Abstract
Both lobeline and lobelane attenuate methamphetamine self-administration in rats by decreasing methamphetamine-induced dopamine release via interaction with vesicular monoamine transporter-2 (VMAT2). A novel derivative of nor-lobelane, cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-110), and its trans-isomers, (2R,5R)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-111) and (2S,5S)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-112), were evaluated for inhibition of [3H]dihydrotetrabenazine binding and [3H]dopamine uptake by using a rat synaptic vesicle preparation to assess VMAT2 interaction. Compounds were evaluated for inhibition of [3H]nicotine and [3H]methyllycaconitine binding to assess interaction with the major nicotinic receptor subtypes. In addition, compounds were evaluated for inhibition of methamphetamine-evoked endogenous dopamine release by using striatal slices. The most promising compound, UKCP-110, was evaluated for its ability to decrease methamphetamine self-administration and methamphetamine discriminative stimulus cues and for its effect on food-maintained operant responding. UKCP-110, UKCP-111, and UKCP-112 inhibited [3H]dihydrotetrabenazine binding (Ki = 2.66 ± 0.37, 1.05 ± 0.10, and 3.80 ± 0.31 μM, respectively) and had high potency inhibiting [3H]dopamine uptake (Ki = 0.028 ± 0.001, 0.046 ± 0.008, 0.043 ± 0.004 μM, respectively), but lacked affinity at nicotinic receptors. Although the trans-isomers did not alter methamphetamine-evoked dopamine release, UKCP-110 inhibited (IC50 = 1.8 ± 0.2 μM; Imax = 67.18 ± 6.11 μM) methamphetamine-evoked dopamine release. At high concentrations, UKCP-110 also increased extracellular dihydroxyphenylacetic acid. It is noteworthy that UKCP-110 decreased the number of methamphetamine self-infusions, while having no effect on food-reinforced behavior or the methamphetamine stimulus cue. Thus, UKCP-110 represents a new lead in the development of novel pharmacotherapies for the treatment of methamphetamine abuse.
Introduction
Lobeline, the principal alkaloid in Lobelia inflata, has a long and varied therapeutic history, ranging from use as a respiratory stimulant for asthmatics to use as an emetic (Dwoskin and Crooks, 2002). Initially, lobeline was categorized as a nicotinic acetylcholine receptor (nAChR) agonist (Decker et al., 1995) and evaluated as a potential smoking cessation agent (Schneider and Olsson, 1996). More recently, studies have shown that lobeline acts as a nAChR antagonist with high affinity for the α4β2* nAChR subtype (Benwell and Balfour, 1998; Miller et al., 2000). Lobeline inhibits nicotine-evoked 86Rb+ efflux from thalamic synaptosomes and also inhibits nicotine-evoked [3H]dopamine (DA) overflow from rat striatal slices (Miller et al., 2000). In contrast to nicotine, lobeline does not produce conditioned place preference and is not self-administered in rats (Fudala and Iwamoto, 1986; Donny et al., 1995; Harrod et al., 2003; Le Foll and Goldberg, 2005).
In addition to nAChR interactions, lobeline inhibits methamphetamine-evoked DA release in the central nervous system, reducing its reinforcing effect. Behavioral studies have demonstrated that lobeline reduces methamphetamine self-administration in rats, while also decreasing food-maintained responding (Harrod et al., 2001). It is noteworthy that tolerance developed to the lobeline-induced decrease in food-maintained responding but not to the decrease in methamphetamine self-administration. Methamphetamine reinforcement results from increases in extracellular DA via interaction with a number of presynaptic proteins, including inhibition of vesicular monoamine transporter-2 (VMAT2), inhibition of monoamine oxidase, and reversal of the dopamine transporter (DAT) (Sulzer et al., 1995; Takahashi et al., 1997). Lobeline is thought to reduce methamphetamine self-administration by inhibiting DA uptake into synaptic vesicles through interactions at the [3H]dihydroxytetrabenazine (DTBZ) binding site on VMAT2 (Teng et al., 1997). Interaction of lobeline with VMAT2 reduces the elevated synaptic DA levels induced by methamphetamine through inhibition of vesicular DA uptake without inhibition of monoamine oxidase, leaving DA more susceptible to metabolism to 3,4-dihydroxyphenylacetic acid (DOPAC) (Dwoskin and Crooks, 2002). Although lobeline-induced VMAT2 inhibition may be the primary mechanism in its attenuation of methamphetamine-induced DA release, a contributing factor also may be its ability to inhibit DAT function (Teng et al., 1997; Baumann et al., 2002; Miller et al., 2004). In this respect, lobeline analogs that selectively target VMAT2 have been proposed as potential treatments for methamphetamine abuse (Dwoskin and Crooks, 2002).
Lobelane, the des-oxy analog of lobeline, is more potent than lobeline in inhibiting DA uptake by VMAT2 and is more selective for VMAT2, as a result of its lower potency as an inhibitor of nAChRs (Miller et al., 2004). Relative to lobeline, the enhanced selectivity for VMAT2 provided by lobelane was accompanied by enhanced specificity for methamphetamine reinforcement, as lobelane decreased methamphetamine self-administration without decreasing food-maintained behavior (Neugebauer et al., 2007). These results suggest that targeting VMAT2 and eliminating interaction at nAChRs results in more specific inhibition of methamphetamine reinforcement. However, repeated dosing of lobelane produced rapid tolerance to the decrease in methamphetamine self-administration (Neugebauer et al., 2007), precluding its consideration as a clinical candidate for the treatment of methamphetamine abuse. Nevertheless, these results prompted the evaluation of a series of lobelane analogs for selective inhibition of VMAT2 function and specific decreases in methamphetamine self-administration.
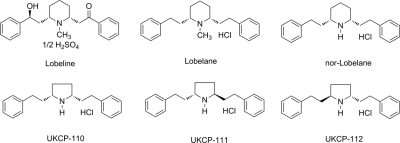
Nor-lobelane, the N-demethylated analog of lobelane (Fig. 1), has little affinity for nAChRs (Miller et al., 2004). To further exploit this property, three novel nor-lobelane analogs (Fig. 1) in which the central piperidine ring was replaced with a smaller pyrrolidine ring were synthesized. These analogs, cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-110) and its trans-isomers, (2R,5R)-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-111) and (2S,5S)-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-112), were evaluated for inhibition of [3H]DTBZ binding to VMAT2, inhibition of [3H]DA uptake at VMAT2, inhibition of [3H]nicotine and [3H]methyllycaconitine (MLA) binding at nAChRs, and inhibition of methamphetamine-evoked endogenous DA release from superfused striatal slices. The compound with the most promising pharmacological profile was evaluated for its ability to decrease methamphetamine self-administration and food-maintained behavior and to inhibit methamphetamine discriminative stimulus effects.
Fig. 1.
Structures of lobeline, lobelane, nor-lobelane, UKCP-110, UKCP-111, and UKCP-112.
Materials and Methods
Drugs and Chemicals
d-Methamphetamine HCl was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in sterile saline (0.9% NaCl) before administration for the behavioral assays. [3H]DA (specific activity, 28.0 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). [3H]DTBZ (specific activity, 20.0 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO). EDTA, EGTA, l-(+)tartaric acid, HEPES, 3-hydroxytyramine (DA), sucrose, magnesium sulfate, polyethyleneimine (PEI), and adenosine 5′-triphosphate magnesium salt were purchased from Sigma-Aldrich. l-Ascorbic acid and sodium bicarbonate were obtained from Aldrich Chemical Co. (Milwaukee, WI). (2R,3S,11bS)-2-ethyl-3-isobutyl-9,10-dimethoxy-2,2,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-ol (Ro4-1284) was a gift from F. Hoffman-La Roche, Ltd. (Basel, Switzerland). All other chemicals used in the assay buffers were purchased from Thermo Fisher Scientific (Waltham, MA). The pyrrolidine analogs, UKCP-110, UKCP-111, and UK-112, were synthesized as described previously (Vartak et al., 2009). Analog doses are expressed as salt weights.
