Abstract
Alcohols and inhaled anesthetics enhance the function of GABAA receptors containing α, β, and γ subunits. Molecular analysis has focused on the role of the α subunits; however, there is evidence that the β subunits may also be important. The goal of our study was to determine whether Asn265, which is homologous to the site implicated in the α subunit (Ser270), contributes to an alcohol and volatile anesthetic binding site in the GABAA receptor β2 subunit. We substituted cysteine for Asn265 and exposed the mutant to the sulfhydryl-specific reagent octyl methanethiosulfonate (OMTS). We used two-electrode voltage-clamp electrophysiology in Xenopus laevis oocytes and found that, after OMTS application, GABA-induced currents were irreversibly potentiated in mutant α1β2(N265C)γ2S receptors [but not α1β2(I264C)γ2S], presumably because of the covalent linking of octanethiol to the thiol group in the substituted cysteine. It is noteworthy that this effect was blocked when OMTS was applied in the presence of octanol. We found that potentiation by butanol, octanol, or isoflurane in the N265C mutant was nearly abolished after the application of OMTS, suggesting that an alcohol and volatile anesthetic binding site at position 265 of the β2 subunit was irreversibly occupied by octanethiol and consequently prevented butanol or isoflurane from binding and producing their effects. OMTS did not affect modulation or direct activation by pentobarbital, but there was a partial reduction of allosteric modulation by flunitrazepam and alphaxalone in mutant α1β2(N265C)γ2S receptors after OMTS was applied. Our findings provide evidence that Asn265 may contribute to an alcohol and anesthetic binding site.
Introduction
Recent investigations of the specific sites of action for alcohols and anesthetics have focused on the role of target proteins in the central nervous system, including the GABAA receptor. Functional GABAA receptors consist of five homologous subunits positioned around a central chloride channel (Hevers and Lüddens, 1998), with the GABA binding site situated at the α-β interface (Newell and Czajkowski, 2003). In the brain, the predominant subunit stoichiometry of GABAA receptors is two α, two β, and one γ (Tretter et al., 1997; Sieghart and Sperk, 2002); however, there is a subpopulation of extrasynaptic receptors in which certain α and β subunits coassemble with the δ subunit (Olsen and Sieghart, 2008). The structure of each subunit consists of an extracellular N-terminal domain, a transmembrane domain with four α-helical segments (TM1, TM2, TM3, and TM4), an intracellular loop between TM3 and TM4, and an extracellular C-terminal domain (Ortells and Lunt, 1995). TM2 amino acid residues from each subunit presumably contribute to the ion pore of the chloride channel (Xu and Akabas, 1996; Miyazawa et al., 2003).
Alcohols and volatile anesthetics positively modulate GABAA receptor function (Lovinger, 1997; Harris, 1999; Franks, 2008), and several studies have identified alcohol-binding sites in this receptor (Mihic et al., 1997; Mascia et al., 2000; Jung et al., 2005; Perkins et al., 2009). Specifically, studies using point mutations and heterologous expression systems provide evidence that homologous TM2 positions in the GABAA receptor α1, α2, and β1 subunits and the glycine receptor α1 subunit are important for the actions of alcohols and anesthetics. For example, Mihic et al. (1997) found that mutant α1(S270I) β1, α2(S270I) β1, and α1β1(S265I) GABAA receptors and α1(S267I) glycine receptors were resistant to enhancement by ethanol or enflurane. Likewise, Ueno et al. (1999) reported that the potentiating effects of ethanol were abolished in mutant α2(S270I)β1γ2L and α2β1(S265I)γ2L receptors, and Jenkins et al. (2001) found that Ser270 of the GABAA receptor α subunits contributes to a common site of action for the inhalation anesthetics chloroform, halothane, and isoflurane. It has further been proposed that these residues, along with residues from the other TM domains, contribute to a water-filled, intrasubunit binding pocket (Yamakura et al., 2001; Trudell and Bertaccini, 2004; McCracken et al., 2010). In addition, residues in the γ and δ subunits may also contribute to alcohol binding sites (Olsen and Sieghart, 2009; Perkins et al., 2009); however, the current role of these subunits is not well defined.
Although recent studies have alternatively proposed the presence of a general anesthetic binding pocket at the GABAA receptor α-β interface (Li et al., 2010), it has been shown that the 265 position of the GABAA receptor β subunit is necessary for the actions of the injectable anesthetic etomidate (Belelli et al., 1997), and two knock-in mice, β2(N265S) and β3(N265M), have been constructed to investigate the behavioral effects of injectable anesthetics in vivo (Jurd et al., 2003; Reynolds et al., 2003; Sanchis-Segura et al., 2007). In contrast to these studies of injectable anesthetics, the role of the GABAA receptor β2 subunit in alcohol and inhaled anesthetic action has not been as extensively studied.
