Peloruside A (1), a potent microtubule stabilizer that acts in a manner synergistic to that of paclitaxel, was first isolated in 2000 by Northcote and coworkers from a marine sponge of the Pelorus Sound in New Zealand.[1] The absolute stereochemistry of 1 was established in De Brabander’s initial total synthesis in 2003,[2] and since then three other total syntheses have been reported.[3–5]
Herein, we describe a convergent total synthesis of peloruside A in which three different enantioenriched epoxides (8, 9, and 11, Figure 1) obtained using asymmetric catalytic methodologies serve as the key building blocks for the stereochemically complex macrocyclic framework. A second key strategic feature is a chiral catalyst-controlled diastereoselective hetero-Diels–Alder reaction for the construction of intermediate 7. The application of direct catalyst control represents a complement to the previous synthetic approaches to peloruside A, which relied primarily on substrate- and auxiliary-based diastereocontrol to establish the relative and absolute stereochemical features of the natural product.[2–5]
Figure 1.
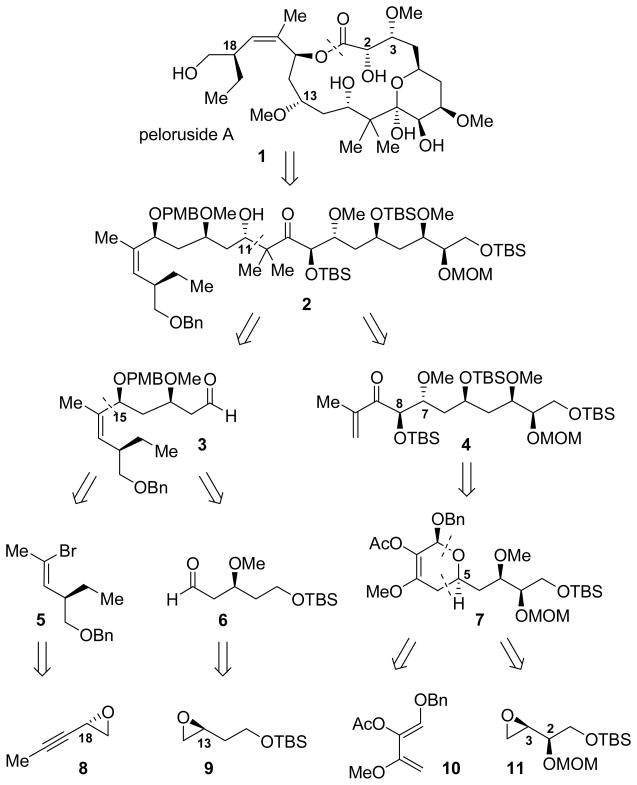
Retrosynthetic analysis of peloruside A
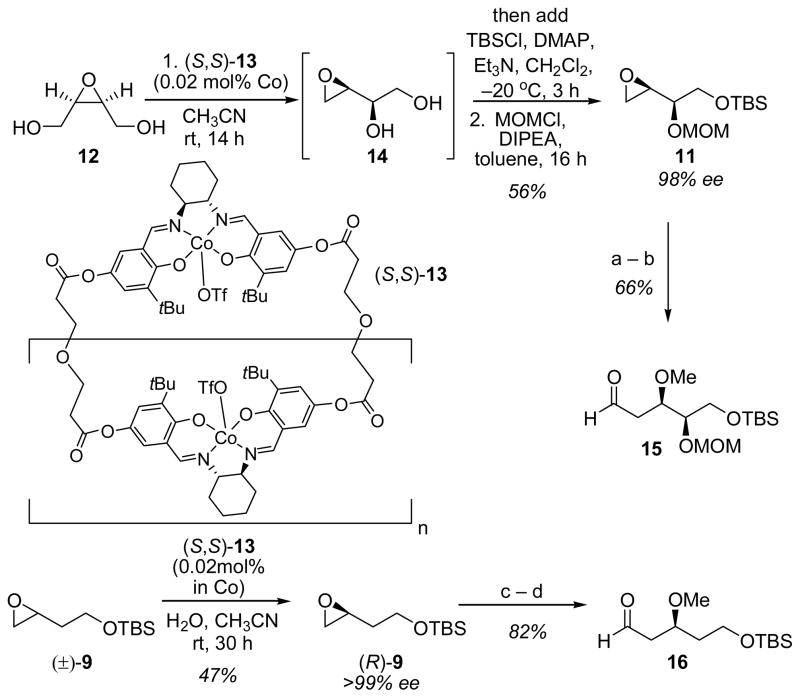
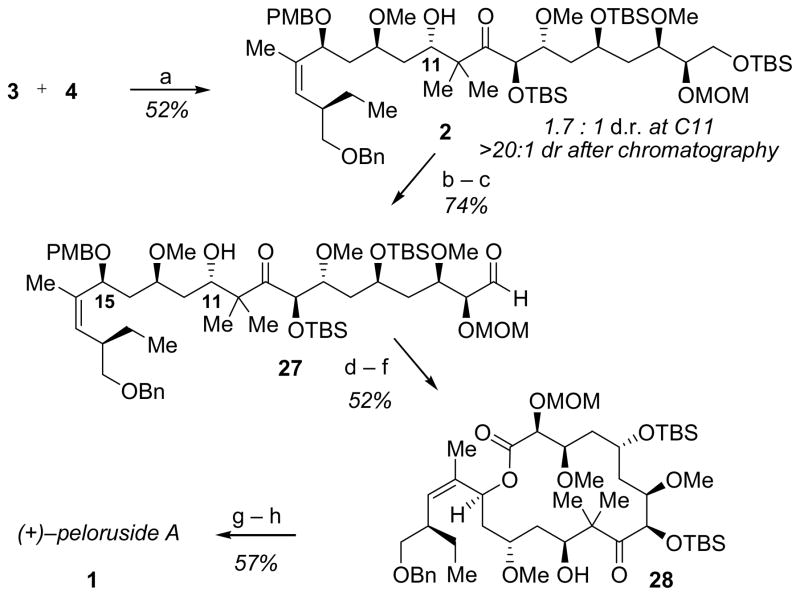