Animals
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually in a temperature- and humidity-controlled environment with a 14-h light/10-h dark cycle (lights on at 6:00 AM) and acclimated to the colony environment for at least 5 days before use. All experiments were conducted during the light phase. For the neurochemical experiments, rats had free access to food and water. For the behavioral experiments, rats were handled for 1 week, and food was restricted to 85% of their free-feeding body weights. Sufficient postsession food was given to maintain this weight. All experimental protocols were in accordance with the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
VMAT2 Binding Assay
Lobelane- and analog-induced inhibition of [3H]DTBZ binding was determined by using modifications of a previously described method (Teng et al., 1998). Whole brains (excluding cerebellum) from individual rats were homogenized in 20 ml of ice-cold 0.32 M sucrose solution with seven up-and-down strokes of a Teflon pestle homogenizer (clearance ≈0.003 inch). Homogenates were centrifuged at 1000g for 12 min at 4°C, and the resulting supernatants were centrifuged at 22,000g for 10 min at 4°C. Resulting pellets were incubated in 18 ml of ice-cold water for 5 min, then 2 ml of a solution of HEPES (25 mM) and potassium tartrate (100 mM) was added. Samples were centrifuged (20,000g for 20 min at 4°C), and 20 μl of MgSO4 (1 mM) was added to the supernatants. Samples again were centrifuged (100,000g for 45 min at 4°C), and pellets were resuspended in ice-cold assay buffer (25 mM HEPES, 100 mM potassium tartrate, 5 mM MgSO4, 0.1 mM EDTA, and 0.05 mM EGTA, pH 7.5). Assays were performed in duplicate by using 96-well plates. Vesicular suspension (15 μg of protein in 100 μl) was added to wells containing 5 nM [3H]DTBZ, 1 nM to 1 mM inhibitor (50 μl), and buffer (50 μl). Nonspecific binding was determined in the presence of Ro4-1284 (10 μM). Reactions were terminated by filtration (Filtermate Harvester; PerkinElmer Life and Analytical Sciences) onto Unifilter-96 GF/B filter plates (presoaked in 0.5% PEI). Filters were washed five times with 350 μl of ice-cold buffer filter, plates were dried and bottom-sealed, and each well was filled with 40 μl of scintillation cocktail (MicroScint 20; PerkinElmer Life and Analytical Sciences). Radioactivity on the filters was determined by liquid scintillation spectrometry (TopCount NXT; PerkinElmer Life and Analytical Sciences).
VMAT2 Uptake Assay
Inhibition of [3H]DA uptake was conducted by using isolated rat synaptic vesicles (Teng et al., 1997). In brief, individual rat striata were homogenized in 0.32 M sucrose solution. Homogenates were centrifuged (2000g for 10 min at 4°C), and the resulting supernatants were centrifuged (10,000g for 30 min at 4°C). Pellets were resuspended in 2 ml of 0.32 M sucrose solution and subjected to osmotic shock followed by immediate restoration of osmolarity. Samples were centrifuged by using the previous parameters, and the resulting supernatants were centrifuged (55,000g for 1 h at 4°C) followed by addition of 100 μl of 10 mM MgSO4, 100 μl of 0.25 M HEPES, and 100 μl of 1.0 M potassium tartrate solution before the final centrifugation (100,000g for 45 min at 4°C). Final pellets were resuspended in 2.4 ml of assay buffer (25 mM HEPES, 100 mM potassium tartrate, 50 μM EGTA, 100 μM EDTA, 1.7 mM ascorbic acid, 2 mM ATP-Mg2+, pH 7.4). Vesicular suspension (100 μl) was added to tubes containing assay buffer, various concentrations of inhibitor (0.1 nM-10 mM), and 0.1 μM [3H]DA in a final volume of 500 μl. Nonspecific uptake was determined in the presence of Ro4-1284 (10 μM). Reactions were terminated by filtration, and radioactivity retained by the filters was determined by liquid scintillation spectrometry.
[3H]Nicotine and [3H]MLA Binding
Analog-induced inhibition of [3H]nicotine and [3H]MLA binding was determined by using previously published methods (Miller et al., 2004). Nicotine and MLA were included in the assays as positive controls for [3H]nicotine and [3H]MLA binding, respectively. Whole brain, excluding cortex and cerebellum, was homogenized in 20 volumes of ice-cold modified Krebs'-HEPES buffer (2 mM HEPES, 14.4 mM NaCl, 0.15 mM KCl, 0.2 mM CaCl2 · 2H2O, and 0.1 mM MgSO4 · 7H2O, pH 7.5). Homogenates were centrifuged at 31,000g for 17 min at 4°C (Avanti J-301 centrifuge; Beckman Coulter, Fullerton, CA). Pellets were resuspended by sonication (Vibra Cell; Sonics and Materials Inc., Danbury, CT) in 20 volumes of Krebs'-HEPES buffer and incubated at 37°C for 10 min (Reciprocal Shaking Bath model 50; Precision Scientific, Chicago, IL). Suspensions were centrifuged by using the above conditions. Resulting pellets were resuspended by sonication in 20 volumes of buffer and centrifuged at 31,000g for 17 min at 4°C. Final pellets were stored in incubation buffer containing 40 mM HEPES, 288 mM NaCl, 3.0 mM KCl, 4.0 CaCl2 · 2H2O, and 2.0 MgSO4 · 7H2O, pH 7.5. Membrane suspensions (100–140 μg of protein/100 μl) were added to tubes containing analog (7–9 concentrations, 1 nM–1 mM) and 3 nM [3H]nicotine or [3H]MLA in a final assay volume of 250 μl. Samples were incubated for 60 min at room temperature. Reactions were terminated by harvesting samples on Unifilter-96 GF/B filter plates presoaked in 0.5% PEI. Samples were washed three times with 350 μl of ice-cold buffer. Filter plates were processed as described previously. Nonspecific binding was determined in the presence of 10 μM cytisine or 10 μM nicotine for the [3H]nicotine and [3H]MLA assays, respectively.
