Abstract
Background
Myocardial fibrosis reflects excess collagen deposition in the extracellular left ventricular (LV) matrix which has been associated with heart failure (HF). No studies have addressed the relation between fibrosis biomarkers and HF in the elderly.
Methods and Results
Serum fibrosis markers were measured in 880 participants of the Cardiovascular Health Study (mean age 77 ± 6 yrs, 48% female). Participants with systolic HF (n = 131, LV ejection fraction < 55%) and those with diastolic HF (n = 179, LV ejection fraction ≥ 55%) were compared to controls (280 with cardiovascular risk factors, and 279 healthy individuals) using a nested case-control design. Fibrosis markers included carboxyl-terminal peptide of procollagen type I (PIP), carboxyl-terminal telopeptide of collagen type I (CITP), and amino-terminal peptide of procollagen type III (PIIINP). Echocardiography was used to document systolic and diastolic function parameters. Analysis of variance and logistic regression (per tertile odds ratios: OR), adjusted by age, gender, race, hypertension, atrial fibrillation, coronary heart disease, baseline serum glucose, serum cystatin C, serum creatinine, C-reactive protein, any ACE inhibitor, spironolactone or any diuretic, NT-proBNP and total bone mineral density were performed.
Systolic HF was associated with significantly elevated CITP (OR = 2.6; 95%CI = 1.2–5.7) and PIIINP (OR = 3.3; 95% CI = 1.6 −5.8), when adjusting for covariates. Associations of diastolic HF were significant for CITP (OR = 3.9, 95% CI = 1.9–8.3) and PIIINP (OR = 2.7; 95% CI = 1.4–5.4). HF was not associated with elevated PIP (p > 0.10), and fibrosis markers did not significantly differ between HF with diastolic versus those with systolic dysfunction (p values > 0.10) whereas NT- proBNP mean values were higher in SHF than in DHF (p <0.0001).
Conclusions
Fibrosis markers are significantly elevated in elderly individuals with diastolic or systolic HF. These associations remained significant when adjusting for covariates relevant to the aging process.
Keywords: heart failure, collagen, population
INTRODUCTION
Myocardial connective tissue plays an important role in defining and preserving normal myocardial architecture and function (1). Excess deposition of collagen in extracellular matrix can lead to increased myocardial stiffness and subsequently to cardiac hypertrophy and left ventricular dysfunction. These pathological changes increase the risk of heart failure and provide an anatomic substrate for life-threatening cardiac arrhythmias (1,2). Type I and III collagens are the major fibrillar collagens in both normal and diseased myocardium (3–4). Both are synthesized as procollagens with a small amino terminal and a larger carboxy terminal propeptide. Serum markers of fibrosis reflecting collagen synthesis include carboxy-terminal propeptide of type I procollagen (PIP) and amino-terminal propeptide of type III procollagen (PIIINP), and degradation markers include carboxy-terminal telopeptide of collagen type I (CITP). Elevations in these fibrosis markers have been shown to reflect intra-myocardial collagen turnover (5–6).
Increased collagen synthesis/degradation has been found in heart failure (HF) (5–6), but the effect of aging on myocardial fibrosis and the role of fibrosis in diastolic and systolic HF in the elderly has not been established. We therefore examined whether fibrosis markers (CITP, PIP and PIIINP) are elevated in HF with systolic dysfunction versus HF with preserved systolic function, and examined whether previously defined predictors of HF (7) mediate the relationship between fibrosis markers and HF. Because osteoporosis is common in the elderly and is associated with increases in fibrosis markers (8–10), we also examined the influence of bone mineral density on the relation between HF and fibrosis markers.
METHODS
Participants and study design
The Cardiovascular Health Study (CHS) is a prospective, community-based, epidemiologic observational study designed to assess cardiovascular risk factors and outcomes in individuals ≥ 65 years old. The design and the rationale of the CHS have been published elsewhere (11). Briefly, 5,201 participants ≥ 65 years old were enrolled in 1989 and 1990, with an additional cohort of 687 ethnic minority participants enrolled in 1992 and 1993. Exclusion criteria were: hospice treatment, wheel-chair bound in the home, and radiation or chemotherapy for cancer. For purposes of the present study, history of chronic liver disease or pulmonary disease were used as additional exclusion criteria to minimize confounding effects of these diseases on fibrosis markers. The baseline examination (1989–1990) included an extensive physical examination, 12–lead electrocardiography, spirometry, and echocardiography. Laboratory assessments included serum-chemistry tests, creatinine, cystatin C, oral glucose-tolerance testing, lipid profile, inflammatory markers. Prevalent coronary heart disease was considered if there was a clinical history of myocardial infarction, angina, coronary-artery bypass grafting, or angioplasty. Prevalent disease status was updated based on adjudicated incident events throughout the study.
