and phenanthrene derivatives that display inhibitory and/or potentiating activity with remarkably different patterns of selectivity at NMDA receptors containing different GluN2 subunits. These agents, and their future derivatives, represent a novel class of NMDA receptor allosteric modulator drugs that do not act at the glutamate or glycine binding sites, the ion channel, or the NTD. They may be acting at the dimer interface between individual subunit ligand-binding domains. This group of compounds should be valuable tools for identifying the physiological roles of distinct NMDA receptor subtypes and serve as lead compounds for a variety of therapeutic applications.
Materials and Methods
Compounds.
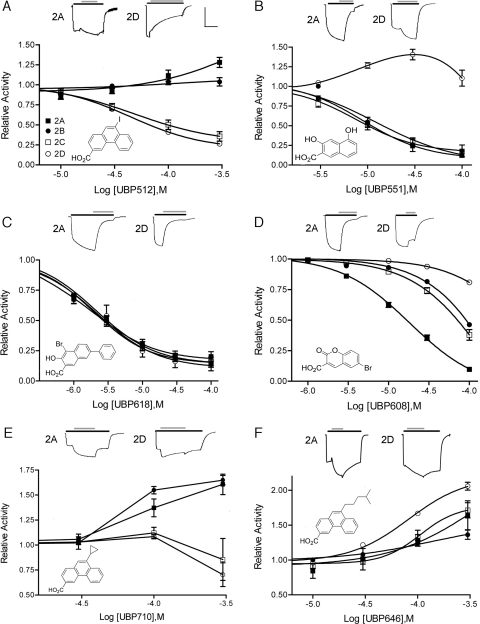
Structures of compounds synthesized and tested for this report are presented in Fig. 1. 9-Iodophenanthrene-3-carboxylic acid (UBP512), 1-bromo-2-hydroxy-6-phenylnaphthalene-3-carboxylic acid (UBP618), 9-(4-methylpent-1-yl)phenanthrene-3-carboxylic acid (UBP646), and 9-cyclopropylphenanthrene-3-carboxylic acid (UBP710) were synthesized and purified by methods to be reported elsewhere. After synthesis and purification, compound structure was verified by 1H NMR and mass spectroscopy. All compounds had elemental analyses in which the determined percentages C, H, and N were less than 0.4% different from theoretical values. The other compounds were obtained from Alfa Aesar [3,5-dihydroxynaphthalene-2-carboxylic acid (UBP551)], Sigma-Aldrich [6-bromo-2-oxo-2H-chromene-3-carboxylic acid (UBP608)], and the National Cancer Institute's Developmental Therapeutics Program Open Repository at http://dtp.cancer.gov [6-, 7-, 8-, and 9-nitro isomers of naphth[1,2-c][1,2,5]oxadiazole-5-sulfonic acid (NSC339614)].
Fig. 1.
A series of two- and three-ring aromatic structures display varied activities on the responses of NMDA receptor subtypes. Representative voltage-clamped (−60 mV) current responses are shown for GluN1/GluN2A (2A) and GluN1/GluN2D (2D) receptors evoked by 10 μM l-glutamate and 10 μM glycine (black bar) plus the addition of a 100 μM concentration of the indicated compound (gray bar). Scale bars: x-axis = 17 s; y-axis = 300 nA (mean values; see Supplemental Table 1 for individual values). In the bottom of each panel is a dose-response curve of compound potentiation (values >1) or inhibition (values <1) of agonist responses by GluN1/GluN2A (■), GluN1/GluN2B (●), GluN1/GluN2C (□), and GluN1/GluN2D (○) receptors. Values represent means ± S.E.M. with n = 4 or more.
NMDA Receptor Constructs.