Endogenous DA Release Assay
Endogenous DA release from rat striatal slices was estimated by using a previously reported method (Teng et al., 1997). Rat coronal striatal slices (0.5 mm, 6–8 mg) were incubated for 60 min in Krebs' buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 1.3 mM CaCl2, 11.1 mM α-d-glucose, 25 mM NaHCO3, 0.11 mM l-ascorbic acid, and 0.004 mM EDTA, pH 7.4, saturated with 95% O2/5% CO2) at 34°C in a metabolic shaker. Each slice was then transferred to superfusion chambers and superfused at 1 ml/min for 60 min with Krebs' buffer. After 1-h superfusion, two basal 1-ml samples were collected into tubes containing 100 μl of 0.1 M perchloric acid. Slices were superfused for 30 min in the absence or presence of a single concentration of analog (0.3–30 μM). Subsequently, methamphetamine (5 μM) was added to the superfusion buffer. Samples were collected to determine methamphetamine-evoked DA release in the absence and presence of analog. Methamphetamine concentration and exposure time were chosen based on the results of pilot experiments. Subsequently, samples were superfused in the absence of methamphetamine for 25 min with the appropriate concentration of analog. In each experiment, one striatal slice was superfused for 90 min in the absence of analog or methamphetamine, which served as the buffer control condition. In each experiment, duplicate slices were superfused with methamphetamine in the absence of UKCP-110, UKCP-111, or UKCP-112, which served as the methamphetamine control condition. Ascorbate oxidase (20 μl; 81 U/1 ml) was added immediately to each superfusate sample (500 μl). Samples (100 μl) were injected into the high-pressure liquid chromatography with electrochemical detection system, which consisted of a pump, autosampler (Beckman Coulter), an ODS Ultrasphere C18 reverse-phase column (80 × 4.6 mm, 3-μm; ESA Inc., Chelmsford, MA), a Coulometric-II detector with guard cell (model 5020; ESA Inc.) maintained at +0.60 V, and an analytical cell (model 5011; ESA, Inc.) maintained at potentials E1 = −0.05 V and E2 = +0.32 V. Analyses were performed at room temperature with 0.07 M citrate/0.1 M acetate buffer (175 mg/liter octylsulfonic acid-sodium salt, 650 mg/l of NaCl, and 7% methanol, pH 4) mobile phase (1.5 ml/min) with a run time of 5 to 6 min to process each sample. DA and DOPAC standards were used to identify the analytes, and peak heights were used to generate standard curves. Analyte concentrations were determined from standard calibration curves. Detection limits for DA and DOPAC were 1 and 2 pg/100 μl, respectively. Height measurement, calibration analysis, and quantification were performed with 32 Karat software (Beckman Coulter).
Methamphetamine Self-Administration
The detailed methods used are described in Neugebauer et al. (2007). Behavioral experiments were conducted in operant conditioning chambers (ENV-008; MED Associates, St. Albans, VT) enclosed within sound-attenuating compartments (ENV-018M; MED Associates). Each chamber was connected to a personal computer interface (SG-502; MED Associates), and chambers were operated by using MED-PC software. A 5 × 4.2-cm recessed food tray was located on the response panel of each chamber. Two retractable response levers were mounted on either side of the recessed food tray (7.3 cm above the metal rod floor). A 28-V, 3-cm diameter white cue light was mounted 6 cm above each response lever.
Rats were initially trained to respond on a lever for food reinforcement. Immediately after food training, rats were allowed free access to food for 3 days. Rats were then anesthetized (100 mg/kg ketamine and 5 mg/kg diazepam, intraperitoneally) and catheters were implanted into the right jugular vein, exiting through a dental acrylic head mount affixed to the skull via jeweler screws. Drug infusions were administered intravenously (0.1 ml over 5.9 s) via a syringe pump (PHM-100; MED Associates) through a water-tight swivel attached to a 10-ml syringe via the catheter tubing, which was attached to the cannula mounted to the head of the rat. After a 1-week recovery period from surgery, rats were trained to press one of two levers for an infusion of methamphetamine (0.03 mg/kg/infusion). Each infusion was followed by a 20-s timeout signaled by illumination of both lever lights. The response requirement was gradually increased to a terminal fixed ratio 5 schedule of reinforcement. Each session was 60 min in duration. Training continued until responding stabilized across sessions. Stable responding was defined as less than 20% variability in the number of infusions earned across three successive sessions, a minimum of a 2:1 ratio of active (drug) lever responses to inactive (no drug) lever responses, and at least 10 infusions per session. Once the stability criteria were reached, an acute dose (0, 0.3, 0.56, or 1.0 mg/kg) of UKCP-110 was administered (subcutaneously) 20 min before the session according to a within-subject Latin square design. Two maintenance sessions (i.e., no pretreatment) were included between each test session to ensure stable responding throughout the experiment. To investigate whether repeated treatment altered the effect of UKCP-110 on methamphetamine self-administration, a separate group of rats (n = 7/group) was administered UKCP-110 (1.0 mg/kg s.c.) or saline 20 min before each of seven consecutive methamphetamine self-administration sessions.
Food-Maintained Responding
The detailed methods used are described in Neugebauer et al. (2007). In brief, rats (n = 12) were trained to respond on one lever (active lever) for food pellet reinforcement (45-mg pellets; Bio-Serv, Frenchtown, NJ), whereas responses on the other lever (inactive lever) had no programmed consequence. Active and inactive levers were counterbalanced across rats. The response requirement was gradually increased, terminating at fixed ratio 5. After lever training, a 20-s signaled timeout (illumination of both lever lights) was included after each pellet delivery. The timeout after each pellet delivery was consistent with the methamphetamine self-administration procedure. However, each food-reinforced session lasted 15 min, rather than 60 min (methamphetamine self-administration sessions) to avoid satiation within the session. Training continued until responding stabilized across sessions. Stable responding was defined as less than 20% variability in the number of pellets earned across three successive sessions and a minimum of a 2:1 ratio of active lever responses to inactive lever responses. After the stability criteria were reached, an acute dose (0, 0.3, 0.56, or 1.0 mg/kg) of UKCP-110 was administered (subcutaneously) 20 min before the session according to a within-subject Latin square design. Two maintenance sessions (i.e., no pretreatment) were included between each test session to ensure stable responding throughout the experiment.
Data Analysis
VMAT2 and nAChR Assays.
For [3H]DTBZ, [3H]nicotine, and [3H]MLA binding assays, specific binding was determined by subtracting nonspecific from total binding. For the [3H]DA uptake assay, specific uptake was determined by subtracting nonspecific from total uptake. Concentrations of inhibitor that produced 50% inhibition of binding or uptake (IC50 values) were determined from the concentration-effect curves via an iterative curve-fitting program (Prism 4.0; GraphPad Software Inc., San Diego, CA). Inhibition constants (Ki values) were determined by using the Cheng-Prusoff equation (Cheng and Prusoff, 1973. In addition, one-way ANOVAs, with compound as a between-subject factor, were used to compare potency between the compounds in the two assays. ANOVAs were conducted by using log transformations of Ki values. Bonferroni adjusted pairwise comparisons were used for all post hoc analyses to determine differences between compound affinity in both the binding and DA uptake assays.
DA Release Assay.
For the endogenous DA release assay, fractional release of DA and DOPAC in each superfusion sample was calculated as pg/ml/mg slice weight. Basal outflow was calculated from the average fractional release during the initial 10-min period (two 1-min samples collected at 5-min intervals) before addition of analog to the superfusion buffer. Total DA or DOPAC overflow was calculated as the sum of the increases in fractional release above basal outflow during superfusion with analog and/or methamphetamine added to the buffer. Intrinsic activity for each analog was determined as DA or DOPAC overflow during the initial 30-min period of superfusion in the absence of methamphetamine.
For each analog, two-way repeated-measures ANOVAs, with analog concentration and time as within-subject factors, were used to determine effect of analog alone and, in a separate analysis, analog-induced inhibition of methamphetamine-evoked DA and DOPAC fractional release. Bonferroni adjusted pairwise comparisons were used for post hoc analyses to determine the time points at which concentrations of analog inhibited methamphetamine-evoked fractional release relative to control (methamphetamine alone). One-way repeated-measures ANOVAs with analog concentration, either in the absence or presence of methamphetamine, determined concentration-dependent effects on DA or DOPAC overflow. Bonferroni-adjusted pairwise comparisons were used for post hoc analyses to determine the concentrations of analog that altered either basal DA or DOPAC overflow or methamphetamine-induced effects on DA or DOPAC overflow. The IC50 value for inhibition of methamphetamine-evoked DA overflow was determined by using a nonlinear least-squares curve-fitting program (Prism version 4.0; GraphPad Software, Inc.).