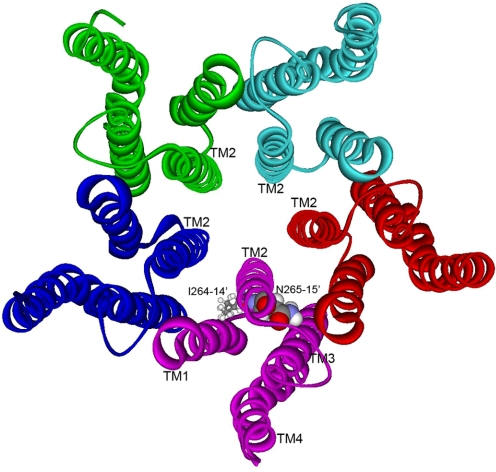
Therefore, the aim of the present study was to investigate whether Asn265 in the GABAA receptor β2 subunit, which is homologous to the implicated sites in TM2 of the α1, α2, and β1 subunits, is important for alcohol and anesthetic action. Figure 1 shows the relative positions of β2(Ile264, 14′) and β2(Asn265, 15′) residues in TM2 of α1β2γ2S GABAA receptors on the basis of the X-ray structure of the prokaryotic proton-gated ion channel GLIC (Bocquet et al., 2009). Specifically, we sought to determine whether the individual mutation of this amino acid residue to cysteine affected receptor sensitivity to alcohols and whether octyl methanethiosulfonate (OMTS), an alcohol/anesthetic analog, covalently reacted with the substituted cysteine and irreversibly altered GABA-induced currents. Propyl methanethiosulfonate covalently reacts with the substituted cysteine in mutant α2(S270C)β1 receptors and irreversibly potentiates GABA-induced currents, mimicking the effects of alcohol and anesthetics (Mascia et al., 2000). We hypothesized that if the substituted cysteine in the homologous position of the β2 subunit contributed to an alcohol and anesthetic binding site, then it would covalently react with OMTS, resulting in the irreversible attachment of octyl thiol to the cysteine thiol group. With the octyl thiol covalently bound at position 265, it was expected that GABA-induced current would be irreversibly potentiated, and subsequently applied alcohols or anesthetics would be unable to bind and modulate receptor function.
Fig. 1.
The five subunits that make up the TM domain of an α1β2γ2S GABAA receptor are rendered as ribbon structures that trace the α-helical backbone. For clarity, the ligand-binding and intracellular domains are not shown. The view is from the extracellular surface, directly down the chloride ion pore. In one subunit, residues Ile264 and Asn265 are rendered with space-filling surfaces. The model was built by replacing the corresponding residues in the prokaryotic proton-gated ion channel GLIC, as described under Materials and Methods.
Materials and Methods
Materials.
GABA was obtained from Sigma/RBI (Natick, MA), and ethanol, 1-butanol, 1-octanol, alphaxalone, chloroform, flunitrazepam, and sodium pentobarbital were purchased from Sigma-Aldrich (St. Louis, MO). OMTS was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada), isoflurane was purchased from Marsam Pharmaceuticals Inc. (Cherry Hill, NJ), and etomidate was purchased from Tocris Bioscience (Ellisville, MO).
Mutagenesis and Transcription.
Mutations were achieved using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Complementary primers containing the desired mutations were designed, and polymerase chain reactions were performed. Subsequently, DpnI was used to digest the parental DNA, and TOP10/P3 competent cells were transformed with the polymerase chain reaction products. The plasmids of interest were obtained by using a QIAGEN (Valencia, CA) miniprep kit and were sent for sequencing to verify the presence of the desired mutations. cRNAs were synthesized using the wild-type and mutant cDNA as a template. Specifically, pCDM8 plasmid encoding human GABAA receptor β2 subunit and pGEMHE plasmids encoding rat α1 or γ2S GABAA receptor subunits were employed to synthesize the cRNAs using T7 RNA polymerase (mMESSAGE mMACHINE T7 Kit; Ambion, Austin, TX).
Oocyte Isolation and Injection.
Adult female Xenopus laevis frogs were obtained from Xenopus Express (Rocksville, FL), and portions of ovary were surgically extracted. Mature oocytes were manually isolated, treated in collagenase (type IA, 0.5 mg/ml), and subsequently injected into the cytoplasm with 30 nl of diethyl pyrocarbonated-treated water and cRNAs (3 ng/subunit/30 nl) encoding wild-type α1, either wild-type or mutant β2, and wild-type γ2S subunit combinations in a 1:1:1 ratio. Injected oocytes were incubated at 13°C in sterile modified Barth's solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 19 mM HEPES, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2, 10,000 units/l penicillin, 50 mg/l gentamicin, 90 mg/l theophylline, and 220 mg/l sodium pyruvate, pH 7.5] for 3 to 4 days.
Electrophysiological Recording.