cDNA encoding the NMDAR1a subunit (GluN1a) was a generous gift of Dr. Shigetada Nakanishi (Kyoto, Japan). cDNA encoding the GluN2A, GluN2C, and GluN2D were kindly provided by Dr. Peter Seeburg (Heidelburg, Germany), and the GluN2B [5′-untranslated receptor] cDNA was the generous gift of Drs. Dolan Pritchett and David Lynch (Philadelphia, PA). GluN2A chimeras containing either the S1 (GluN2A2CS1) or the S2 domain (GluN2A2CS2) of GluN2C were constructed by overlap-extension polymerase chain reaction. In GluN2A2CS1, the GluN2C S1 domain, amino acids 352 to 535 replaced the corresponding sequence in GluN2A (Monyer et al., 1992). In GluN2A2CS2, the region between M3 and M4, GluN2C amino acids 634 to 795, replaced the corresponding sequence in GluN2A (Monyer et al., 1992). Constructs were verified by sequencing by the University of Nebraska Medical Center Sequencing Facility. The NTD-deleted NR1 (NR1ΔNTD) and the NTD-deleted NR2 constructs (NR2AΔNTD and NR2DΔNTD) were kindly provided by Dr. Bodo Laube (Madry et al., 2008) and Dr. Pierre Paoletti (Rachline et al., 2005), respectively. Plasmids were linearized with NotI (GluN1a, GluN2C, GluN2D, and NR1ΔNTD), EcoRI (GluN2A, GluN2A2CS1, and GluN2A2CS2), or SalI (GluN2B, NR2AΔNTD, and NR2DΔNTD) and transcribed in vitro with T7 (GluN1a, GluN2A, GluN2C, GluN2D, GluN2A2CS1, and GluN2A2CS2) or SP6 (NR1ΔNTD, NR2AΔNTD, NR2DΔNTD, and GluN2B) RNA polymerase using the mMessage mMachine transcription kits (Ambion, Austin, TX).
NR Subunit Expression and Electrophysiology in Xenopus laevis Oocytes.
Oocytes from mature female X. laevis (Xenopus One, Ann Arbor, MI) were removed and isolated. GluN1a and GluN2 RNAs were dissolved in sterile distilled H2O and mixed in a molar ratio of 1:1-3. Then, 50 nl of the final RNA mixture was microinjected (15–30 ng total) into the oocyte cytoplasm. Oocytes were incubated in ND-96 solution at 17°C before electrophysiological assay (1–5 days). Electrophysiological responses were measured using a standard two-microelectrode voltage clamp (model OC-725B; Warner Instruments, Hamden, CT). The recording buffer contained 116 mM NaCl, 2 mM KCl, 0.3 mM BaCl2, and 5 mM HEPES, pH 7.4. Agonist-evoked responses were clamped at −60 mV unless stated otherwise. Response amplitudes for the four heteromeric complexes were generally between 0.1 and 3 μA. After obtaining a steady-state response to agonist application, test compounds were bath applied (16-channel perfusion system; AutoMate Scientific, Inc., Berkeley, CA), and the responses were digitized for quantification (Digidata 1440A and pClamp-10; Molecular Devices, Sunnyvale, CA). Dose-response relationships were fit to a single site with variable slope (Prism; GraphPad Software, San Diego, CA), using a nonlinear regression to calculate IC50 or EC50 and percentage maximal inhibition. All experiments were performed a minimum of four times.
Results
A variety of structures containing either two or three fused aromatic rings were evaluated for their ability to modulate NMDA receptor responses evoked by 10 μM l-glutamate and 10 μM glycine. GluN1/GluN2A, GluN1/GluN2B, GluN1/GluN2C, and GluN1/GluN2D receptors were expressed in X. laevis oocytes, and receptor activity was determined by two-electrode voltage clamp. Of the compounds screened, seven compounds represent the different activities that were observed. Four of these compounds were novel and were synthesized. UBP512 inhibited GluN1/GluN2C and GluN1/GluN2D receptors, had minimal effect on GluN2B-containing receptors, and caused a small potentiation of GluN1/GluN2A receptor responses (Fig. 1A). At 3 to 10 μM, UBP512 weakly inhibited GluN1/GluN2A and GluN1/GluN2B receptor responses (∼10–15%). At higher doses, UBP512 potentiated GluN1/GluN2A receptor-mediated responses and inhibited responses at GluN1/GluN2C (IC50 = 51 ± 11 μM; Hill coefficient = 1.3 ± 0.3) and GluN1/GluN2D receptors (IC50 = 46 ± 6 μM; Hill coefficient = 1.35 ± 0.1). Under these conditions, UBP512 maximally inhibited 69 ± 6 and 72 ± 2% of the total GluN1/GluN2C and GluN1/GluN2D receptor responses, respectively.
In contrast to UBP512, UBP551 inhibited responses at receptors containing GluN2A, GluN2B, or GluN2C subunits and potentiated activity at GluN1/GluN2D receptors (Fig. 1B). UBP551 displayed IC50 values of 9.7 ± 0.2, 9.4 ± 0.6, and 15 ± 6 μM for receptors containing GluN2A-C subunits, respectively, and Hill coefficients of 1.4 ± 0.1, 1.8 ± 0.2, and 1.2 ± 0.3, respectively, with maximal inhibition of 91 ± 1.3, 83.9 ± 7.1, and 85.0 ± 2.3%, respectively. Maximal potentiation of GluN1/GluN2D responses was found at a concentration of 30 μM; higher concentrations resulted in reduced potentiating activity.