Behavioral Experiments
Two-way repeated-measures ANOVAs with dose and time as within-subject variables determined dose-response effects of acute UKCP-110 pretreatment on methamphetamine self-administration, food-maintained behavior, and drug discrimination. Linear mixed-effects modeling was used to further analyze time course effects of UKCP-110 pretreatment on methamphetamine self-administration and food-maintained behavior. A two-way mixed-factor ANOVA with pretreatment day as a within-subject factor was used to analyze the effect of repeated dosing of UKCP-110 on methamphetamine self-administration. A one-way repeated-measures ANOVA with dose as a within-subject factor was used to analyze the effects of UKCP-110 pretreatment on methamphetamine self-administration within the first 15 min of the 60-min session (for comparison to the data obtained from the 15-min food-maintained session) and on percentage drug appropriate responding and response rate for drug discrimination (see Supplemental Figure). Bonferroni-adjusted pairwise comparisons were used for post hoc analyses.
Results
Inhibition of [3H]DTBZ Binding and [3H]DA Uptake at VMAT2.
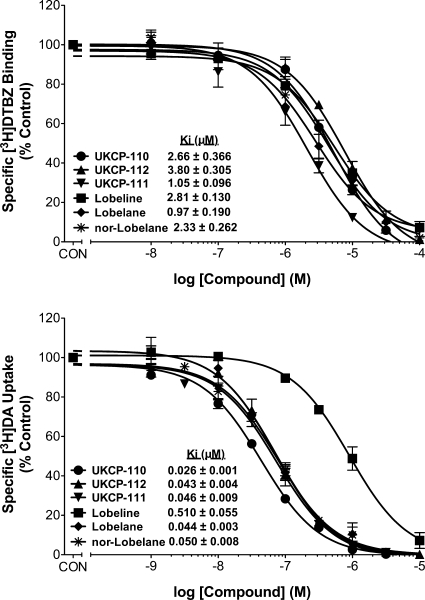
UKCP-110 and its trans-isomers UKCP-111 and UKCP-112 (Fig. 1) were evaluated for their ability to inhibit [3H]DTBZ binding to its high-affinity site on VMAT2 (Fig. 2, top) and inhibit [3H]DA uptake by VMAT2 (Fig. 2, bottom) in comparison with lobeline, and the structurally related compounds, lobelane and nor-lobelane. One-way ANOVA revealed differences in potencies to inhibit [3H]DTBZ binding (F5,15 = 21.20; p < 0.05). UKCP-111 and lobelane had higher potency for inhibiting [3H]DTBZ binding than UKCP-110, UKCP-112, nor-lobelane, and lobeline (p < 0.05). In addition, one-way ANOVA revealed differences between compounds in potency to inhibit [3H]DA uptake (F5,19 = 74.31; p < 0.05). Post hoc analyses indicated that UKCP-110 (Ki = 0.028 μM) was more potent than lobelane and nor-lobelane in inhibiting [3H]DA uptake, and all of the compounds evaluated were 10- to 20-fold more potent inhibiting [3H]DA uptake than lobeline.
Fig. 2.
Effect of UKCP-110 and its trans-isomers (UKCP-111 and UKCP-112) on [3H]DTBZ binding (top) and [3H]DA uptake (bottom) into synaptic vesicles. Concentration-dependent increases in inhibition of [3H]DTBZ binding and inhibition of vesicular [3H]DA uptake by UKCP-110 and its trans-isomers are shown. For the binding assays, control represents specific [3H]DTBZ binding in the absence of compound. Data are mean (± S.E.M.) specific [3H]DTBZ binding presented as a percentage of the respective control (1100 ± 96.2 fmol/mg control, n = 22 rats; n = 3–5 rats/compound). For the uptake assays, control represents [3H]DA uptake in the absence of compound. Data are mean (± S.E.M.) specific [3H]DA uptake as a percentage of the respective control (35.8 ± 5.33 pmol/min/mg, control, n = 24 rats; n = 4 rats/compound). Previous results for [3H]DTBZ binding (Zheng et al., 2008) and [3H]DA uptake (Nickell et al., 2010) for lobeline and lobelane are illustrated.
Inhibition of [3H]Nicotine and [3H]MLA Binding.
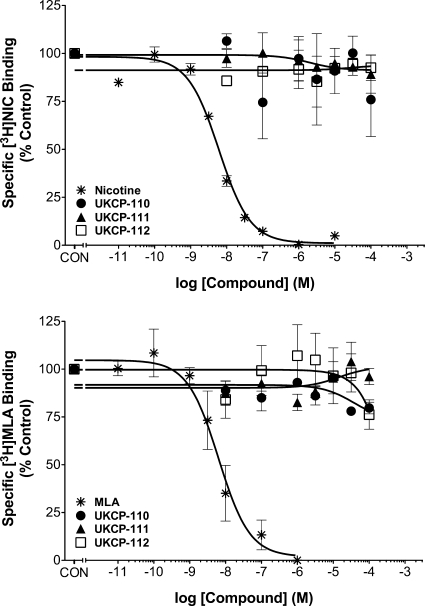
Because lobeline interacts with nAChRs, the novel compounds were evaluated for inhibition of [3H]nicotine and [3H]MLA binding to rat brain membranes (Fig. 3). Ki values for inhibition by nicotine and MLA were consistent with previous reports (Ki = 3.00 ± 0.20 and 3.89 ± 1.02 nM, respectively) (Miller et al., 2004). None of the novel analogs evaluated inhibited [3H]nicotine or [3H]MLA binding, indicating greater selectivity for VMAT2 compared with lobeline.
Fig. 3.
Analogs do not inhibit [3H]nicotine (top) or [3H]MLA (bottom) binding. Analog concentration response curves for inhibition of [3H]nicotine (NIC) and [3H]MLA binding to rat whole brain membranes are shown. Nonspecific binding was determined in the presence of 10 μM cytisine or 10 μM NIC for the [3H]NIC and [3H]MLA assays, respectively. Control represents [3H]NIC or [3H]MLA binding in the absence of analog (46.4 ± 4.20 and 49.1 ± 2.31 fmol/mg protein, respectively). Data are mean ± S.E.M. (n = 3–4 rats/compound).
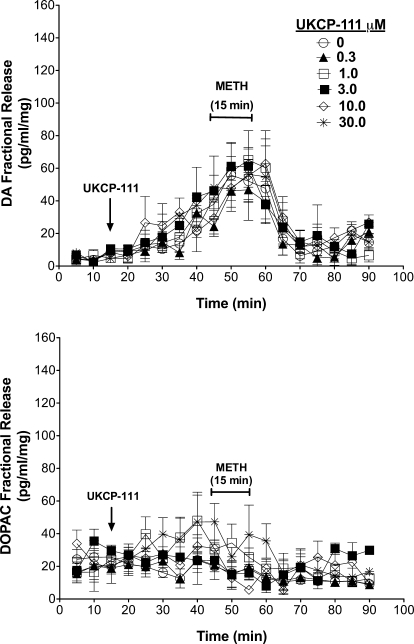
Inhibition of Methamphetamine-Evoked DA and DOPAC Release.