Fibrosis markers were assessed in 880 participants, selected using a nested case control design based on HF status of the total CHS cohort (N=5888). Assays for fibrosis markers were obtained in CHS Year 5 (1992–1993; n = 638) or CHS Year 9 (1996–1997; n = 242). All clinical and biochemistry measures were obtained at the same evaluation visit for each patient (CHS year 5 or 9) to ensure simultaneous assessments of fibrosis markers and covariates. The presence of HF, prevalent at the 1993–4 examination cycle or before, was determined by expert adjudication of clinical records, and updated to reflect clinical status in CHS Year 9 (1996–1997).
Four groups were examined: (1) HF with systolic dysfunction (decreased left ventricular ejection fraction –LVEF- < 55%; n = 131); (2) HF with preserved systolic function (LVEF≥ 55%; n = 179); (3) Controls with cardiovascular disease risk factors but without HF (Control; n = 280); and (4) Controls (Healthy Control) without HF, and also without hypertension, diabetes, or hypercholesterolemia (n = 279). Prevalent cardiovascular risk factors in the Control – cardiovascular risk factors group were based on the overall prevalence of diabetes, hypertension, and hypercholesterolemia in the CHS. Prevalent cardiovascular risk factors in the Control group included diabetes, hypertension, and hypercholesterolemia.
All echocardiograms were interpreted at a centralized core echocardiography laboratory (Georgetown University, Washington, D.C.). Qualitative LVEF was estimated based on echocardiographic data obtained either at the baseline CHS examination or at the point of care as abstracted from clinical records. LV mass was estimated by ECG using methods published elsewhere (12). Systolic HF was defined as HF with a documented LVEF < 55%, and HF with preserved systolic function (diastolic HF) as a positive history of HF with a LVEF ≥ 55% (7).
Determination of plasma CITP, PIP, and PIIINP
Phlebotomy methods, blood processing, and handling of samples have been described previously (11). Aliquots were frozen at −70°C until analysis.
CITP was measured using the CITP RIA from Orion Diagnostica on serum samples. Intra-and inter-assay variability are 3.5–9.5% and 5.6–9.0%, respectively. The lower detection limit is 0.4 ug/L.
PIP was measured using an enzyme immunoassay kit (Takara Mirus Bio Inc., Madison, WI). The assay range is 10 – 640 ng/mL with a lower detection limit of 10 µg/L. Intra-assay and inter-assay CVs range from 4.5–7.4% and 4.3–6.3%, respectively.
PIIINP was determined by a coated-tube radioimmunoassay as described previously by Risteli et al (13), using commercial antisera specifically directed against the terminal amino terminal peptide (Orion Diagnostica, Finland). The interassay and intra-assay variations for determining PIIIP are both about 5%. The sensitivity (lower detection limit) is 1.5 µg/L.
Bone mineral density
Total bone mineral density (head, left and right arm, left and right rib, left and right leg, thoracic spine, lumbar spine and pelvic) was measured in a subset of 273 participants as part of an ancillary study by dual-energy x-ray absorptiometry (DXA;QDR 2000 or 2000+;Hologic, Inc, Bedford, MA). Standardized positioning and utilization of QDR software was based on the manufacturer’s recommended protocol. Scans were read blindly at the University of California, San Francisco reading center with Hologic software version 7.10 (14).
NT-proBNP
This biomarker was measured in serum collected in 1992–1993 from the main CHS and supplementary minority cohorts and measured on the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN). The coefficient of variation for the NT-proBNP assay was 2–5% during the testing period, and the analytical measurement range for NT-proBNP was 5–35,000 pg/mL.