Two-electrode voltage-clamp electrophysiological methods were used to measure GABA-induced currents from oocytes 3 to 4 days after injection with cRNAs. Each oocyte was placed in a rectangular chamber (∼100 μl) and continuously perfused at a rate of 2 ml/min with ND96 buffer (96 mM NaCl, 1 mM CaCl2, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.5) at room temperature (23°C). Two glass electrodes containing 3 M KCl were used to achieve a −70-mV holding potential using a Warner Instruments oocyte clamp (Hamden, CT). All solutions were prepared immediately before use and applied by bath perfusion.
Concentration Response Curves.
Increasing concentrations of GABA were applied to oocytes from each condition for 20 to 30 s, followed by a 5- to 15-min washout. From the resulting concentration response curves, the concentrations eliciting the half-maximal response (EC50) were calculated for the wild type and the mutants tested.
Effect of Alcohols and Anesthetics on GABA-Induced Current.
The GABA EC5 GABA (the concentration that produced 5% of the maximal response) was determined for each oocyte after application of the maximal GABA concentration and served as the test GABA concentration. All test GABA applications were 30 s and were followed by a 5-min washout. The test GABA concentration was applied twice, and the modulator was then preapplied for 1 min, coapplied with GABA for 30 s, and followed by a 5-min washout. The test GABA concentration was applied again, and the percentage potentiation of the GABA-induced current by the modulator was calculated for each oocyte. This procedure was used to test 200 mM ethanol; 11, 22, and 32 mM butanol; and 57, 114, and 171 μM octanol. The potentiation of EC5 GABA-induced current by 1 μM etomidate and 2 mM chloroform was also measured using this protocol.
Effect of OMTS on GABA-Induced Current.
Two consecutive applications of the test GABA concentration were administered, with the second application representing the initial response. OMTS (50 μM) was applied alone for 1 min and was followed by a 5-min washout. Subsequently, three applications of the test GABA concentration were applied, and currents were measured as percentages of the initial response. To test the effect of OMTS when the receptor was in the open/desensitized state, the same protocol was followed, except that 50 μM OMTS was coapplied with maximal GABA, and the washout time immediately afterward was extended to 15 min. In addition, we tested the ability of 114 μM octanol or 0.4 μM flunitrazepam to block the effects of OMTS on the N265C mutant using the protocol described above, except that octanol or flunitrazepam was preapplied for 1 min alone and then coapplied with 50 μM OMTS.
Effect of Allosteric Modulators Pre- and Post-OMTS Application.
Oocytes received two applications of the test GABA concentration, followed by a 30-s coapplication of the test GABA concentration and the modulator. After a 5-min washout, the test GABA concentration was applied alone, and the potentiation of GABA-induced current by the modulator was calculated. After a 1-min application of 50 μM OMTS and a 5-min washout, the maximal GABA concentration was reapplied, and the test concentration was recalculated. The potentiation by the modulator was then measured again as described above. This protocol was used to test 114 μM octanol, 0.6 mM isoflurane, 0.4 μM flunitrazepam, 3 μM alphaxalone, and 50 μM pentobarbital. However, 11 mM butanol and 0.6 mM isoflurane were applied alone for 1 min before being coapplied with the test GABA concentration.
Direct Activation by Etomidate and Pentobarbital.
The maximal GABA concentration was applied for 20 s and was followed by a 15-min washout. Etomidate (10 μM) was then applied for 1 min, and the resulting current was measured as a percentage of the maximal response. The same procedure was used to measure the direct activation after the application of 50 μM OMTS. Likewise, direct activation by 300 μM pentobarbital was also measured.
Modeling.
The relative positions of β2(Ile264, 14′) and β2(Asn265, 15′) residues in α1β2γ2S GABAA receptors were modeled using the prokaryotic proton-gated ion channel GLIC (Protein Data Bank ID 3EAM), the best available template for the ion pore of the Cys-loop superfamily of receptors (Bocquet et al., 2009). Given that the structure of GLIC now provides the best information about the likely orientation and position of residues in TM2 (Nury et al., 2010), the model of GABAA β2 residues was made by simply substituting the two corresponding residues in GLIC with Ile264 and Asn265. The positions of the side chains were then optimized automatically with the side chain refinement module of Discovery Studio 2.5.5 using the CHARMm force field (Accelrys Inc., San Diego, CA).
Results
The goal of our study was to determine whether Asn265 (15′) contributes to an alcohol and anesthetic binding site in the GABAA receptor β2 subunit. We substituted cysteine for the candidate amino acid and exposed the cysteine mutant to sulfhydryl-specific reagents. In addition, we constructed a second mutant in which cysteine was substituted at the neighboring residue, Ile264. Two tryptophan mutants, I264W and T266W, were previously constructed by Nishikawa et al. (2002), and the effects of volatile anesthetics were tested. Although submaximal GABA currents were significantly potentiated by both halothane and isoflurane in the mutant tryptophan receptors, the effects were small in the I264W compared with the wild type. Therefore, we included the I264C mutant (14′) in our study to rule out the possibility that Ile264 also participates in alcohol and volatile anesthetic binding.