Dissection of the seco ester form of peloruside A into fragments of roughly equal size and complexity suggested aldehyde 3 and enone 4 as potentially useful late-stage intermediates (Figure 1).[6] The synthesis of enone 4 began with a highly enantioselective Payne rearrangement of meso-epoxy diol 12, available in one step from commercial cis-2,3-butenediol, to enantioenriched terminal epoxide 14 (Scheme 1).[7] This transformation was catalyzed by oligomeric cobalt salen catalyst 13,[8] which establishes an equilibrium favoring terminal epoxide 14 over meso epoxide 12 in a 7:3 ratio. Epoxide 14 was unstable to purification, but protection as the primary silyl ether in situ and subsequent alkylation of the secondary alcohol provided the functionally-rich bis-protected epoxide 11 in good overall yield.[9]
Scheme 1.
Asymmetric Payne rearrangement and elaboration. Reagents and conditions: a) CuBr (10 mol%), vinyl magnesium bromide, −40 °C, 2 h; then HMPA, Me2SO4, rt, 48 h; b) O3, CH2Cl2, −78 °C; then PPh3, rt, 3 h; c) CuBr (10 mol%), vinyl magnesium bromide, −40 °C, 2 h; then HMPA, Me2SO4, 4 °C, 48 h; d) O3, CH2Cl2, −78 °C; then PPh3, rt, 3 h. DIPEA=diisopropylethylamine, TBSCl=t-butyldimethylsilyl chloride, MOMCl=methoxymethyl chloride, HMPA= hexamethylphosphoramidite, PPh3=triphenylphosphine.
Epoxide 11 was subjected to a one-pot vinyl cuprate addition/methylation, followed by ozonolysis to provide aldehyde 15 in 66% overall yield. In an analogous manner, enantiopure aldehyde 16 was obtained from racemic epoxide 9 via a high-yielding hydrolytic kinetic resolution (HKR)/vinylation/alkyation/ozonolysis sequence.