Covariates
Demographic, clinical , and laboratory covariates utilized in analyses were: age, gender, race, diabetes, hypertension, hyperlipidemia, atrial fibrillation, myocardial revascularization, stroke or TIA, coronary heart disease, myocardial infarction, total bone mineral density, baseline serum glucose, 2-hour glucose tolerance test, baseline serum insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, serum creatinine, urine microalbumin, microalbumin/creatinine ratio, cystatin C, hemoglobin, hematocrit, white blood count, C-reactive protein, albumin, fibrinogen, factor VII, NT-proBNP, and self report of current use of ACE-inhibitors, spironolactone, or any diuretics. To assure the reliability of medication taken, the medication inventory method has been employed utilizing specially-written computer program (15).
The echocardiographic variables analyzed were LV diastolic dimension, LVEF, septal and posterior wall thickness, left atrial antero-posterior systolic diameter, end-systolic wall stress, peak early and late diastolic transmitral flow velocity, E/A.
Statistical Analysis
Data are presented as mean ± standard deviation or percentages. Biomarkers were not normally distributed and results are therefore presented as medians with interquartile ranges. Group comparisons were made using non-parametric Kruskal-Wallis tests or ANOVA for continuous variables and chi-square test for dichotomous variables, using Bonferroni corrections for multiple comparisons. To adjust for effects of covariates which were significant in table 1, logistic regression methods were used and odd ratios per tertile were reported for each fibrosis marker, using HF status as outcome measure and the healthy control group as reference. Five logistic regression models were performed: model 1 unadjusted; model 2 adjusted for age, gender and race; model 3 adjusted for age, gender, race, and bone mineral density; model 4 adjusted for age, gender, race, hypertension, atrial fibrillation, coronary heart disease, baseline serum glucose, serum cystatin C, serum creatinine, C reactive protein, use of ACE inhibitor, spironolactone or any diuretic, and model 5: adjusted by age, gender, total cholesterol, triglycerides, diabetes, hypertension.
Table 1.
Demographic, Clinical and Biochemistry Variables by Study Group
| VARIABLE | Healthy Control N = 279 | Control N = 280 | Diastolic Heart Failure N = 179 | Systolic Heart Failure N = 131 | p-value | Pair wise comparison |
|---|---|---|---|---|---|---|
| Age | 78 ± 6 | 76 ± 6 | 76 ± 5 | 77 ± 6 | 0.01 | 2* |
| Gender: Male (%) | 51 | 52 | 45 | 63 | 0.02 | 3*,5*,6* |
| Race: White (%) | 95 | 55 | 85 | 88 | <0.0001 | 1†,2‡,3‡,4‡,5‡ |
| Diabetes (%) | 0 | 21 | 27 | 30 | <0.0001 | 1‡,2‡,3†,4* |
| Hypertension (%) | 1 | 57 | 53 | 48 | <0.0001 | 1‡,2‡,3‡,4†,5‡ |
| Total cholesterol (mg/dl) | 198±36 | 199±39 | 194±41 | 195±44 | 0.62 | NS |
| LDL-cholesterol (mg/dl) | 127±35 | 127±36 | 120±33 | 122±32 | 0.06 | NS |
| HDL-cholesterol (mg/dl) | 55±14 | 53±14 | 50±14 | 47±12 | <0.0001 | 2†,3‡,4‡ |
| Triglyceride (mg/dl) | 127±60 | 132±88 | 154±87 | 168±12 | <0.001 | 2†,3‡,4*,5‡ |
| Atrial fibrillation (%) | 1 | 2 | 10 | 9 | 0.0007 | 2‡,3‡,4‡,5‡ |
| Stroke or TIA (%) | 0 | 13 | 21 | 29 | 0.001 | 1‡,2‡,3‡,4*,5‡ |
| Myocardial revascularization (%) | 0 | 9 | 25 | 36 | <0.0001 | 1‡,2‡,3‡,4‡,5‡ |