UBP608 and UBP618 displayed only inhibitory activity when tested against receptor responses evoked by 10 μM l-glutamate plus 10 μM glycine (Fig. 1, C and D). UBP608 fully inhibited (maximal inhibition = 104 ± 0.6%) GluN1/GluN2A responses with an IC50 of 18.6 ± 1.4 μM and a Hill coefficient of 1.08 ± 0.02. Concentrations of UBP608 several-fold higher were required to inhibit GluN1/GluN2B (IC50 = 90 ± 4 μM, Hill coefficient = 1.25 ± 0.06) and GluN1/GluN2C responses (IC50 = 68 ± 9 μM, Hill coefficient = 1.22 ± 0.07). GluN2D-containing receptors were least affected with an extrapolated IC50 of 426 ± 40 μM and a Hill coefficient of 1.16 ± 0.1. UBP618 was a relatively potent, nonselective inhibitor at NMDA receptors (Fig. 1C) with IC50 values as follows: GluN1/GluN2A, 1.8 ± 0.2 μM; GluN1/GluN2B, 2.4 ± 0.1 μM; GluN1/GluN2C, 2.0 ± 0.08 μM; and GluN1/GluN2D, 2.4 ± 0.3 μM. Corresponding Hill coefficients were 0.98 ± 0.07, 0.94 ± 0.04, 0.98 ± 0.05, and 1.48 ± 0.15, respectively, and maximal inhibitions were 83 ± 4, 88 ± 2.0, 87 ± 2, and 87 ± 5%, respectively.
In contrast to UBP512, UBP710 displayed greater activity in potentiating GluN2B-containing receptors than those containing GluN2A (Fig. 1E). UBP710 potentiated responses at receptors containing GluN2A and GluN2B subunits (approximately 50–150%) and, at 100 μM, usually caused a weak potentiation of responses at GluN2C- and GluN2D-containing receptors (GluN2C, 8 of 12 cells; GluN2D, 8 of 12 cells). A more universal potentiator was UBP646. This agent most effectively potentiated GluN1/GluN2D receptors and consistently potentiated the other three subtypes (Fig. 1F).
A different pattern of potentiation activity was observed for the compound NSC339614. This compound potentiated GluN1/GluN2C and GluN1/GluN2D receptor responses but inhibited GluN1/GluN2A and GluN1/GluN2B receptor activity (Supplemental Fig. 1). Chemical characterization revealed that this compound is a mixture of the 6-, 7-, 8-, and 9-nitro isomers of naphth[1,2-c][1,2,5]oxadiazole-5-sulfonic acid potassium salt (Supplemental Fig. 2).
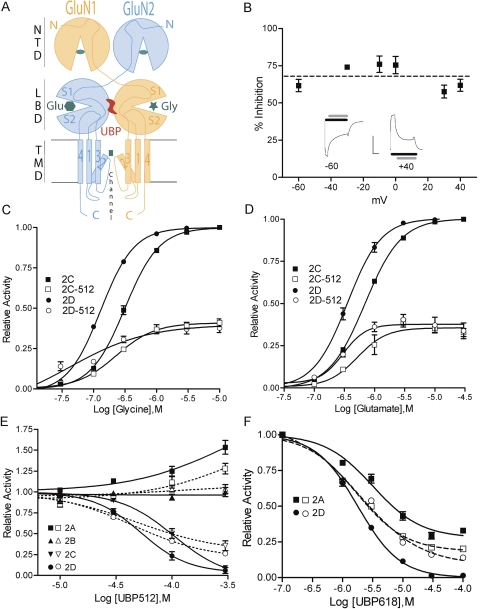
The structural features of these compounds do not conform to any known group of NMDA receptor antagonists or modulators. Thus, further studies were directed at defining the site of action. UBP512 does not appear to act as an NMDA receptor ion-channel blocker. The ability of UBP512 to inhibit GluN1/GluN2D responses was not voltage-dependent (Fig. 2B), suggesting that UBP512 does not block by binding within the ion channel pore that is exposed to the transmembrane electric field. Voltage dependence of inhibition was also evaluated for UBP618; 100 μM UBP618 inhibited GluN1/GluN2A responses 76 ± 4% at +40 mV and by 66 ± 3% at −60 mV.
Fig. 2.