The relative positions of β2(Ile264, 14′) and β2(Asn265, 15′) residues in α1β2γ2S GABAA receptors are shown in a model that is based on the prokaryotic proton-gated ion channel GLIC (Bocquet et al., 2009) (Fig. 1). Although less is known about the structure of GABAaR subunits, compared with nAChR, there is a consensus that residues in TM2 at positions 13′ and 17′ face into the ion pore. Because the C-α atoms of an α-helix rotate approximately 100° clockwise with respect to the long axis of the helix, it is reasonable that Ile264 (14′) should face toward TM1 of the same subunit and Asn265 (15′) should face into the center of the subunit, but slightly toward TM3. We should note that Fig. 1 depicts the α-helices as a ribbon that traces the helical backbone carbons. This representation provides a clear depiction of Ile264 and Asn265 but is somewhat incomplete because, when all the side chains are rendered as space-filling surfaces, the entire TM domain is essentially filled with atoms, except for spaces in the center of each four-helical bundle and spaces at the interfaces between subunits.
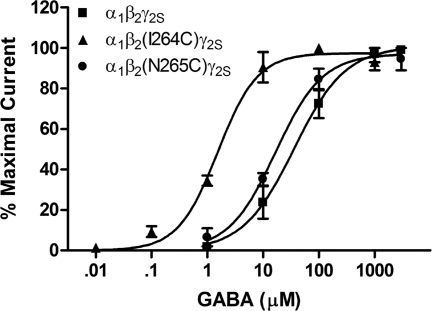
The GABA concentration-response curves show that the β2(I264C) and β2(N265C) mutations both produced an increase in apparent affinity for GABA (Fig. 2). This increase was larger for the I264C mutation than N265C; the GABA EC50 values were 1.5 μM for α1β2(I264C)γ2S and 17 μM for α1β2(N265C)γ2S versus 36 μM for the wild type. In each of the cysteine mutants tested, there was also a slight decrease in the maximal current elicited by saturating concentrations of GABA [5337 nA for α1β2(I264C)γ2S and 6114 nA for α1β2(N265C)γ2S versus 7361 for the wild type].
Fig. 2.
GABA concentration response curves for wild-type α1β2γ2S and mutant GABAA receptors expressed in X. laevis oocytes. All values are presented as mean ± S.E.M. from four to five oocytes.
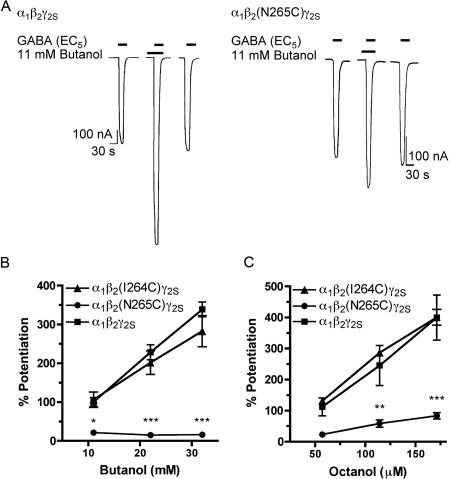
We next examined whether the β2(I264C) or β2(N265C) point mutations had an effect on alcohol modulation of GABA-induced currents. The β2(N265C) mutation eliminated the enhancement of GABA-induced current by 200 mM ethanol; instead, inhibition was produced (−14 ± 3% versus 51 ± 5% in wild type) (data not shown). In addition, the potentiation of GABA-induced current by butanol was significantly reduced in mutant α1β2(N265C)γ2S receptors compared with the wild type at all concentrations tested (Fig. 3, A and B). Likewise, potentiation by octanol was markedly reduced in mutant α1β2(N265C)γ2S receptors (Fig. 3C). In contrast to the β2(N265C) mutation, the β2(I264C) mutation did not significantly alter receptor modulation by any of the alcohols tested (Fig. 3, B and C).
Fig. 3.
The β2(N265C) point mutation reduced alcohol modulation of GABAA receptor function. A, representative tracings showing the potentiation of EC5 GABA-induced current by 11 mM butanol in wild-type α1β2γ2S and mutant α1β2(N265C)γ2S GABAA receptors expressed in X. laevis oocytes. B, wild-type and mutant α1β2(I264C)γ2S receptors were potentiated by butanol (11, 22, and 32 mM) in a concentration-dependent manner, unlike mutant α1β2(N265C)γ2S receptors, which were significantly less sensitive to the potentiating effects of butanol, compared with the wild type at all concentrations tested. C, the enhancement of GABAA receptor function by octanol was also less in the α1β2(N265C)γ2S mutant than the wild type, and this effect was statistically significant at 114 and 171 μM octanol concentrations. Octanol modulation of mutant α1β2(I264C)γ2S receptors did not differ significantly from the wild type. All values are presented as mean ± S.E.M. from four to five oocytes. *, p < 0.05; **, p < 0.01; ***, p < 0.001 denotes values significantly different from the wild type, as tested by two-way analysis of variance with Bonferroni's post-test.