| Coronary Heart Disease (%) | 0 | 28 | 66 | 83 | <0.0001 | 1‡,2‡3‡,4‡,5‡,6‡ |
| Myocardial Infarction (%) | 0 | 14 | 38 | 64 | <0.0001 | 1‡,2‡3‡,4‡,5‡,6‡ |
| Left ventricular hypertrophy (%) | 2 | 10 | 10 | 17 | <0.0001 | 1‡,2‡3‡ |
| PIP (ng/mL)* | 403 | 384 | 395 | 406 | 0.25 | NS |
| (336,491) | (321, 467) | (329, 503) | (353, 477) | |||
| CITP (µg/L )* | 3.8 | 4.5 | 5.9 | 5.7 | <0.0001 | 1‡,2‡,3‡,4‡,5‡ |
| (2.8, 5.3) | (3.2, 5.9) | (4.3, 8.6) | (4.0, 8.3) | |||
| PIIINP (µg/L)* | 3.1 | 3.8 | 4.2 | 4.7 | <0.0001 | 1‡,2‡,3‡,4‡,5‡ |
| (2.4,4.0) | (2.8,5.0) | (3.0, 6.9) | (3.4, 7.2) | |||
| PIP / CITP* | 111 | 90 | 68 | 72 | <0.0001 | 1‡,2‡,3‡,4‡,5† |
| (74, 149) | (62,119) | (45, 93) | (51, 104) | |||
| Baseline serum glucose (mg/dl) | 95 | 100 | 103 | 102 | <0.0001 | 1‡,2‡,3‡ |
| (90,100) | (91,114) | (94,122) | (92,124) | |||
| Two-hour glucose tolerance test (mg/dl) | 123±35 | 156±64 | 161±71 | 167±87 | <0.0001 | 1‡,2‡,3‡ |
| Baseline serum insulin (IU/ml) | 8 | 10 | 11 | 11 | <0.0001 | 1‡,2‡,3‡ |
| (6,11) | (8,15) | (8,17) | (7,17) | |||
| Serum creatinine (mg/dl) | 1.00±0.2 | 1.05±0.3 | 1.09±0.33 | 1.2±0.5 | <0.0001 | 2*,3‡,5‡,6† |
| Cystatin C (mg/l) | 1.09±0.2 | 1.09±0.28 | 1.25±0.37 | 1.4±0.5 | <0.0001 | 2‡,3‡,4‡,5‡,6† |
| Microalbumin/Creatinine (mg/g ) | 7 | 12 | 19 | 18 | <0.0001 | 1‡,2‡,3‡,4‡,5‡ |
| (5,13) | (6,27) | (8,64) | (10,56) | |||
| Serum microalbumin (mg/dl) | 0.6 | 1.1 | 1.5 | 1.8 | <0.0001 | 1‡,2‡,3‡,5* |
| (0.3,1.2) | (0.5,3.0) | (0.5,6.4) | (0.6,6.1) | |||
| Hemoglobin (g/dl) | 13.6±1.3 | 13.7±1.5 | 13.3±1.5 | 13.5±1.5 | 0.03 | 3* |
| Hematocrit (%) | 40±4 | 41±4 | 40±4 | 40±4 | 0.15 | NS |
| White blood count (x1,000/ mm3) | 6.00±1.4 | 5.9±1.8 | 6.8±2.2 | 6.98±1.7 | <0.0001 | 2‡,3‡,4‡,5‡ |
| C-reactive protein (mg/l) | 1.7 | 2.8 | 4.1 | 3.3 | <0.0001 | 1‡,2‡,3‡,4*,5* |
| (0.8,4.4) | (1.2,5.5) | (1.4,9.1) | (1.5,7.7) | |||
| Albumin (g/dl) | 3.93±0.27 | 3.96±0.27 | 3.92±0.28 | 3.94±0.29 | 0.28 | NS |
| Fibrinogen (mg/dl) | 323±66 | 328±65 | 341±79 | 346±76 | 0.005 | 2*,3* |
| Factor VII (%) | 110±22 | 106±26 | 108±32 | 102±33 | 0.05 | 3* |
| Any ACE inhibitor (%) | 0.4 | 15 | 28 | 45 | <0.0001 | 1‡,2‡3‡,4‡,5‡,6‡ |
| Potassium-sparing agents (%) | 0.4 | 0 | 1.2 | 0.9 | 0.34 | NS |
| Any diuretic (%) | 8 | 33 | 53 | 66 | <0.0001 | 1‡,2‡,3‡,4‡,5‡,6* |
| VARIABLE | Healthy Control N = 82 | Control N = 108 | Diastolic Heart Failure N = 52 | Systolic Heart Failure N = 31 | p-value | Pair wise comparison |
| Total bone mineral density (g/cm2) | 0.99 ± 0.14 | 1.08 ± 0.15 | 1.04 ± 0.16 | 1.11 ± 0.16 | <0.0001 | 1‡,3‡ |
| VARIABLE | Healthy Control N = 217 | Control N = 220 | Diastolic Heart Failure N = 147 | Systolic Heart Failure N = 95 | p-value | Pair wise comparison |
| NT-proBNP (pg/ml) | 106 (60,207) | 128 (66,233) | 300 (136,602) | 841 (340,233) | <0.0001 | 2‡,3‡,4‡,5‡,6‡ |
mean ± SD for normal distributed continuous variables; the median value (interquartile range) for skewed continuous variables; the percentage for categorical variables
1=healthy control vs.control, 2=healthy control vs. DHF, 3= healthy control vs. SHF, 4 = control vs. DHF, 5 = control vs. SHF, 6 = DHD vs. SHF
p < 0.05
p < 0.01
p < 0.001
Two-sided p-values are reported with p < 0.05 considered as statistically significant. All analyses were performed using SAS 9.1.3 (SAS Institute Inc., Cary, North Carolina).