Compound inhibition of NMDA receptor responses is not voltage-dependent and does not compete with l-glutamate or glycine binding to NMDA receptors. A, a schematic illustrating a GluN1/GluN2 dimer and the domain structure and binding sites for l-glutamate (hexagon), glycine (star), NTD ligands (oval), and channel blockers (square). B, UBP512 (100 μM) inhibition of GluN1/GluN2D receptor responses at different membrane potentials. Insets: current traces showing agonist (black bar) and UBP512 application (gray bar), scale bars (x-axis = seconds, y-axis = microamperes): −60 mV (180 s, 1.1 μA); +40 mV (72 s, 2.0 μA). C and D, GluN1/GluN2C (2C) or GluN1/GluN2D (2D) receptors were activated by increasing concentrations of glycine (C) or l-glutamate (D), and 10 μM concentrations of the other agonist in the absence (filled symbols) or presence (open symbols) of 100 μM UBP512. E and F, UBP512 (E) and UBP618 (F) modulation of NMDA receptor responses evoked by low (10 μM l-glutamate and 10 μM glycine; open symbols) or high agonist concentrations (300 μM l-glutamate/300 μM glycine; closed symbols). UBP512 more effectively inhibited GluN1/GluN2C (inverted triangles) and GluN1/GluN2D (circles) receptor responses and more effectively potentiated GluN1/GluN2A (squares) receptor responses evoked by high agonist concentrations than by low concentrations. F, UPB618 displays greater maximal inhibition of GluN1/GluN2D receptor responses and decreased maximal inhibition of GluN1/GluN2A receptor responses in the presence of high agonist concentrations.
UBP512 is not a competitive antagonist at either the l-glutamate or glycine binding sites. The blockade of GluN2C or GluN2D by 100 μM UBP512 could not be overcome by increasing concentrations of glycine (Fig. 2C) or l-glutamate (Fig. 2D). At the highest doses of l-glutamate in the presence of UBP512, there was a small reduction in both GluN1/GluN2C and GluN1/GluN2D receptor activation (Fig. 2D). In converse experiments, UBP512 activity was tested with a range of concentrations in the presence of low (10 μM) or high (300 μM) concentrations of l-glutamate and glycine (Fig. 2E). High agonist concentrations did not significantly alter UBP512 potency for inhibition (GluN1/GluN2C and GluN1/GluN2D) or potentiation (GluN1/GluN2A). GluN1/GluN2C and GluN1/GluN2D receptor responses to high agonist concentrations (300 μM l-glutamate/300 μM glycine) were inhibited by UBP512 with IC50 values of 108 ± 12 and 53 ± 6 μM, respectively. However, in the presence of high agonist concentrations, UBP512 blockade became more effective. Under these conditions, UBP512 fully blocked GluN2C- and GluN2D-containing receptor responses (104 ± 8% and 97 ± 7%, respectively), and displayed Hill coefficients near 2 (1.8 ± 0.3 and 2.1 ± 0.5, respectively). The greater blockade by UBP512 in the presence of high agonist concentrations can account for the decrease in response size that occurs in the presence of 100 μM UBP512 when increasing l-glutamate concentration (Fig. 2D). Increasing agonist concentrations also increased the magnitude of UBP512 potentiation of GluN1/GluN2A receptors (Fig. 2E).
In a similar manner, the potency of UBP618 for inhibiting GluN1/GluN2A and GluN1/GluN2D responses was mostly unaffected by increasing agonist concentration (Fig. 2F; GluN1/GluN2A IC50 = 3.2 ± 0.4 μM; GluN1/GluN2D IC50 = 1.8 ± 0.1 μM), whereas the maximal percentage inhibition of GluN1/GluN2A responses was decreased from 83 ± 4 to 72 ± 1%, and the maximal percentage inhibition of GluN1/GluN2D responses was increased from 92.0 ± 0.7 to 99.9 ± 0.4%. These results indicate that the inhibitory actions of UBP512 and UBP618 are not due to a competitive interaction at either the l-glutamate or glycine binding sites. However, agonist binding does alter the ability of UBP512 and UBP618 to inhibit channel function.
Zn2+ is a high-affinity negative modulator of GluN1/GluN2A receptors that binds to the NTD of GluN2A (Paoletti et al., 2000). Thus, the selective potentiation of GluN2A-containing receptors by UBP512 could potentially be due to the reversal of Zn2+-inhibition by Zn2+ chelation. However, UBP512 potentiation was not affected by the presence of a potent Zn2+ chelator or the addition of 100 nM Zn2+ (Supplemental Fig. 3A). Conversely, UBP512 addition did not alter the EC50 values for the high- or low-affinity components of Zn2+ inhibition at GluN1/GluN2A receptors (Supplemental Fig. 3B).