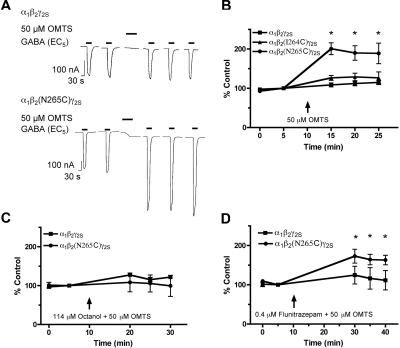
Next, we tested the β2(I264C) and β2(N265C) mutants to determine whether the reaction of MTS reagents with the substituted cysteines consequently altered receptor function. We found that after a 1-min application of 50 μM OMTS, the GABA-induced currents of mutant α1β2(N265C)γ2S receptors were irreversibly enhanced (Fig. 4, A and B), suggesting that OMTS reacted with the substituted cysteine and resulted in the covalent attachment of octanethiol to the cysteine thiol group. The GABA responses of the β2(I264C) mutant, like those of the wild type, were not altered after OMTS application (Fig. 4B). Moreover, OMTS had no effect on the β2(N265C) mutant when it was applied in the presence of 114 μM octanol (Fig. 4C), suggesting a competitive interaction between octanol and OMTS at the 265 site. However, 0.4 μM flunitrazepam, which presumably acts at a distinct site from 265, did not block the reaction of OMTS with the substituted cysteine (Fig. 4D).
Fig. 4.
Effect of OMTS on the EC5 GABA responses of wild-type and mutant GABAA receptors expressed in X. laevis oocytes. A, representative tracings showing EC5 GABA-induced current from wild-type α1β2γ2S and mutant α1β2(N265C)γ2S GABAA receptors, before and after 1-min application of 50 μM OMTS. B, OMTS irreversibly enhanced the GABA responses of mutant α1β2(N265C)γ2S GABAA receptors. The GABA responses of wild-type and mutant α1β2(I264C)γ2S GABAA receptors were not affected by OMTS. C, the effect of 50 μM OMTS on the α1β2(N265C)γ2S mutant was blocked in the presence of 114 μM octanol. D, the GABA responses of α1β2(N265C)γ2S receptors were irreversibly enhanced in the presence of 0.4 μM flunitrazepam. All values are presented as mean ± S.E.M. from three to six oocytes. *, p < 0.05 denotes values significantly different from the pre-OMTS condition, as tested by one-way analysis of variance with Bonferroni's post-test.
To address the possibility that Ile264 is accessible only when the receptor is in the activated or desensitized state, we measured EC5 GABA responses before and after applying 50 μM OMTS in the presence of maximal GABA. These results were similar to our findings when OMTS was applied while the receptor was in the resting state, and again we observed an irreversible enhancement of the GABA responses of the β2(N265C) mutant but no change in the GABA responses of the β2(I264C) mutant or the wild type (data not shown). Propyl methanethiosulfonate, a smaller sulfhydryl-specific reagent, was also tested on the I264C and N265C mutants in the resting and activated/desensitized states. The potentiating effects on the N265C mutant were similar to those observed with OMTS, although not as robust, and there was no effect on the I264C mutant or the wild type (data not shown). Because the β2(I264C) mutation did not affect receptor modulation by alcohols, and MTS reagents had no apparent effect on the function of these mutant receptors, we focused on the α1β2(N265C)γ2S mutant in the remainder of our experiments.
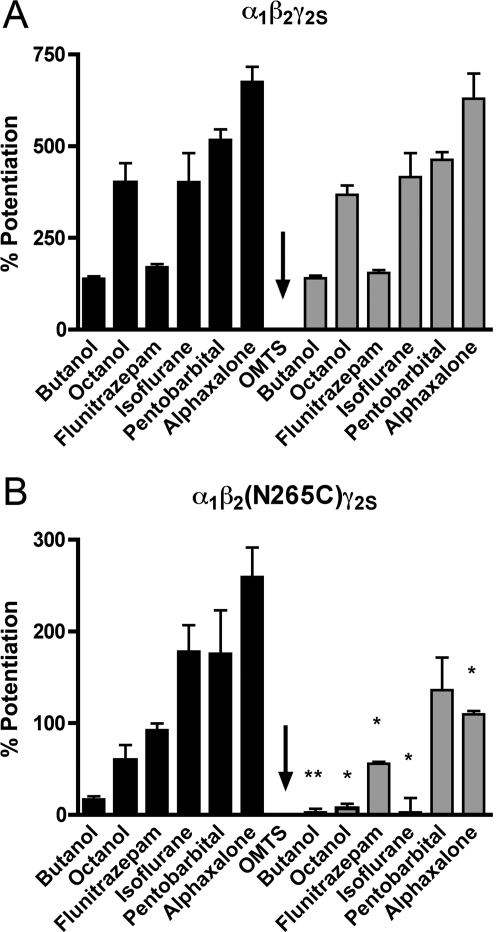
Subsequently, we examined whether the N265C mutation or the application of OMTS altered allosteric modulation. It is noteworthy that all of the compounds tested (11 mM butanol, 0.4 μM flunitrazepam, 0.6 mM isoflurane, 50 μM pentobarbital, and 3 μM alphaxalone) produced less potentiation of GABA-induced current in α1β2(N265C)γ2S receptors compared with the wild type before the application of OMTS. Nevertheless, the potentiation was still measurable in all cases. In wild-type receptors, OMTS did not alter the potentiation of GABA responses by any of the modulators tested (Fig. 5A). In mutant α1β2(N265C)γ2S receptors, the application of OMTS abolished the effects of octanol and isoflurane, and the potentiation by flunitrazepam and alphaxalone was reduced (Fig. 5B). However, OMTS did not significantly affect mutant receptor modulation by pentobarbital (Fig. 5B).