The study was approved by the institutional review committees of the participating medical centers. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Subjects characteristics
Table 1 shows demographic, clinical and laboratory variables. Compared to the healthy control group, participants in the HF groups had higher prevalence of coronary heart disease, stroke, atrial fibrillation, and worse metabolic, renal and inflammatory measures. ACE-inhibitors and diuretics were used more in the HF groups compared to both control groups whereas the difference between participants with HF and controls in the use of potassium–sparing agents did not reach statistical significance.
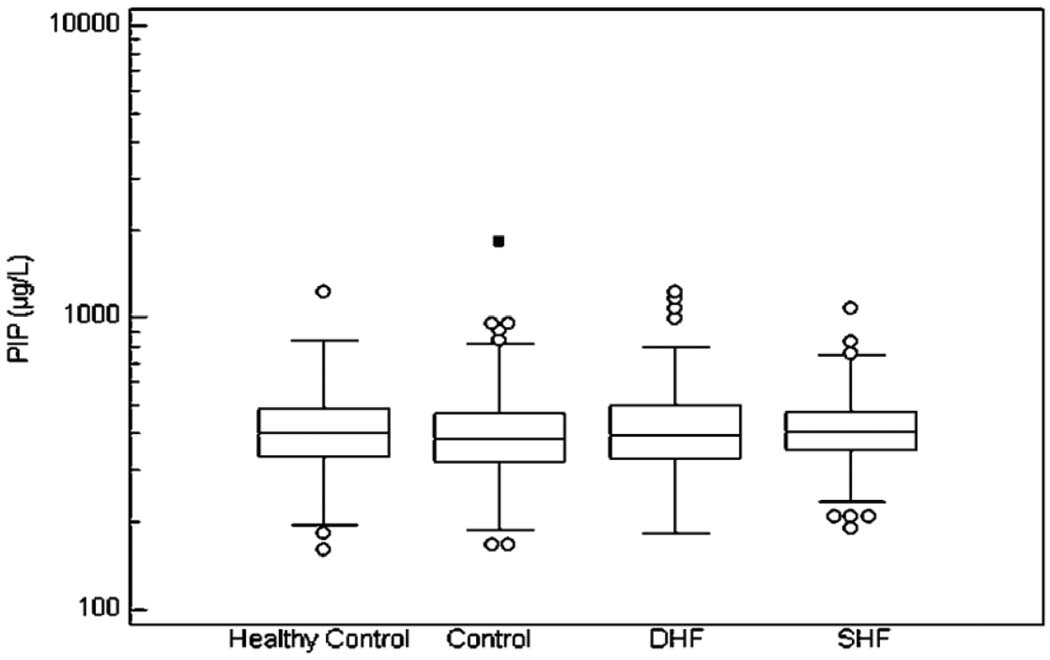
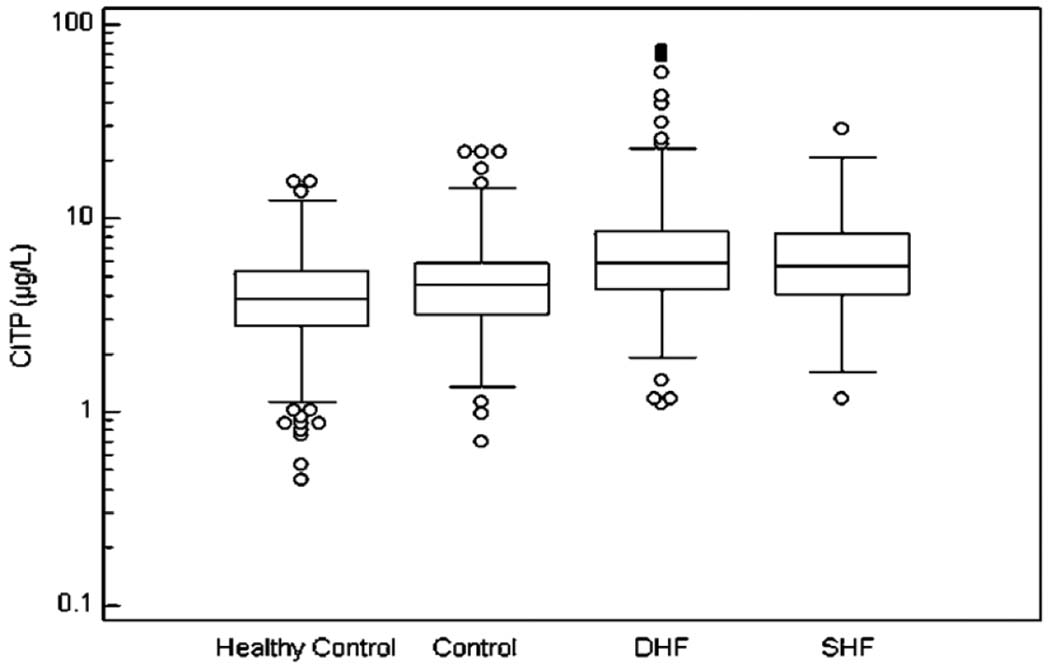
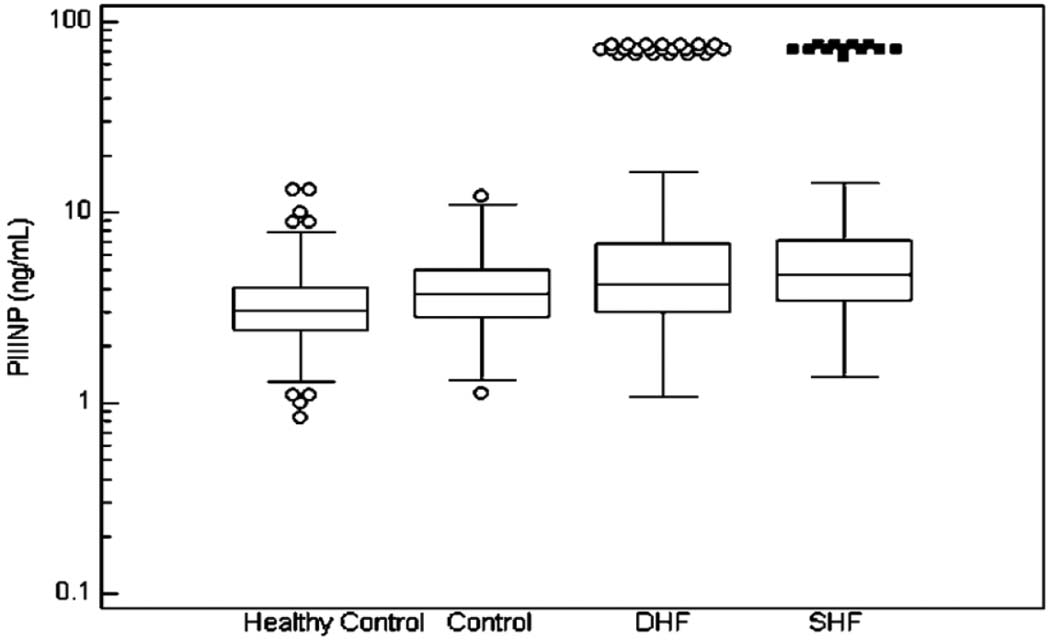
The median and interquartile range of the fibrosis markers in the 4 analyzed groups are illustrated in Figure 1, Figure 2, and Figure 3.
Figure 1.