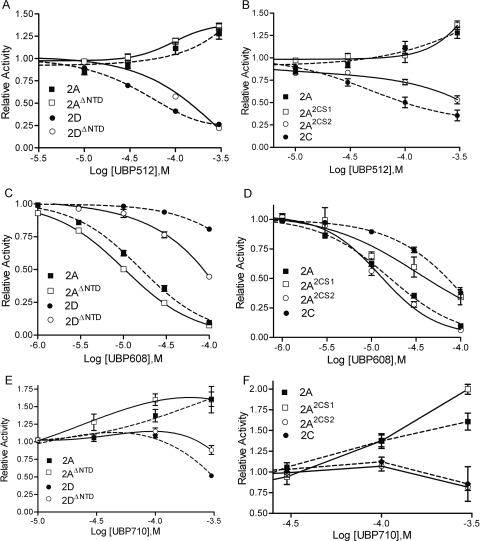
UBP512, UBP608, and UBP710 do not require the NTD for their modulatory activity. Removal of the NTD region of both GluN1 and GluN2 subunits enhances, rather than blocks, UBP512 potentiation of GluN1/GluN2A receptors (Fig. 3A). Likewise, NTD deletion of GluN1/GluN2D receptors does not eliminate UBP512 inhibitory activity, but does reduce UBP512 inhibitory potency a few fold (Fig. 3A). NTD deletion also does not block UBP608's inhibitory activity (Fig. 3C) or the ability of UBP710 to potentiate responses at GluN1/GluN2A receptors (Fig. 3E).
Fig. 3.
A, C, and E, compound activity was tested on responses evoked by 10 μM l-glutamate/10 μM glycine of wild-type GluN1/GluN2A (2A) and GluN1/GluN2D (2D) receptors (dashed lines) or receptors without NTDs of both GluN1 and GluN2 subunits (solid lines)—GluN1/GluN2A (2AΔNTD) and GluN1/GluN2D (2DΔNTD). B, D, and E, compounds were tested on responses by wild-type GluN1/GluN2A (2A) and GluN1/GluN2C (2C) receptors (dashed lines) and by chimeric receptors (solid lines) where the GluN2A subunit has the GluN2C S1 (2A2CS1) or the GluN2C S2 (2A2CS2) domain.
Because the NTD region is not necessary for modulator activity, these compounds are most likely binding on the remaining extracellular region that comprises the ligand-binding domain—either segment 1 (S1) between the NTD and the first intramembrane domain (M1) and/or on segment 2 (S2), the extracellular loop between the third (M3) and fourth (M4) intramembrane domain (see Fig. 2A). The GluN1/GluN2A potentiators UBP512 and UBP710 and the inhibitor UBP608 were tested on GluN2A chimeras containing either the S1 or the S2 domain of GluN2C (Fig. 3). GluN2A-containing NMDA receptors with the S1 domain of GluN2C were still potentiated by UBP512 and UBP710; however, GluN2A-containing receptors with the S2 domain of GluN2C were inhibited, instead of potentiated, by UBP512 and UBP710. In contrast, the inhibitory actions of UBP608 (which has higher potency at GluN2A-containing than GluN2C-containing receptors) were reduced in receptors having the GluN2A subunit with the GluN2C S1 domain. The S2 domain of GluN2C in GluN2A had a negligible affect on UBP608 inhibitory activity. Thus, the S2 domain is important for the binding and/or the downstream potentiating actions of UBP512 and UBP710, whereas the S1 domain seems to be more important for the inhibitory actions of UBP608.
All compounds were also evaluated for agonist or partial agonist activity by testing for excitatory activity alone or when paired with glycine or l-glutamate at each of the four GluN1/GluN2 receptors (Supplemental Table 2). Compounds were found to be devoid of agonist or partial agonist activity. Because the inhibitory modulators generally have maximal inhibitions of 70 to 90%, the absence of partial agonist activity confirms that these compounds are not acting at either of the agonist (l-glutamate or glycine) binding sites.
Discussion
Over the past 30 years, there have been more than 30,000 publications characterizing NMDA receptor function. This work has established that NMDA receptors are critically involved in many physiological and pathophysiological activities and has led to many preclinical and clinical studies attempting to develop NMDA receptor therapeutic agents for the treatment of epilepsy, schizophrenia, depression, pain, drug addition, alcoholism, Alzheimer's disease, Huntington's disease, Parkinson's disease, ALS, traumatic brain injury, stroke, and other conditions (Koutsilieri and Riederer, 2007; Kalia et al., 2008; Sanacora et al., 2008). Unfortunately, results from most clinical trials have been disappointing because of adverse effects and limited therapeutic efficacy (Villmann and Becker, 2007; Kalia et al., 2008). Optimal therapeutic effectiveness of NMDA receptor pharmacological agents may require targeting the most appropriate subtypes of NMDA receptors. Thus, it is significant that most NMDA receptor agents that have been evaluated in the clinic, other than GluN2B-selective agents, are not subtype-selective. Because of the highly conserved nature of their respective binding sites, compounds that inhibit glutamate or glycine binding, or that block the ion channel, have very low subtype selectivity.