The effect of UKCP-111 on DA and DOPAC fractional release is illustrated in Fig. 4. A two-way repeated-measures ANOVA on DA fractional release during the period when striatal slices were exposed to UKCP-111 alone (20–40 min; Fig. 4, top) indicated a main effect of time (F4,60 = 13.34; p < 0.05), but no main effect of concentration (F5,60 = 0.69; p > 0.05) and no time × concentration interaction (F20,60 = 1.17; p > 0.05). In addition, a two-way repeated-measures ANOVA on DOPAC fractional release during the period when striatal slices were exposed to UKCP-111 alone (Fig. 4, bottom) indicated no main effect of time (F4,60 = 2.09; p > 0.05), concentration (F5,60 = 1.62; p > 0.05), and time × concentration interaction (F20,60 = 0.78; p > 0.05). Thus, UKCP-111 had no effect alone on DA or DOPAC fractional release. Likewise, a two-way repeated-measures ANOVA on DA fractional release during the period when the slices were exposed to UKCP-111 concurrently with methamphetamine (45–75 min; Fig. 4, top) revealed a main effect of time (F6,90 = 25.95; p < 0.05), but no main effect of concentration (F5,90 = 0.27; p > 0.05) or a time × concentration interaction (F30,90 = 0.42; p > 0.05). Furthermore, a two-way repeated-measures ANOVA on DOPAC fractional release during this same period (Fig. 4, bottom) indicated a main effect of time (F6,90 = 6.94; p < 0.05), but no main effect of concentration (F5,90 = 1.69; p > 0.05) and no time × concentration interaction (F30,90 = 1.56; p > 0.05). These results suggest that UKCP-111 did not alter the effect of methamphetamine on DA or DOPAC fractional release.
Fig. 4.
Effect of exposure to UKCP-111 alone and in combination with methamphetamine on DA (top) and DOPAC (bottom) fractional release. DA and DOPAC fractional release represents the amount of DA and DOPAC in each 5-min sample. The arrows indicate the point in time at which analog was added to the superfusion buffer and analog remained in the buffer until the end of the experiment. Methamphetamine (METH) was added to the buffer for 15 min as indicated by the brackets. Data are mean ± S.E.M pg/ml/mg slice weight (n = 3).
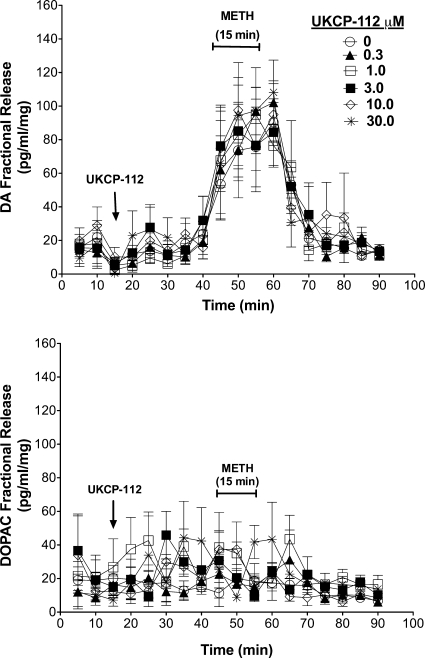
Figure 5 illustrates the effects of UKCP-112 on endogenous DA and DOPAC fractional release and its ability to inhibit methamphetamine-evoked release. A two-way repeated-measures ANOVA on DA fractional during the period when striatal slices were exposed to UKCP-112 alone (20–40 min; Fig. 5, top) indicated no main effect of time (F4,60 = 2.06; p > 0.05), concentration (F5,60 = 1.23; p > 0.05), and time × concentration interaction (F20,60 = 0.29; p > 0.05). In addition, a two-way repeated-measures ANOVA on DOPAC fractional release during the same period (Fig. 5, bottom) indicated no main effect of time (F4,60 = 0.69; p > 0.05), concentration (F5,60 = 0.99; p > 0.05), and time × concentration interaction (F20,60 = 1.69; p > 0.05). Thus, UKCP-112 had no effect alone on DA or DOPAC fractional release. Likewise, a two-way repeated-measures ANOVA on DA fractional release during the period when the slices were exposed to UKCP-112 concurrently with methamphetamine (45–75 min; Fig. 5, top) revealed a main effect of time (F6,90 = 32.04; p < 0.05), but no main effect of concentration (F5,90 = 0.07; p > 0.05) and no time × concentration interaction (F30,90 = 0.62; p > 0.05). Furthermore, a two-way repeated-measures ANOVA on DOPAC fractional release during the same period (Fig. 5, bottom) indicated a main effect of time (F6,90 = 2.55; p < 0.05), but no main effect of concentration (F5,90 = 1.19; p > 0.05) and no time × concentration interaction (F30,90 = 1.44; p > 0.05). These results suggest that, like UKCP-111, UKCP-112 did not alter the effect of methamphetamine on DA or DOPAC fractional release.
Fig. 5.
Effect of exposure to UKCP-112 alone and in combination with methamphetamine on DA (top) and DOPAC (bottom) fractional release. The arrows indicate the point in time at which analog was added to the superfusion buffer and analog remained in the buffer until the end of the experiment. METH was added to the buffer for 15 min as indicated by the brackets. Data are mean ± S.E.M pg/ml/mg slice weight (n = 3).
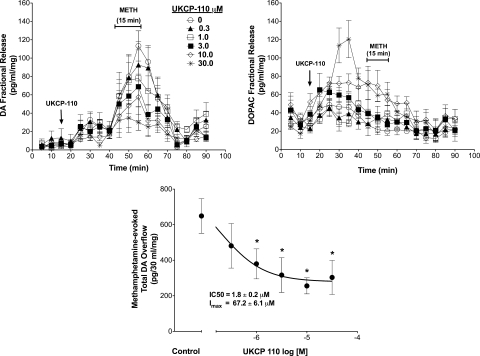
The effect of UKCP-110 on DA and DOPAC fractional release is illustrated in Fig. 6. A two-way repeated-measures ANOVA on DA fractional release during the period when striatal slices were exposed to UKCP-110 alone (20–40 min; Fig. 6, left) indicated a main effect of time (F4,144 = 7.73; p < 0.05), no main effect of concentration (F5,144 = 0.63; p > 0.05), and no time × concentration interaction (F20,144 = 0.76; p > 0.05). In addition, UKCP-110 alone had no effect on DA overflow (F5,30 = 0.64; p > 0.05; data not shown). Two-way repeated-measures ANOVA on DOPAC fractional release during this period (Fig. 6, right) indicated no main effect of time (F4,144 = 2.19; p > 0.05) and no time × concentration interaction (F20,144 = 2.43; p > 0.05), but a main effect of concentration (F5,144 = 11.85; p > 0.05) was found. Post hoc analysis indicated that 10 and 30 μM UKCP-110 increased DOPAC fractional release from 30 to 40 min (Fig. 6, right). In addition, UKCP-110 increased DOPAC overflow (F5,30 = 12.31; p < 0.05; data not shown). Post hoc analysis indicated that 10 and 30 μM UKCP-110 increased DOPAC overflow, relative to the buffer control (absence of analog). Thus, UKCP-110 alone did not alter DA fractional release, but increased DOPAC fractional release and overflow.
Fig. 6.
Effect of UKCP-110 alone and in combination with methamphetamine on DA (top left) and DOPAC (top right) fractional release and total methamphetamine-evoked DA overflow (bottom). The arrows indicate the point in time at which analog was added to the superfusion buffer and analog remained in the buffer until the end of the experiment. METH was added to the buffer for 15 min as indicated by the brackets. Data are mean ± S.E.M pg/ml/mg slice weight (n = 6).