The Median Values and Interquartile Range of PIP. No differences have been found between the 4 groups.
Figure 2.
The Median Values and Interquartile Range of CITP. Significant differences have been found between: a) healthy control group and diastolic HF, systolic HF and control group (p<0.0001, p<0.0001, and p=0.0006, respectively); b) control group and diastolic HF and systolic HF (p<0.0001 for both).
Figure 3.
The Median Values and Interquartile Range of PIIINP.
Significant differences have been found between: a) healthy control group and diastolic HF, systolic HF and control (p<0.0001 for all three); b) control group and diastolic HF and systolic HF ( p=0.0008 and p<0.0001, respectively).
In the Box-and-whisker plot, the central box represents the values from the lower to upper quartile (25 to 75 percentile). The middle line represents the median. The horizontal line extends from the minimum to the maximum value, excluding outside and far out values which are displayed as separate points.
Relationship between heart failure and fibrosis markers
CITP and PIIINP were significantly higher in both HF groups compared to both control groups (p < 0.0001), whereas differences in PIP were not significant (p= 0.40). No differences were found between systolic HF versus diastolic HF (p > 0.20). Controls with CV disease had higher values of CITP (p =0.0006) and PIIINP (p <0.0001 ) than healthy controls.
In the multivariate adjusted logistic regression models, systolic HF was associated with increased presence of elevated CITP and PIIINP (OR = 2.6 and 2.9, respectively; Table 2). Diastolic HF was associated with increased prevalence of elevated CITP and PIIINP (OR per tertile = 3.8 and 2.5, respectively).
Table 2.
The Association Between Serum Fibrosis Biomarkers with Heart Failure Groups Analyzed by Tertiles
| HEART FAILURE TYPE | CITP OR (95% CI) | PIIINP (OR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Diastolic HF | 3.1 (2.4, 4.0) | 3.5 (2.6, 4.6) | 2.3 (1.5,3.8) | 3.9 (1.9,8.3) | 2.7 (1.9, 4.0) | 2.2 (1.7, 2.8) | 2.4 (1.9, 3.1) | 2.1 (1.4, 3.5) | 2.7 (1.4, 5.4) | 2.5 (1.7, 3.7) |
| Systolic HF | 2.6 (2.0, 3.4) | 2.9 (2.2, 3.9) | 2.9 (1.6, 5.6) | 2.6 (1.2, 5.7) | 2.3 (1.6, 3.3) | 2.7 (2.0, 3.5) | 2.7 (2.0, 3.6) | 2.1 (1.2, 4.0) | 3.3 (1.6, 5.8) | 2.8 (1.9, 4.2) |
Model 1: unadjusted logistic regression model
Model 2: adjusted by age, gender and race
Model 3: adjusted by age, gender, race and bone mineral density
Model 4: adjusted by age, gender, race, hypertension, atrial fibrillation, CHD, baseline serum glucose, serum cystatin C, serum creatinine, C reactive protein, any ACE inhibitor, spironolactone or any diuretic
Model 5: adjusted by age, gender, total cholesterol, triglycerides, diabetes, hypertension
Association of Serum Fibrosis Markers with Age, Gender, and Race
No association was found between age and fibrosis markers in participants with systolic HF. Among individuals with diastolic HF, CITP was associated with age (r =0.15, p = 0.05). Similar results were found for CITP in controls with cardiovascular risk factors (r= 0.27, p < 0.0001) and in healthy controls (r = 0.19, p = 0.001). PIP was associated with age in participants with diastolic HF (r = 0.25, p=0.001), but not in other groups. PIIINP was weakly associated with age in the control group (r = 0.12, p = 0.04).
In systolic HF, males had higher PIIINP levels than females (5.3 vs. 3.7µg/L, p = 0.0002) whereas, in diastolic HF, females had higher levels of PIP than males (419 vs. 370 ng/mL, p = 0.03). The same relationships were maintained in controls, where PIP was also higher in females than in males (431 vs. 380 µg/L, p =0.001) and serum levels of PIIINP were higher in males than in females (3.3 vs. 2.9 ng/mL, p = 0.004). No gender differences in fibrosis markers were observed among healthy controls.
Differences between White versus Black participants were observed only in the healthy control group: CITP and PIIINP were higher in Black than in White participants (4.7 vs. 4.1 µg/L, p = 0.04 and 4.3 vs. 3.5 µg/L, p = 0.002, respectively).