In the present study, we have identified a class of allosteric modulators with a novel mechanism of action that imparts greater subtype selectivity than the other classes of NMDA receptor agents. For UBP512 and UBP710 (and NSC339614; Supplemental Fig. 1), there is a general separation of activities at GluN2A/GluN2B versus GluN2C/GluN2D-containing receptors consistent with their relative degree of sequence homology (Dingledine et al., 1999). Some of the compounds described here can also distinguish between GluN2A and GluN2B (e.g., UBP512 and UBP608) and between GluN2C and GluN2D (e.g., UBP551 and UBP608). Thus, the corresponding pharmacophores for these agents seem to vary among the GluN2 subunits, especially between GluN2A/B and GluN2C/D subunits. The degree of selectivity by these compounds is already greater than that displayed by glutamate and glycine binding site antagonists and channel blockers. Thus, this class of agents should be a valuable approach for further development of subtype-selective agents.
Because NMDA receptors are involved in a wide variety of psychiatric and neurological conditions, there are many potential applications of subtype-selective positive and negative NMDA receptor modulators. Most clinical interest has focused on the use of NMDA receptor blockers as neuroprotective agents. Overactivation of NMDA receptors can lead to neuronal cell death in stroke, in head injury, and, probably, in neurodegenerative diseases. It is noteworthy that several studies have indicated that NMDA receptor subtypes differ in their ability to cause cell death. GluN2B-containing NMDA receptors initiate cell death, whereas GluN2A-containing receptors have been reported to contribute to neuroprotection signaling in traumatic mechanical injury and ischemia models (DeRidder et al., 2006; Chen et al., 2008; Terasaki et al., 2010). This may correspond to an enrichment of GluN2A and GluN2B subunits in synaptic and extrasynaptic compartments, respectively (Tovar and Westbrook, 1999; Lozovaya et al., 2004), and the ability of synaptic NMDA receptors to promote neuroprotection, whereas extrasynaptic NMDA receptor activation signals to neuronal cell death (Hardingham and Bading, 2003; Papadia et al., 2008). (See, however, Thomas et al., 2006; von Engelhardt et al., 2007.) Thus, the neuroprotective properties of GluN2B-selective antagonists have been actively studied.
Multiple lines of evidence also suggest that GluN2D may have a special role in initiating cell death in various conditions. As mentioned above, extrasynaptic NMDA receptors may preferentially contribute to cell death (Hardingham and Bading, 2003). Thus, it is noteworthy that GluN2D is found exclusively in the extrasynaptic compartment at some CNS synapses (Momiyama, 2000; Brickley et al., 2003; Lozovaya et al., 2004). Consistent with an excitotoxic role, we find that GluN2D knockout mice display reduced cerebral cortical damage, but unchanged hippocampal damage in the middle cerebral artery occlusion stroke model (Monaghan et al., 2010). Related to this observation, tissue plasminogen activator-enhanced stroke damage in the cerebral cortex seems to be dependent specifically on GluN2D subunits (Baron et al., 2010). GluN2C and/or GluN2D may also play a specific role in white matter injury (Salter and Fern, 2005) and (specifically GluN2D) a role in Creutzfeldt-Jakob disease (Khosravani et al., 2008) and Alzheimer's disease (Khosravani et al., 2008; Laurén et al., 2009). Hence, compounds with partial GluN2D selectivity, such as UBP512, may have neuroprotective actions in some brain regions without affecting the larger populations of GluN2A- and GluN2B-containing receptors. GluN2-selective inhibitors of NMDA receptor signaling may also be useful for treating pain. GluN2D subunits are essential for the expression of pain in the sciatic nerve ligation neuropathic pain model and in the prostaglandin F2-α-induced pain model, whereas GluN2A is important in the expression of tonic inflammatory pain (Hizue et al., 2005).
The compound class identified here has several additional therapeutic applications because of its ability to potentiate NMDA receptor activity. One intriguing possibility is that the potentiation of synaptic NMDA receptors containing the GluN2A subunit may stimulate neuroprotective-signaling pathways (Chen et al., 2008; Terasaki et al., 2010). In an in vivo context, direct agonist activation would activate inappropriate receptors, whereas a potentiator should specifically increase the response of endogenously activated receptors, thus enhancing an appropriate biological response. Consequently, compounds that selectively potentiate GluN2A subunits, and at the same time inhibit GluN2D-containing receptors (e.g., UBP512), may have combined prosurvival and neuroprotective properties. Such an activity may also have cognitive enhancement properties by promoting synaptic plasticity.