Fig. 5.
Allosteric modulation of wild-type α1β2γ2S and mutant α1β2(N265C)γ2S GABAA receptors expressed in X. laevis oocytes before and after the application of 50 μM OMTS. A, in wild-type α1β2γ2S receptors, there was no change in the potentiation of EC5 GABA responses by 114 μM octanol, 0.4 μM flunitrazepam, 0.6 mM isoflurane, 50 μM pentobarbital, or 3 μM alphaxalone after a l-min application of OMTS. B, in mutant α1β2(N265C)γ2S receptors, OMTS abolished the effects of 11 mM butanol, 114 μM octanol, and 0.6 mM isoflurane. The potentiating effects of 0.4 μM flunitrazepam and 3 μM alphaxalone were also reduced after OMTS was applied. However, OMTS did not affect the enhancement of EC5 GABA responses by 50 μM pentobarbital. All values are presented as mean ± S.E.M. from four to seven oocytes. *, p < 0.05; **, p < 0.01 denotes values significantly different from the pre-OMTS condition, as tested by Student's t test.
The effects of the cysteine mutation on direct channel activation by 10 μM etomidate and 300 μM pentobarbital were also measured (Table 1). Activation by etomidate was significantly less in mutant α1β2(N265C)γ2S receptors compared with the wild type. However, activation by pentobarbital did not differ significantly from the wild type, and this effect was not altered after the application of OMTS.
TABLE 1.
Direct activation of wild-type α1β2γ2S and mutant α1β2(N265C)γ2S GABAA receptors expressed in X. laevis oocytes
All values represent percentages of the maximal GABA response and are given as the mean ± S.E.M. from four to five oocytes.
| α1β2γ2S | α1β2γ2S Post-OMTS | α1β2(N265C)γ2S | α1β2(N265C)γ2S Post-OMTS | |
|---|---|---|---|---|
| 10 μM Etomidate | 10 ± 2 | 9 ± 1 | 0.1 ± 0.1* | 0.2 ± 0.1 |
| 300 μM Pentobarbital | 18 ± 1 | 16 ± 2 | 19 ± 4 | 17 ± 3 |
Significant effect of the β2(N265C) mutation, P < 0.001 comparing α1β2(N265C)γ2S with the wild type before OMTS application.
Discussion
Our results from cysteine mutagenesis and the sulfhydryl-specific reagent OMTS support the role of Asn265 in the binding of alcohols and volatile anesthetics. Although the residue at position 265 (15′) of the β2 subunit is presumably facing away from the channel, the negatively charged sulfhydryl-specific reagent p-chloromercuribenzenesulfonate has previously been shown to covalently react when cysteine is substituted at this site, indicating that the residue at position 265 of the β2 subunit is water-accessible (Bali and Akabas, 2004). Accordingly, we observed an irreversible enhancement of submaximal GABA-induced current in mutant α1β2(N265C)γ2S receptors after OMTS application in both the open and closed states. This suggests that the substituted cysteine was water accessible and covalently reacted with OMTS, resulting in the irreversible attachment of octanethiol to the cysteine thiol group. Our finding is also consistent with previous work showing that the position homologous to GABAA β2(Asn265) in the α1 subunit of the glycine receptor (Ser267) is accessible to OMTS (Lobo et al., 2004). Additional studies on glycine receptors and GABAA receptors have also shown that MTS reagents, such as propyl MTS or hexyl MTS, produce similar effects on receptor function when cysteine is substituted at important sites for alcohol and volatile anesthetic action (Mascia et al., 2000; Jung et al., 2005; Crawford et al., 2007). Notably in our study, the ability of octanol, but not flunitrazepam, to block the effects of OMTS in the β2(N265C) mutant is consistent with a competitive interaction and provides compelling evidence that Asn265 participates in an alcohol and volatile anesthetic binding pocket. Knock-in mice containing a β2(N265S) mutation have been constructed and are resistant to the sedative effects of etomidate, but the effects of alcohols or volatile anesthetics have not been tested in these animals (Reynolds et al., 2003). In addition, β3(N265M) mice have been constructed; however, this subunit does not seem to have a major role in the effects of ethanol or isoflurane (Liao et al., 2005; Sanchis-Segura et al., 2007).