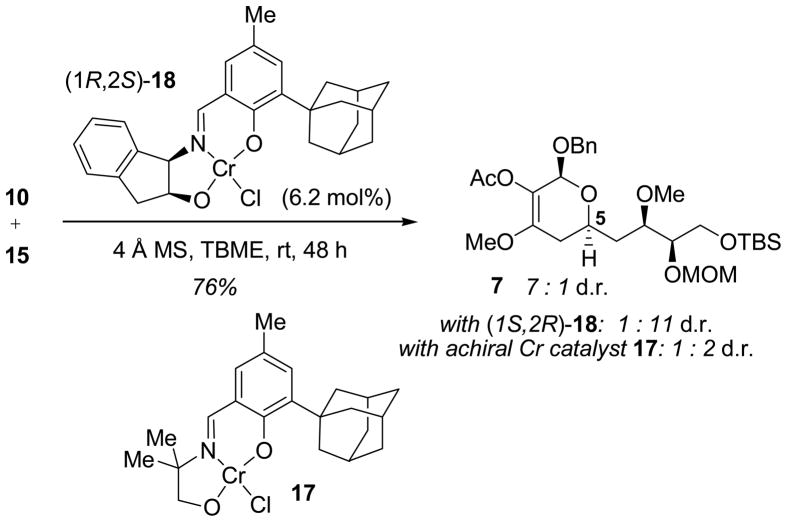
Aldehyde 15 was then engaged in a hetero-Diels–Alder (HDA) reaction with trioxy-substituted diene 10, available in two steps from methyl benzyloxyacetate (see Supporting Information). Diene 10 proved highly sensitive to decomposition in the presence of strong Lewis acids, but cycloadditions catalyzed by (Schiff-base)chromium complexes were found to proceed cleanly. The degree of intrinsic substrate diastereocontrol was poor, as reaction with achiral chromium catalyst 17 afforded cycloadduct in 1:2 dr favoring the undesired isomer. However, the chiral chromium Schiff-base complex (1R,2S)-18[10] catalyzed formation of the desired product 7 in good yield and 7:1 dr favoring the desired isomer. Conversely, the enantiomeric catalyst (1S,2R)-18 provided the undesired diastereomer in high (1:11) selectivity. This represents one of the most demanding applications reported to date of the application of catalyst 18 in an HDA reaction between stereochemically and functionally complex substrates.[11]
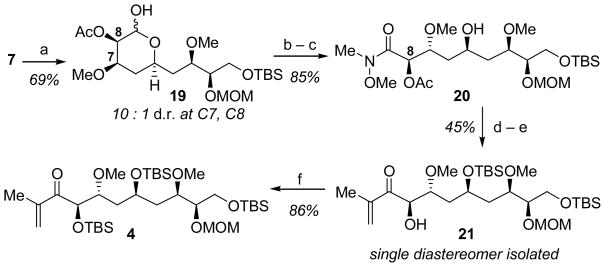
Hydrogenation of hetero-Diels–Alder adduct 7 took place diastereoselectively, and concomitant hydrogenolysis of the O-benzyl acetal provided 19 in 69% yield and in 10:1 dr.[12] Oxidation of lactol 19 and opening of the resulting lactone with N,O-dimethylamine hydrochloride afforded Weinreb amide 20, which was protected as the secondary TBS ether. Addition of isopropenylmagnesium bromide occurred with cleavage of the C8 acetate ester to provide hydroxyenone 21, which was purified chromatographically to >20:1 dr. The C8 hydroxyl group was then re-protected as the TBS ether to provide aldol coupling partner 4 (Scheme 3).
Scheme 3.
Elaboration of the hetero-Diels–Alder adduct 7 to enone 4. Reagents and conditions: a) Pd/C, i-PrOH, pH 7 buffer, H2 (200 psi), 48 h; b) KBr, TEMPO, NaOCl, pH 7 buffer, CH2Cl2, 0 °C, 90 min; c) N,O-dimethylamine hydrochloride, AlMe3, toluene, −10 °C, 90 min; d) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 2 h; e) isopropenyl-magnesium bromide, THF, 5 h; f) TBSOTf, 2,6-lutidine, CH2Cl2, −10 °C, 4 h. TEMPO=,2,6,6-Tetramethylpiperidine-1-oxyl (free radical), THF=tetrahydrofuran, TBSOTf=t-butyldimethylsilyl trifluoromethanesulfonate.