NMDA receptor potentiators might also be useful in treating post-traumatic stress disorder and schizophrenia. The reversal of post-traumatic stress disorder has been reported to be enhanced by increasing NMDA receptor function (Davis et al., 2006). Schizophrenia is thought to be associated with NMDA receptor hypofunction (Lindsley et al., 2006). Thus, the ability to selectively potentiate the most appropriate subpopulations of NMDA receptors may be useful in these conditions.
The structure-activity relationships for these compounds are unusual and thus represent a novel class (or classes) of compounds. The structural features corresponding to the activities described here do not conform to any known group of NMDA receptor antagonists or modulators. They do not contain an amino group α to a carboxylic acid group, as is common for either glutamate or glycine binding site ligands (Jane et al., 2000). These compounds also do not have a T-shaped hydrophobic multiring system with a positive charge center commonly found in NMDA receptor channel blockers. They also do not have an extended structure with an aromatic ring containing a proton donor linked via a basic nitrogen to another aromatic ring, which is a structure typical of ifenprodil-like agents that act at the NTD (Jane et al., 2000).
Consistent with these structure-activity considerations, UBP512 and related compounds do not act as competitive ligands at either the l-glutamate or glycine binding sites. It is noteworthy, however, that high agonist concentrations differentially affect modulator activity at GluN1/GluN2A and GluN1/GluN2D receptors by enhancing modulator potentiation (or reducing inhibition) at GluN1/GluN2A and enhancing modulator inhibition at GluN1/GluN2D receptors. This dichotomy parallels the differential actions of the NTD on NMDA receptors—removing the NTD domains of GluN2A and GluN2D has opposite actions on both channel open probability and glutamate affinity (Gielen et al., 2009; Yuan et al., 2009). The NTD also influences modulator activity, but it is not required for either the inhibitory or potentiating actions (Fig. 3). Thus, the modulator binding site is not at the l-glutamate or glycine binding sites or on the NTD, although these sites interact allosterically with the modulator site.
The potentiating actions of UBP512 and UBP710 become inhibitory at GluN1/GluN2A receptors that have the GluN2A S2 domain replaced by GluN2C's S2 domain (Fig. 3). Thus, these modulators might be binding to this domain, or this domain contributes to transducing the effect of modulator binding to its effect on receptor function. In contrast, the GluN2 subunit's S1 domain, but not the S2 domain, influence the subunit-specific inhibitory actions of UBP608. These findings suggest that the potentiating and inhibiting activities are at different binding sites. This would be consistent with the biphasic effect that some compounds display upon adding or removing the modulator (Fig. 1, A, E, and F).
The dimer interface between the agonist ligand-binding domains may be the site of action for UBP512 and related compounds. In the AMPA receptor family, several positive and negative modulators have been identified. Site-directed mutagenesis and crystallography studies indicate that the inhibitory 2,3-benzodiazepines [e.g., 4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzeneamine dihydrochloride (GYKI-52466)] bind at the dimer interface formed by the ligand-binding domains (Balannik et al., 2005; Ahmed and Oswald, 2010). Also in the ligand-binding dimer interface is a binding site for the allosteric potentiator cyclothiazide (Ahmed and Oswald, 2010). Consistent with this possible location, we find that GluN2 identity of the S2 domain influences UBP512 and UBP710 potentiation, whereas the S1 domain is important for the subunit-selective inhibitory actions of UBP608.
The compounds described here represent several novel lead compounds for a variety of activities at NMDA receptors. We have found that small structural modifications of these compounds lead to significant changes in potency and selectivity. Hence, these compounds should be useful tools for determining the function of discrete NMDA receptor subtypes, and they also serve as a unique starting point for developing highly specific NMDA receptor modulator agents for a variety of neuropsychiatric and neurological conditions.
Supplementary Material
Acknowledgments
We thank Drs. Bodo Laube, Technische Universität Darmstadt, Darmstadt, Germany, David Lynch, University of Pennsylvania Philadelphia, PA, Shigetada Nakanishi, Kyoto University, Kyoto, Japan, Pierre Paoletti, Ecole Normale Supérieure, Paris, France, and Peter Seeburg, Max-Planck-Institute, Heidelberg, Germany for providing NMDA receptor cDNA constructs; the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute for providing chemicals; and Dr. John Wood, University of Bristol, Bristol, United Kingdom for assisting in the synthesis of an intermediate for the preparation of UBP710.