The effects of the I264C and N265C mutations on alcohol and anesthetic modulation further suggest that Asn265 is a critical site for the effects of these drugs. Previous studies have shown that the ability of alcohols to modulate receptor function is dependent on specific amino acids located in TM2 of the glycine receptor and GABAA receptor subunits (Mihic et al., 1997; Wick et al., 1998), and amino acid volume has been hypothesized to be an important factor governing alcohol and anesthetic enhancement of GABAA and glycine receptors (Ye et al., 1998; Jenkins et al., 2001; Nishikawa et al., 2002). Although cysteine and asparagine differ in volume by only approximately 5 Å3, we found that the single point mutation in α1β2(N265C)γ2S receptors dramatically reduced alcohol and anesthetic potentiation of GABA-induced currents despite causing only a modest change in receptor sensitivity to GABA. The N265C mutation, however, did produce a change in polarity such that a nonpolar amino acid was substituted for an uncharged polar amino acid. In contrast, isoleucine and cysteine are both nonpolar amino acids but differ in volume by approximately 35 Å3, and the substitution of cysteine for isoleucine at position 264 in our study had a greater effect on receptor sensitivity to GABA than the N265C mutation. However, despite the previous finding that volatile anesthetic potentiation is reduced in mutant α1β2(I264W)γ2S receptors expressed in human embryonic kidney 293 cells (Nishikawa et al., 2002), the I264C mutation in our study did not significantly affect alcohol or volatile anesthetic modulation of mutant α1β2(I264C)γ2S receptors. Because alcohol or anesthetic potentiation of GABA-induced current was not affected by the I264C mutation, and there were not apparent functional effects of OMTS on mutant α1β2(I264C)γ2S receptors, we propose that Ile264 is not critical for alcohol and anesthetic action on the GABAA receptor. As depicted in Fig. 1, it is possible that Ile264 is buried between adjacent TM2 α-helices and TM1 of its own subunit. As a result, it may not be available for interaction with alcohols or reaction with MTS reagents. Therefore, we focused exclusively on Asn265 in the remainder of our experiments.
Additional evidence that Asn265 is a critical site of action for alcohols and volatile anesthetics is provided by our experiments examining the effects of OMTS on allosteric modulation. It is noteworthy that we found that butanol, octanol, and isoflurane potentiation were reduced by the cysteine mutation alone and were nearly abolished after the application of OMTS. We propose that an alcohol and volatile anesthetic binding site at position 265 of the β2 subunit was irreversibly occupied by the covalent attachment of octanethiol to N265C and consequently prevented butanol, octanol, or isoflurane from binding and further enhancing receptor function. Our finding is in accordance with previous studies on other GABAA receptor subunits, glycine receptors, and nicotinic acetylcholine receptors, suggesting that the addition of an alkylthiol group to a substituted cysteine at an alcohol- and volatile anesthetic-binding site causes the site to no longer be accessible to alcohols and anesthetics (Mascia et al., 2000; Borghese et al., 2003; Crawford et al., 2007). Direct activation of the N265C mutant by pentobarbital did not differ from the wild type and was not altered after OMTS was applied. These findings are supported by previous work proposing that the pre-TM1 region of the β2 subunit contributes to a pentobarbital-mediated activation pathway (Mercado and Czajkowski, 2008).
It is noteworthy that allosteric modulation of GABAA receptor function was less in mutant α1β2(N265C)γ2S receptors compared with the wild type for all of the modulators tested before the application of OMTS. In addition, the modulation produced by both flunitrazepam and alphaxalone was reduced in the N265C mutant after the application of OMTS. Because there is considerable evidence that modulators such as benzodiazepines and neurosteroids bind to sites distinct from Asn265 on the GABAA receptor (Teissére and Czajkowski, 2001; Hosie et al., 2006), we have considered the alternative hypothesis that our results are entirely a consequence of altered channel gating as a result of the allosteric effects of a cysteine mutation at position 265. Although this possibility cannot be completely eliminated on the basis of the current study, our finding that octanol, but not flunitrazepam, blocks the effects of OMTS in the β2(N265C) mutant provides strong evidence for a competitive interaction at position 265. Therefore, we favor the hypothesis that Asn265 is a critical site for alcohol and volatile anesthetic binding. Based on previous work implicating the α subunit as a site of alcohol and volatile anesthetic action and by our finding that Asn265 of the β2 subunit also seems to contribute to an alcohol/anesthetic binding site, we speculate that water-filled cavities need to be present in both the α and β subunits for alcohols or volatile anesthetics to bind and produce their effects. In summary, we conclude that Asn265 of the GABAA receptor β2 subunit contributes to an intrasubunit alcohol and volatile anesthetic binding site.
Acknowledgments
We thank Kathy L. Carter, Marni Martinez, and Chelsea R. Geil for technical assistance.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA06399, AA13378] and the National Institutes of Health National Institute of General Medical Sciences [Grant GM47818].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.170472.