UKCP-110, in contrast to both trans-isomers, decreased methamphetamine-evoked DA and DOPAC fractional release as illustrated in Fig. 6. Two-way repeated-measures ANOVA indicated a main effect of time (F6,216 = 15.51; p < 0.05), concentration (F5,216 = 2.90; p < 0.05), and time × concentration interaction (F30,216 = 2.34; p < 0.05; Fig. 6, left). Post hoc analysis indicated a decrease in methamphetamine-evoked DA fractional release at 55 to 60 min for 1 μM UKCP-110, at 55 to 60 min for 3 μM UKCP-110, at 45 to 60 min for 10 μM UKCP-110, and at 50 to 60 min for 30 μM UKCP-110. In addition, UKCP-110 decreased total methamphetamine-evoked DA overflow (F5,30 = 7.13; p < 0.05; Fig. 6, bottom). Post hoc analysis indicated that with the exception of 0.3 μM, all UKCP-110 concentrations decreased methamphetamine-evoked DA overflow. An IC50 value of 1.8 ± 0.2 μM and Imax of 67.2 ± 6.1% were derived from the concentration-response curve for inhibition of methamphetamine-evoked total DA overflow. In addition, a two-way repeated-measures ANOVA on DOPAC fractional release during the period when the slices were exposed to UKCP-110 concurrently with methamphetamine (45–75 min; Fig. 6, right) revealed a main effect of time (F6,216 = 10.14; p < 0.05) and a main effect of concentration (F5,216 = 4.14; p < 0.05), but no time × concentration interaction (F30,216 = 1.095; p > 0.05). Post hoc analysis indicated an increase in DOPAC fractional release in the presence of methamphetamine at 45 to 55 and 65 min at 10 μM UKCP-110 and at 45 to 55 min for 30 μM UKCP-110. In addition, in the presence of methamphetamine, UKCP-110 increased total DOPAC overflow (F5,30 = 12.21; p < 0.05; data not shown). Post hoc analysis indicated that 10 and 30 μM UKCP-110 increased DOPAC overflow in the presence of methamphetamine.
Inhibition of Methamphetamine Self-Administration.
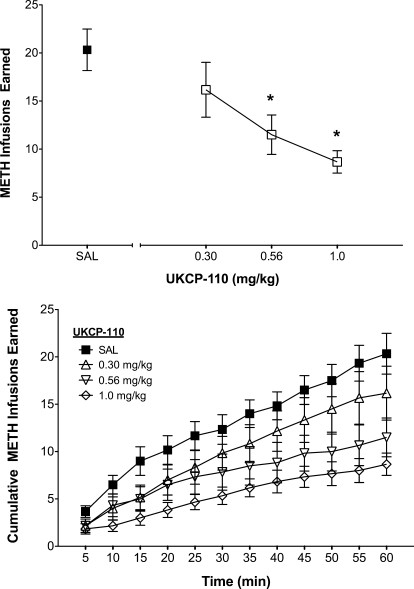
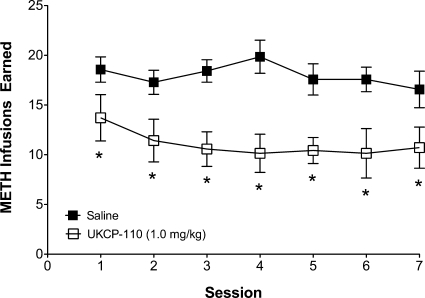
Given its ability to inhibit methamphetamine-evoked endogenous DA release, UKCP-110 was evaluated for its ability to decrease methamphetamine self-administration. The dose-dependent effect of UKCP-110 on the total number of methamphetamine infusions earned within a 60-min session and the time course for this effect are shown in Fig. 7. A two-way ANOVA with dose and time as within-subject factors revealed a main effect of dose (F3,15 = 7.09; p < 0.05), time (F11,55 = 65.10; p < 0.05), and a significant dose × time interaction (F33,165 = 4.36; p < 0.05). Subsequent pairwise comparisons indicated that the 0.56 and 1.0 mg/kg doses reduced the number of methamphetamine infusions relative to saline control. A linear mixed-effects analysis was used to estimate both the line intercept and the slope for each UKCP-110 dose as a function of time. Differences in slope (F3,257 = 6.79; p < 0.05) as a function of dose were found; however, no differences were found for intercept (F3,257 = 1.67; p > 0.05). Subsequent pairwise comparisons of slope estimates indicated that the slopes for each dose of UKCP-110, except 0.3 mg/kg, were different from the saline dose (saline = 0.28 ± 0.04; 0.3 mg/kg = 0.26 ± 0.03; 0.56 mg/kg = 0.15 ± 0.04; 1.0 mg/kg = 0.13 ± 0.04). In addition, UKCP-110 produced no effect on the number of responses on the inactive lever during methamphetamine self-administration sessions (results not shown), although the number of responses on the inactive lever was low (< five responses per session in saline control condition).
Fig. 7.
Effect of UKCP-110 on the number of methamphetamine infusions (top) and time course of methamphetamine self-administration (bottom). UKCP-110 dose-dependently decreased the number of METH infusions (0.03 mg/kg/infusion) over the 60-min session. Total and cumulative METH infusions earned in 5-min blocks are presented as mean ± S.E.M. *, different from saline (n = 6).
To compare the effects of UKCP-110 on methamphetamine self-administration with its effects on food-reinforced behavior, the data collected during the first 15 min of the self-administration session were analyzed separately (results not shown). A two-way repeated-measures ANOVA with dose and time as within-subject factors revealed a main effect of dose (F3,15 = 4.64; p < 0.05), a main effect of time (F2,10 = 52.70; p < 0.05), and a dose × time interaction (F6,30 = 4.25; p < 0.05). Subsequent pairwise comparisons revealed that 1.0 mg/kg UKCP-110 reduced infusions relative to saline within the first 15 min of the self-administration session.
Food-Maintained Responding.
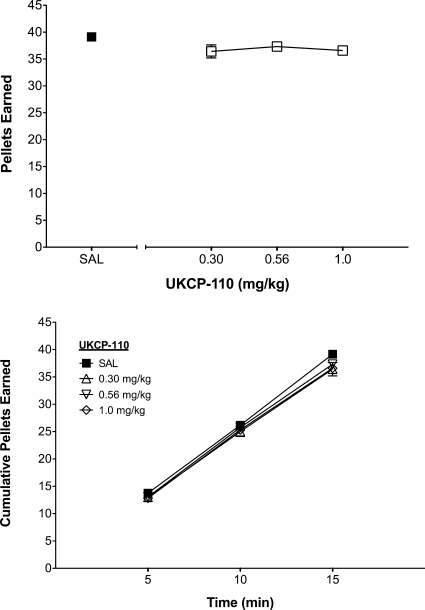
The dose-dependent effect of UKCP-110 on the total number of food pellets earned and the time course of the effect are shown in Fig. 8. A two-way repeated-measures ANOVA with dose and time as within-subject factors revealed no main effect of dose (F3,33 = 2.37; p > 0.05) on the total number of pellets earned, but a significant main effect of time (F2,22 = 2634.60; p < 0.05) and a significant dose × time interaction (F6,66 = 3.20; p < 0.05). To further evaluate this interaction, linear mixed-effects analysis was used to estimate both line intercept and slope for each UKCP-110 dose over the course of the 15-min session and revealed no significant differences in slope (F3,89 = 2.69; p > 0.05) or intercept (F3,89 = 0.33; p > 0.05) as a function of dose. Thus, UKCP-110 did not alter cumulative food intake across the 15-min session. In addition, UKCP-110 produced no alterations in responding on the inactive lever during food-maintained behavior (data not shown).
Fig. 8.