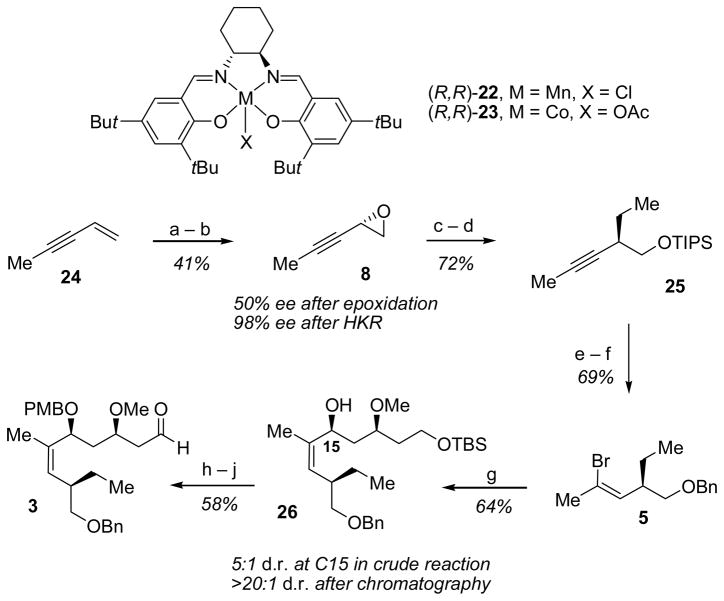
In the approach to aldehyde 3, epoxide 8 was prepared in high ee from enyne 24, available in two steps from commercial 3-pentyn-1-ol,[13] via a (salen)Mn-catalyzed epoxidation[14]/hydrolytic kinetic resolution (HKR) sequence.[15] Epoxide 8 was then opened stereospecifically and regioselectively at the propargylic position, and the resulting primary alcohol was protected as the triisopropylsilyl ether to provide alkyne 25 in 72% yield over two steps (Scheme 4). This strategy of opening a terminal epoxy-alkyne at the internal position with a simple Grignard reagent provides a concise and convenient method for the stereocontrolled synthesis of homopropargylic primary alcohols.
Scheme 4.
Synthesis of key aldehyde fragment 3. Reagents and conditions: a) 22 (5.0 mol%), NaOCl, CH2Cl2, 0 °C, 6.5 h;b) 23 (0.50 mol%), H2O, Et2O, 0 °C to rt, 24 h; c) ethylmagnesium chloride, THF, −78 °C to rt, 4 h; d) TIPSCl, imidazole, DMF, rt, 16 h; e) catecholborane, 40 to 50 °C, 48 h; then bromine, CH2Cl2, −78 °C, 10 min.; then TBAF, THF, 40 °C, 2.5 h; f) 2-benzyloxy-1-methylpyridinium triflate, MgO, trifluorotoluene, 83 °C, 24 h; g) sec-butyllithium, THF, Et2O, −78 °C; then 16, THF, −78 °C to rt, 16 h; h) PMBBr, NaH, DMF, rt, 2 h; i) acetic acid, H2O, THF, rt, 16 h; j) Dess–Martin periodinane, CH2Cl2, rt, 4 h. TBAF=tetrabutylammonium fluoride, TIPSCl=triisopropylsilyl chloride, PMBBr=p-methoxybenzyl bromide.
Silyl ether 25 was further elaborated to vinyl bromide 5 via a one-pot hydroboration/bromination/elimination/silyl-deprotection sequence (Scheme 4).[16] Protection of the resultant primary alcohol as the benzyl ether provided compound 5 in 69% overall yield from 25.[17] This protecting-group exchange on the C20 hydroxyl proved advantageous because a large silyl protecting group was required for attaining high regioselectivity (9:1) in the hydroboration of compound 25, while the presence of a benzyl protecting group led to improved diastereoselctivity in the addition of the vinyl lithium reagent derived from 5 into aldehyde 17. In this manner, alcohol 26 was obtained in 5:1 dr and isolated in 64% yield following chromatographic purification. In contrast, analogous silyl-protected vinyl bromides (TIPS, TBDPS) led to the corresponding allylic alcohols in only 2:1 dr. Alcohol 26 was then protected as the paramethoxybenzyl ether, and the primary alcohol was selectively unmasked and oxidized with the Dess-Martin periodinane to provide aldehyde 3 in 58% yield over the 3 steps.