- TM
- transmembrane
- OMTS
- octyl methanethiosulfonate
- GLIC
- Gloeobacter violaceus ion channel.
References
- Bali M, Akabas MH. (2004) Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol 65:68–76 [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. (1997) The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA 94:11031–11036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114 [DOI] [PubMed] [Google Scholar]
- Borghese EM, Henderson LA, Bleck Y, Trudell JR, Harris RA. (2003) Sites of excitatory and inhibitory actions of alcohols on neuronal alpha2beta4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther 307:42–52 [DOI] [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. (2007) Evidence that ethanol acts on a target in Loop 2 of the extracellular domain of alpha1 glycine receptors. J Neurochem 102:2097–2109 [DOI] [PubMed] [Google Scholar]
- Franks NP. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386 [DOI] [PubMed] [Google Scholar]
- Harris RA. (1999) Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res 23:1563–1570 [PubMed] [Google Scholar]
- Hevers W, Lüddens H. (1998) The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol 18:35–86 [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489 [DOI] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. (2001) Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci 21:RC136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Akabas MH, Harris RA. (2005) Functional and structural analysis of the GABAA receptor alpha 1 subunit during channel gating and alcohol modulation. J Biol Chem 280:308–316 [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, et al. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor beta3 subunit. FASEB J 17:250–262 [DOI] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Cohen JB, Olsen RW. (2010) Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem 285:8615–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Sonner JM, Jurd R, Rudolph U, Borghese CM, Harris RA, Laster MJ, Eger EI., 2nd (2005) Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg 101:412–418 [DOI] [PubMed] [Google Scholar]
- Lobo IA, Mascia MP, Trudell JR, Harris RA. (2004) Channel gating of the glycine receptor changes accessibility to residues implicated in receptor potentiation by alcohols and anesthetics. J Biol Chem 279:33919–33927 [DOI] [PubMed] [Google Scholar]
- Lovinger DM. (1997) Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn Schmiedebergs Arch Pharmacol 356:267–282 [DOI] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, McCracken ML, Gong DH, Trudell JR, Harris RA. (2010) Linking of glycine receptor transmembrane segments three and four allows assignment of intrasubunit-facing residues. ACS Chem Neurosci 1:482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado J, Czajkowski C. (2008) Gamma-aminobutyric acid(GABA) and pentobarbital induce different conformational rearrangements in the GABAA receptor alpha1 and beta2 pre-M1 regions. J Biol Chem 283:15250–15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955 [DOI] [PubMed] [Google Scholar]
- Newell JG, Czajkowski C. (2003) The GABAA receptor alpha 11 subunit Pro174-Asp191 segment is involved in GABA binding and channel gating. J Biol Chem 278:13166–13172 [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. (2002) Volatile anesthetic actions on the GABAA receptors: contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology 42:337–345 [DOI] [PubMed] [Google Scholar]
- Nury H, Poitevin F, Van Renterghem C, Changeux JP, Corringer PJ, Delarue M, Baaden M. (2010) One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc Natl Acad Sci USA 107:6275–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60:243–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2009) GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortells MO, Lunt GG. (1995) Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci 18:121–127 [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Asatryan L, Alkana RL, Davies DL. (2009) Loop 2 structure in glycine and GABA(A) receptors plays a key role in determining ethanol sensitivity. J Biol Chem 284:27304–27314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, et al. (2003) Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci 23:8608–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline B, Jurd R, Rudolph U, Spanagel R. (2007) Etomidate and propofol-hyposensitive GABAA receptor beta3(N265M) mice show little changes in acute alcohol sensitivity but enhanced tolerance and withdrawal. Neurosci Lett 416:275–278 [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795–816 [DOI] [PubMed] [Google Scholar]
- Teissére JA, Czajkowski C. (2001) A (beta)-strand in the (gamma)2 subunit lines the benzodiazepine binding site of the GABA A receptor: structural rearrangements detected during channel gating. J Neurosci 21:4977–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci 17:2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell JR, Bertaccini E. (2004) Comparative modeling of a GABAA alpha1 receptor using three crystal structures as templates. J Mol Graph Model 23:39–49 [DOI] [PubMed] [Google Scholar]
- Ueno S, Wick MJ, Ye Q, Harrison NL, Harris RA. (1999) Subunit mutations affect ethanol actions on GABA(A) receptors expressed in Xenopus oocytes. Br J Pharmacol 127:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, Ye Q, Harrison NL, Harris RA. (1998) Mutations of gamma-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc Natl Acad Sci USA 95:6504–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Akabas MH. (1996) Identification of channel-lining residues in the M2 membrane-spanning segment of the GABA(A) receptor alpha1 subunit. J Gen Physiol 107:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Bertaccini E, Trudell JR, Harris RA. (2001) Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol 41:23–51 [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine W, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem 273:3314–3319 [DOI] [PubMed] [Google Scholar]