Effect of UKCP-110 on food maintained responding (top) and time course of responding (bottom). Total and cumulative number of pellets earned in 5-min blocks across a 15-min session are presented as mean ± S.E.M. (n = 12).
Lack of Tolerance to the Inhibition of Methamphetamine Self-Administration.
The decrease in methamphetamine self-administration produced by UKCP-110 (1.0 mg/kg) persisted across seven consecutive sessions (Fig. 9). A two-way mixed-factor ANOVA with session as a within-subject factor and treatment as a between-subjects factor revealed a main effect of treatment (F1,12 = 13.35; p < 0.05); however, no main effects of session (F6,72 = 0.97; p > 0.05) and no treatment × session interaction (F6,72 = 0.87; p > 0.05) were found. Thus, tolerance did not develop to the effect of UKCP-110 to decrease methamphetamine self-administration.
Fig. 9.
No tolerance to repeated treatment with UKCP-110 on methamphetamine self-administration. Repeated administration of UKCP-110 decreased METH self-administration over the course of seven sessions. Data are presented as mean ± S.E.M. number of METH infusions (0.03 mg/kg/infusion) earned over seven consecutive 60-min sessions. *, different from saline (n = 7/group).
Methamphetamine Drug Discrimination.
UKCP-110 (0.3 or 1.0 mg/kg) did not alter the percentage of methamphetamine-appropriate responses (see Supplemental Figure for methods; Wooters and Bardo, 2007); thus, UKCP-110 did not block the methamphetamine discriminative cue. In addition, UKCP-110 did not alter response rate. A one-way repeated-measures ANOVA revealed no differences in response rate on the drug appropriate lever as a function of dose (F2,6 = 1.74; p > 0.05).
Discussion
The current experiments demonstrate that UKCP-110 and its trans-isomers UKCP-111 and UKCP-112 have affinity for the [3H]DTBZ binding site on VMAT2 that was comparable with lobeline, lobelane, and nor-lobelane. Similar to lobelane, these novel analogs did not exhibit affinity for α4β2 and α7 nAChRs, indicative of selectivity for VMAT2. In addition, these novel analogs potently inhibited [3H]DA uptake at VMAT2, exhibiting a 10-fold greater potency than lobeline, but comparable potency to lobelane and nor-lobelane. High concentrations (10 and 30 μM) of the cis-isomer UKCP-110 alone produced a concentration-dependent increase in DOPAC overflow. It is noteworthy that in a concentration-dependent manner UKCP-110 decreased methamphetamine-evoked DA release. Thus, UKCP-110 potently inhibits VMAT2 function and the in vitro effects of methamphetamine on DA release. Consistent with these in vitro observations, UKCP-110 dose-dependently decreased methamphetamine self-administration, and this decrease did not generalize to food reinforcement or alter the discriminative stimulus effects of methamphetamine (Supplemental Figure), indicating a specific inhibition of the reinforcing effects of methamphetamine. Furthermore, upon repeated administration of UKCP-110, tolerance did not develop to the decrease in methamphetamine self-administration. Collectively, these results demonstrate that the VMAT2-selective inhibitor UKCP-110 has a preclinical profile that meets the criteria for consideration as a candidate for the treatment of methamphetamine abuse.
Previous research has demonstrated the importance of VMAT2 function in methamphetamine-evoked striatal DA release (Wang et al., 1997; Fumagalli et al., 1999). Lobeline, a VMAT2 inhibitor and nAChR antagonist, decreases d-amphetamine-evoked DA overflow from rat striatal slices (Miller et al., 2001). The lack of selectivity with respect to the pharmacological mechanism of lobeline prompted the discovery of novel analogs that selectively inhibit VMAT2 function (Zheng et al., 2008; Vartak et al., 2009). Lobelane was identified as a defunctionalized analog of lobeline that had diminished nAChR activity and greater selectivity as a VMAT2 inhibitor (Miller et al., 2001). Furthermore, lobelane decreased methamphetamine self-administration in rats (Neugebauer et al., 2007). Unfortunately, lobelane exhibited poor water solubility, necessitating further optimization to afford analogs with more drug-like properties. The analogs reported herein represent compounds in which the flexibility of the central piperidine ring in lobelane has been restricted to afford more conformationally defined pyrrolidine analogs and provide improved water solubility. These analogs are a subset of a larger library of pyrrolidine analogs of lobelane (Vartak et al., 2009).
Of the three conformationally restricted lobelane analogs, only UKCP-110 decreased methamphetamine-evoked DA release from striatal slices. Both trans-isomers, UKCP-111 and UKCP-112, inhibited DA uptake at VMAT2, but did not alter methamphetamine-evoked DA release. This difference in inhibition of the in vitro effects of methamphetamine between the cis-and trans-isomers is intriguing and indicates that the difference in stereochemistry between these analogs is an important factor in the mediation of the inhibition of the effect of methamphetamine. The cis-scaffold is structurally quite different from the trans-scaffold with respect to spatial arrangement of the 2,5-diphenethyl substituents. Even with the trans-compounds, UKCP-111 and UKCP-112, which are mirror image isomers, differences in the manner in which these optical isomers interact with the pharmacological target are expected. The site of interaction on the cytosolic face of VMAT2, which translocates DA from the cytosol into the vesicle, may have a different pharmacophoric requirement than the DA recognition site on the intravesicular face of VMAT2. The DA recognition site on the cytosolic face may not be sensitive to stereochemistry, because all three analogs equipotently inhibited DA uptake by VMAT2, whereas the DA recognition site on the intravesicular face of VMAT2 may have specific stereochemical requirements in which only the cis-isomer, and not the trans-isomers, are recognized. Alternatively, another site with specific stereochemical requirements may be involved, which allosterically modulates the region of VMAT2 protein that recognizes methamphetamine. Support for stereochemically defined sites on VMAT2 comes from previous work showing that lobelane, which also incorporates a cis-diphenethyl scaffold, inhibits both DA translocation and methamphetamine-evoked DA release (Miller et al., 2001). Reports from the literature showing that DA translocation sites on opposite faces of the DAT are regulated differently (Gnegy, 2003) provide precedence for the differentially regulated DA recognition sites on VMAT2 protein.
Previous research has shown the importance of VMAT2 function in amphetamine reward and reinforcement, as measured by conditioned place preference (Takahashi et al., 1997) and methamphetamine self-administration (Harrod et al., 2001; Neugebauer et al., 2007), respectively. The present results further support the role of VMAT2 in methamphetamine reinforcement, because UKCP-110 potently inhibited VMAT2 function and decreased methamphetamine self-administration without generalizing to another reinforcer (i.e., food) and without altering the discriminative stimulus properties of methamphetamine. This result indicates that the interoceptive cues that the animal experiences upon administration of methamphetamine are not altered by UKCP-110. Thus, the animal is aware that methamphetamine has been administered, but is not willing to work for the drug (i.e., methamphetamine reinforcement is decreased). Collectively, these results demonstrate that VMAT2 may be a useful target for the development of compounds that specifically inhibit the reinforcing effects of methamphetamine.
Current results from the behavioral assays reveal some important differences between UKCP-110 and its parent compounds, lobeline and lobelane (Harrod et al., 2001; Neugebauer et al., 2007). While lobeline attenuated methamphetamine self-administration, this compound within a similar dose range also decreased food-reinforced responding, presumably because of its activity at nicotinic receptors (Harrod et al., 2001). In contrast, UKCP-110 specifically decreased methamphetamine self-administration, probably resulting from its enhanced selectivity in inhibiting VMAT2 and its lack of affinity for α4β2 and α7 nAChRs. Although lobelane also provided greater selectivity for VMAT2 (Miller et al., 2004) and specifically decreased methamphetamine self-administration, rapid tolerance was observed across repeated treatment (Neugebauer et al., 2007), which may have been caused by pharmacokinetic and/or pharmacodynamic factors. In contrast, tolerance did not develop to the ability of UKCP-110 to decrease methamphetamine self-administration across repeated treatment. For these reasons, UKCP-110 has both improved physicochemical properties and pharmacological profile compared with either lobeline or lobelane, suggesting that it would be a superior clinical candidate for treating methamphetamine addiction.