Enone 4 and aldehyde 3 were coupled via a reductive aldol reaction similar to that utilized in the Ghosh synthesis of peloruside A[4] to afford 2 in 1.7:1 dr. Despite the modest stereoselectivity in this step, 2 could be isolated in diastereomerically pure form and in 52% yield following chromatographic purification (Scheme 5). The primary TBS ether was then removed selectively using buffered HF·pyridine and the resulting alchol oxidized to provide aldehyde 27 in 74% yield over the 2 steps. Aldehyde 27 was then oxidized to the corresponding acid, and the crude reaction mixture was subjected to pH 7 buffered DDQ to cleave the C15 PMB ether and afford the macrolaconization substrate. The seco acid was subjected without purification to Yamaguchi conditions to provide macrolactone 28 in 52% yield for the 3 steps from aldehyde 27. This macrolactonization strategy drew direct inspiration from the Evans approach to peloruside A employing a similarly protected seco acid,[5] wherein differentiation between free hydroxyl groups at C11 and C15 was also observed. Finally, the benzyl protecting group at the C20 hydroxyl was removed under transfer hydrogenolysis conditions, and a subsequent global deprotection of the remaining protecting groups under strongly acidic conditions[18] afforded (+)-peloruside A (1) in 57% isolated yield, with characterization data matching those reported for the natural product.[1]
Scheme 5.
Synthesis of (+)-peloruside A. Reagents and conditions: a) L-Selectride, THF, −78 °C, 2 h; then −40 °C, 2 h; b) HF·pyridine, pyridine, THF, 0 °C to rt, 2 h; c) bis-acetoxyiodobenzene, TEMPO, CH2Cl2, rt, 16 h; d) NaClO2, NaH2PO4, isoamylene, H2O, t-BuOH; e) DDQ, pH 7 buffer, CH2Cl2, 5 h; f) 2,4,6-trichlorobenzoyl chloride, DIPEA, THF, rt, 16 h; then DMAP, toluene, 60 °C, 48 h; g) Pd/C, formic acid, EtOAc, MeOH, rt, 1 h; h) 1N HCl, THF, rt, 16 h; then 4 N HCl, THF, rt, 3.5 h. DDQ=2,3-dichloro-5,6-dicyano-1,4-bezoquinone, DMAP=4-(dimethylamino)pyridine.
This convergent synthesis of (+)-peloruside A required 20 steps in the longest linear sequence from commercially available materials. The approach relies on the availability of both simple (e.g. 8 and 9) and relatively complex (i.e., 11) terminal epoxides via (salen)Co-catalyzed ring-opening reactions, and on chiral catalyst-induced diastereocontrol in a key hetero-Diels–Alder cycloaddition reaction between advanced intermediates. The route provides a useful illustration of the applicability of modern asymmetric catalytic methods in the total synthesis of stereochemically complex polyketides.
Experimental Section
Full experimental details are provided as Supporting Information.
Supplementary Material
Scheme 2.
Diastereoselective hetero-Diels–Alder Reaction.
Acknowledgments
This work was supported by the NIH (GM-59316 and GM-43214). We are grateful to Dr. Dieter Muri, Dr. Kristine Nolin, Ms. Tavé van Zyl, and Mr. Charles Goodhue for key experimental contributions, and to Prof. D. A. Evans, Prof. J. K. De Brabander, Dr. D. S. Welch, and Mr. A. W. S. Speed for helpful discussions.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Meredeth A. McGowan, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138
Christian P. Stevenson, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138
Matthew A. Schiffler, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138
Prof. Dr. Eric N. Jacobsen, Email: jacobsen@chemistry.harvard.edu, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138, Fax: (+1) 617-496-1880
References
- 1.a) West LM, Northcote PT. J Org Chem. 2000;65:445–449. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]; b) Hood KA, West LM, Rouwe B, Northcote PT, Berridge MV, Wakefield StJ, Miller JH. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- 2.Liao X, Wu Y, De Brabander JK. Angew Chem. 2003;115:1686–1652. doi: 10.1002/anie.200351145. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2003;42:1648–1652. doi: 10.1002/anie.200351145. [DOI] [PubMed] [Google Scholar]
- 3.a) Taylor RE, Jin M. Org Lett. 2005;7:1303–1305. doi: 10.1021/ol050070g. [DOI] [PubMed] [Google Scholar]; b) Taylor RE, Jin M. Org Lett. 2003;5:4959–4961. doi: 10.1021/ol0358814. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh AK, Xu X, Kim JH, Xu CX. Org Lett. 2008;10:1001–1004. doi: 10.1021/ol703091b. [DOI] [PubMed] [Google Scholar]
- 5.Evans DA, Welch DS, Speed AWS, Moniz GA, Reichelt A, Ho S. J Am Chem Soc. 2009;131:3840–3841. doi: 10.1021/ja900020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A similar fragment-coupling strategy was used by Ghosh and coworkers in their synthesis of peloruside A (see ref. 4).