This work was supported by the National Institutes of Health National Institutes of Mental Health [Grant MH60252] and the UK Medical Research Council [Grant G0601812].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.174144.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- CNS
- central nervous system
- NMDA
- N-methyl-d-aspartate
- NR2
- NMDA receptor subunit 2
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NTD
- N-terminal regulatory domain
- NSC339614
- 6-, 7-, 8-, and 9-nitro isomers of naphth[1,2-c][1,2,5]oxadiazole-5-sulfonic acid
- UBP512
- 9-iodophenanthrene-3-carboxylic acid
- UBP551
- 3,5-dihydroxynaphthalene-2-carboxylic acid
- UBP608
- 6-bromo-2-oxo-2H-chromene-3-carboxylic acid
- UBP618
- 1-bromo-2-hydroxy-6-phenylnaphthalene-3-carboxylic acid
- UBP646
- 9-(4-methylpent-1-yl)phenanthrene-3-carboxylic acid
- UBP710
- 9-cyclopropylphenanthrene-3-carboxylic acid
- S
- segment
- M
- intramembrane domain
- GYKI-52466
- 4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)benzeneamine dihydrochloride.
References
- Ahmed AH, Oswald RE. (2010) Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem 53:2197–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balannik V, Menniti FS, Paternain AV, Lerma J, Stern-Bach Y. (2005) Molecular mechanism of AMPA receptor noncompetitive antagonism. Neuron 48:279–288 [DOI] [PubMed] [Google Scholar]
- Baron A, Montagne A, Cassé F, Launay S, Maubert E, Ali C, Vivien D. (2010) NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ 17:860–871 [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. (2003) NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23:4958–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. (1994) The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci 14:5471–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. (2008) Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 39:3042–3048 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335 [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. (2006) Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 60:369–375 [DOI] [PubMed] [Google Scholar]
- DeRidder MN, Simon MJ, Siman R, Auberson YP, Raghupathi R, Meaney DF. (2006) Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis 22:165–176 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61 [PubMed] [Google Scholar]
- Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. (2009) Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26:81–89 [DOI] [PubMed] [Google Scholar]
- Hizue M, Pang CH, Yokoyama M. (2005) Involvement of N-methyl-d-aspartate-type glutamate receptor epsilon1 and epsilon4 subunits in tonic inflammatory pain and neuropathic pain. Neuroreport 16:1667–1670 [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. (2000) Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci (Online) 20:RC81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane DE, Tse HW, Skifter DA, Christie JM, Monaghan DT. (2000) Glutamate receptor ion channels: activators and inhibitors. Handb Exp Pharmacol 147:415–478 [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. (2008) NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol 7:742–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, Chen L, Villemaire M, Ali Z, Jirik FR, et al. (2008) Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Gen Physiol 131:i5. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Riederer P. (2007) Excitotoxicity and new antiglutamatergic strategies in Parkinson's disease and Alzheimer's disease. Parkinsonism Relat Disord 13 (Suppl 3):S329–S331 [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. (1998) Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci 18:2954–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457:1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, Kinney GG. (2006) Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem 6:771–785 [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze TSh, Feng B, Monaghan DT, Krishtal OA. (2004) Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol 558:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Betz H, Geiger JR, Laube B. (2008) Supralinear potentiation of NR1/NR3A excitatory glycine receptors by Zn2+ and NR1 antagonist. Proc Natl Acad Sci USA 105:12563–12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A. (2000) Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol 523:621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. (1989) The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol 29:365–402 [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Zhao H, Gautam V, Sun H, Mayhan W. (2010) Deletion of the NMDA receptor subunit GluN2D leads to reduced cell death in the cerebral cortex in the mouse middle cerebral artery occlusion model. Soc Neurosci Abstr 36:873.2 [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529–540 [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256:1217–1221 [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59:467–477 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. (2000) Molecular organization of a zinc binding N-terminal modulatory domain in a NMDA receptor subunit. Neuron 28:911–925 [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, et al. (2008) Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. (2005) The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci 25:308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. (2005) NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438:1167–1171 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. (2008) Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7:426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki Y, Sasaki T, Yagita Y, Okazaki S, Sugiyama Y, Oyama N, Omura-Matsuoka E, Sakoda S, Kitagawa K. (2010) Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J Cereb Blood Flow Metab 30:1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. (2006) Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95:1727–1734 [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19:4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmann C, Becker CM. (2007) On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist 13:594–615 [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Köhr G, Seeburg PH, Monyer H. (2007) Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology 53:10–17 [DOI] [PubMed] [Google Scholar]
- Watkins JC, Davies J, Evans RH, Francis AA, Jones AW. (1981) Pharmacology of receptors for excitatory amino acids. Adv Biochem Psychopharmacol 27:263–273 [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. (2009) Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci 29:12045–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.