Although the effects of UKCP-110 were specific to methamphetamine self-administration, some caution is needed in comparing results from methamphetamine self-administration with those from food-maintained responding. Rats responding for food reinforcement were not exposed to either methamphetamine or surgery, and food-reinforced operant sessions were 15 min in duration in contrast to the 60-min sessions for methamphetamine self-administration. In addition, because the baseline rate of responding was higher for food-maintained responding than for methamphetamine self-administration, the possibility of rate-dependent effects requires consideration (Dews, 1958). Based on the notion of rate dependence, higher ongoing rates of behavior are typically more easily disrupted than low rates. Following this logic, UKCP-110 would be expected to disrupt food-maintained responding more readily than methamphetamine self-administration; however, in the present study, the data obtained indicated that this was not the case. Thus, the observed differences between the effects of UKCP-110 on food-reinforced behavior and methamphetamine-reinforced behavior are unlikely to be caused by differences in baseline rates of responding.
In the current study, UKCP-110 did not block the discriminative stimulus effects of methamphetamine, in contrast to lobeline (Miller et al., 2001). Mechanisms that govern cue effects in drug discrimination are often similar to mechanisms that govern the reinforcing effects of a particular compound (Schuster et al., 1981), although dissociation between these two effects have been observed. For example, the nAChR antagonist N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, which has been reported to selectively inhibit α6β2-containing nAChRs, effectively attenuates nicotine self-administration without blocking nicotine cue effects in drug discrimination assays (Neugebauer et al., 2006; Dwoskin et al., 2008). This dissociation suggests that nicotine drug discrimination and self-administration may be governed by separate underlying neurochemical mechanisms. In contrast, methamphetamine drug discrimination and self-administration have been demonstrated to depend, at least in part, on DA function (Munzar et al., 1999; Munzar and Goldberg, 2000). However, the selective VMAT2 inhibitor UKCP-110 attenuated methamphetamine-evoked DA release and methamphetamine self-administration without altering methamphetamine drug discrimination. Taken together, these results suggest that the discriminative stimulus effects of methamphetamine invoke mechanisms other than or in addition to VMAT2-regulated DA function. Thus, VMAT2 plays a more important role in methamphetamine reinforcement than in the methamphetamine discriminative cue, and selectively targeting VMAT2 function specifically inhibits the reinforcing properties of methamphetamine.
In conclusion, the current preclinical studies show that the novel cis-pyrrolidine analog UKCP-110 has improved physicochemical, pharmacological, and behavioral characteristics over both lobeline and lobelane and also demonstrate that compounds selectively targeting VMAT2 may provide potential pharmacotherapies for treating methamphetamine abuse.
Supplementary Material
Acknowledgments
We thank Kate Fischer for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA13519, T32-DA01617].
The University of Kentucky holds patents on lobeline and the analogs described in the current work. A potential royalty stream to L.P.D. and P.A.C. may occur consistent with University of Kentucky policy.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172742.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- nAChR
- nicotinic acetylcholine receptor
- VMAT2
- vesicular monoamine transporter-2
- METH
- methamphetamine
- DA
- dopamine
- DAT
- DA transporter
- DTBZ
- dihydrotetrabenazine
- UKCP-110
- cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride
- UKCP-111
- (2R,5R)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride
- UKCP-112
- (2S,5S)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride
- MLA
- methyllycaconitine
- NIC
- nicotine
- PEI
- polyethyleneimine
- Ro4-1284
- (2R,3S,11bS)-2-ethyl-3-isobutyl-9,10-dimethoxy-2,2,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-ol
- DOPAC
- 3,4-dihydroxyphenylacetic acid
- ANOVA
- analysis of variance.
References
- Baumann MH, Phillips JM, Ayestas MA, Ali SF, Rice KC, Rothman RB. (2002) Preclinical evaluation of GBR12909 decanoate as a long-acting medication for methamphetamine dependence. Ann NY Acad Sci 965:92–108 [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. (1998) The influence of lobeline on nucleus accumbens dopamine and locomotor responses to nicotine in nicotine-pretreated rats. Br J Pharmacol 125:1115–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. (1995) Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci 56:545–570 [DOI] [PubMed] [Google Scholar]
- Dews PB. (1958) Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther 122:137–147 [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. (1995) Nicotine self-administration in rats. Psychopharmacology (Berl) 122:390–394 [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. (2002) A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol 63:89–98 [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, Manda VK, Ayers JT, Zhang Z, Deaciuc AG, et al. (2008) N,N′-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity. J Pharmacol Exp Ther 326:563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. (1986) Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav 25:1041–1049 [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. (1999) Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci 19:2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy ME. (2003) The effect of phosphorylation on amphetamine-mediated outward transport. Eur J Pharmacol 479:83–91 [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. (2001) Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther 298:172–179 [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. (2003) Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 165:397–404 [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178:481–492 [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Dwoskin LP. (2000) Lobeline inhibits nicotine-evoked [3H]dopamine overflow from rat striatal slices and nicotine-evoked 86Rb+ efflux from thalamic synaptosomes. Neuropharmacology 39:2654–2662 [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. (2001) Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther 296:1023–1034 [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm SD, Dwoskin LP. (2004) Lobeline analogs with enhanced affinity and selectivity for plasmalemma and vesicular monoamine transporters. J Pharmacol Exp Ther 310:1035–1045 [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. (2000) Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 148:209–216 [DOI] [PubMed] [Google Scholar]
- Munzar P, Baumann MH, Shoaib M, Goldberg SR. (1999) Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology (Berl) 141:287–296 [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. (2007) Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol 571:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. (2006) Effect of a novel nicotinic receptor antagonist, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 184:426–434 [DOI] [PubMed] [Google Scholar]
- Nickell JF, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. (2010) Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther 332:612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider FH, Olsson TA. (1996) Clinical experience with lobeline as a smoking cessation agent. Med Chem Res 6:562–570 [Google Scholar]
- Schuster CR, Fischman MW, Johanson CE. (1981) Internal stimulus control and subjective effects of drugs. NIDA Res Monogr 37:116–129 [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15:4102–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. (1997) VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA 94:9938–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Dwoskin LP. (1998) Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: comparison with d-amphetamine. J Neurochem 71:258–265 [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. (1997) Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther 280:1432–1444 [PubMed] [Google Scholar]
- Vartak AP, Nickell JR, Chagkutip J, Dwoskin LP, Crooks PA. (2009) Pyrrolidine analogs of lobelane: relationship of affinity for the tetrabenazine binding site on the vesicular monoamine transporter 2 (VMAT2) with function of VMAT2. J Med Chem 52:7878–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. (1997) Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 19:1285–1296 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. (2007) The monoamine oxidase inhibitor phenelzine enhances the discriminative stimulus effect of nicotine in rats. Behav Pharmacol 18:601–608 [DOI] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Deaciuc AG, Crooks PA. (2008) Synthesis and evaluation of a series of homologues of lobelane at the vesicular monoamine transporter-2. Bioorg Med Chem Lett 18:6509–6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.