- 7.Initial report: Wu MH, Hansen KB, Jacobsen EN. Angew Chem. 1999;111:2167–2170. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<2012::AID-ANIE2012>3.0.CO;2-H.Angew Chem Int Ed. 1999;38:2012–2014.
- 8.a) White DE, Jacobsen EN. Tetrahedron: Asymmetry. 2003;14:3633–3638. [Google Scholar]; b) Ready JM, Jacobsen EN. J Am Chem Soc. 2001;123:2687–2688. doi: 10.1021/ja005867b. [DOI] [PubMed] [Google Scholar]
- 9.A similar strategy to the acetonide-protected analog of 11 was employed by White and coworkers in the context of a general synthetic approach to hexose derivatives: D. J. Covell, N. A. Vermeulen, N. A. Labenz, M. C. White, Angew Chem. 2006, 118, 8391–8400; Angew. Chem. Int. Ed. 2006. 45, 8217–8220.
- 10.Dossetter AG, Jamison TF, Jacobsen EN. Angew Chem. 1999;111:2549–2552. doi: 10.1002/(sici)1521-3773(19990816)38:16<2398::aid-anie2398>3.0.co;2-e.Angew Chem, Int Ed. 1999;38:2398–2400. doi: 10.1002/(sici)1521-3773(19990816)38:16<2398::aid-anie2398>3.0.co;2-e.Gademann K, Chavez DE, Jacobsen EN. Angew Chem. 2002;114:3185–3187. doi: 10.1002/1521-3773(20020816)41:16<3059::AID-ANIE3059>3.0.CO;2-I.Angew Chem Int Ed. 2002;41:3059–3061. doi: 10.1002/1521-3773(20020816)41:16<3059::AID-ANIE3059>3.0.CO;2-I.Chavez DE, Jacobsen EN. Org Synth. 2004;82:34–39.For a recent review of applications of 18 in complex-molecule synthesis, see: Pellissier H. Tetrahedron. 2009;65:2839–2877.
- 11.For examples of catalyst-controlled diastereoselectivity in HDA reactions with chiral aldehydes catalyzed by 18, see: Joly GD, Jacobsen EN. Org Lett. 2002;4:1795–1798. doi: 10.1021/ol0258785.Wender PA, Baryza JL, Bennett CE, Bi FC, Brenner SE, Clarke MO, Horan JC, Kan C, Lacôte E, Lippa B, Nell PG, Turner TM. J Am Chem Soc. 2002;124:13648–13669. doi: 10.1021/ja027509+.Patterson I, Luckhurst CA. Tetrahedron Lett. 2003;44:3749–3754.Loh TP, Fu F. Tetrahedron Lett. 2009;50:3530–3533.
- 12.The minor diastereomer was the result of hydrogenation from the more hindered face of dihydropyran 7. For seminal examples of the use of stereoselective dihydropyran manipulation leading to acyclic stereochemical relationships, see: Meng D, Sorensen EJ, Bertinato P, Danishefsky SJ. J Org Chem. 1996;61:7998–7999. doi: 10.1021/jo961673w.Myles DC, Danishefsky SJ. Pure Appl Chem. 1989;61:1235–1242.Danishefsky SJ. Aldrichimica Acta. 1986;19:59–69.
- 13.Poutsma ML, Ibarbia PA. J Org Chem. 1970;35:4038–4047. [Google Scholar]
- 14.Jacobsen EN, Zhang W, Muci AR, Ecker JR, Deng L. J Am Chem Soc. 1991;131:7063–7064.Enyne epoxidation: Lee NH, Jacobsen EN. Tetrahedron Lett. 1991;32:6533–6536.
- 15.For an analysis of practical considerations associated with this type of two-step process, see: Brandes BD, Jacobsen EN. Tetrahedron: Asymmetry. 1997;8:3927–3933.
- 16.This reaction sequence was adapted from a similar method for converting an intetrnal alkyne to the Z vinyl bromide: Brown HC, Subrahmanyam C, Hamaoka T, Ravindran N, Bowman DH, Misumi S, Unni MK, Somayaji V, Bhat NG. J Org Chem. 1989;54:6068–6075.
- 17.The vinyl bromide was exteremely base-sensitive and required the mild benzylation conditions of the Dudley reagent: Poon KWC, Dudley GB. J Org Chem. 2006;71:3923–3927. doi: 10.1021/jo0602773.
- 18.An analogous deprotection strategy was adopted by Ghosh et al. See Ref. 4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.