Association of Fibrosis Markers with Echocardiographic Variables
Table 3 presents the echocardiographic variables analyzed and the comparison of their mean values between the 4 groups. In general, there was a modest correlation between the echo-Doppler variables and fibrosis markers. The strongest correlations were between PIP with LVEF (r = −0.22, p =0.007), CITP with interventricular septum diastolic thickness (IVS) (r = 0.23, p= 0.01) and PIIINP with IVS (r = 0.25, p = 0.006) in the participants with DHF. In the control group, PIIINP was associated with LA diameter (r = 0.15, p =0.02). In the healthy control group, the only association was found between PIIINP with peak velocity of E wave (r = 0.18, p = 0.01), and in the participants with SHF, no association was found between fibrosis markers and the echo-Doppler parameters.
Table 3.
The Echocardiographic Data of the Participants Population
| VARIABLE | Healthy Control N = 279 | Control N = 280 | Diastolic Heart Failure N = 179 | Systolic Heart Failure N = 131 | p-value | Pair wise comparison |
|---|---|---|---|---|---|---|
| LV end –diastolic diameter (cm) | 4.6±0.6 | 4.8± 0.6 | 4.9 ±0.6 | 6.0± 1.0 | <0.0001 | 1*,2†,3‡,5‡,6‡ |
| LV end-systolic diameter (cm) | 2.7±0.6 | 2.7±0.7 | 3±0.7 | 4.5±1.2 | <0.0001 | 2†,3‡,4*,5‡,6‡ |
| Posterior wall diastolic thickness (cm) | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | <0.0001 | 1†,2‡,3‡ |
| Interventricular septum diastolic thickness (cm) | 0.9±0.1 | 0.9±0.1 | 1.0±0.2 | 0.9±0.2 | 0.0003 | 1†,2‡ |
| Left atrial anterior-posterior wall diameter (cm) | 3.9±0.6 | 4.1±0.7 | 4.3±0.7 | 4.4±0.7 | <0.0001 | 1†,2‡,3‡,4‡,5† |
| ESWS (dyne.cm−5) | 52.5±17.4 | 56±29 | 62.4±28.5 | 110.4±46.6 | <0.0001 | 2†,3†,4*,5† |
| Peak velocity E wave (cm/sec) | 0.67±0.17 | 0.68±0.17 | 0.74±0.22 | 0.75±0.28 | 0.002 | 2†,3‡,5‡,6‡ |
| Peak velocity A wave (cm/sec) | 0.79±0.19 | 0.82±0.18 | 0.83±0.22 | 0.82±0.29 | 0.49 | NS |
| E/A | 1.05±0.75 | 0.93±0.42 | 0.86±0.22 | 0.93±0.42 | 0.0002 | 3‡,5‡,6* |
ESWS = left ventricular end-systolic wall stress
1 = healthy control vs. control, 2 = healthy control vs. DHF, 3 = healthy control vs. SHF, 4 = control vs. DHF, 5 = control vs. SHF, 6 = DHD vs. SHF
p < 0.05
p < 0.01
p < 0.001
NS = non significant
Association of Fibrosis Markers with Renal Function, Inflammatory Markers, NT-proBNP and Total Bone Mineral Density
Table 4 shows CITP was associated with renal function measures, whereas PIP displayed weak inverse correlations with serum creatinine. In the multivariate adjusted model (age, gender, race, hypertension, diabetes, coronary artery disease, spironolactone, any diuretic, any ACE-inhibitor) the strongest association was found between CITP and cystatin C in both HF groups.
Table 4.
Correlation Coefficients of the Analyses Between Serum Biomarkers of Fibrosis and Renal Function Parameters and Inflammatory Markers
| VARIABLE | HEALTHY CONTROL | CONTROL | DIASTOLIC HEART FAILURE | SYSTOLIC HEART FAILURE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIP | CITP | PIIINP | PIP | CITP | PIIINP | PIP | CITP | PIIINP | PIP | CITP | PIIINP | |
| Serum Creatinine | −0.16* | 0.10 | 0.22 | 0.11 | 0.26* | 0.29* | 0.03 | 0.32*† | 0.15‡ | −0.10 | 0.36*† | 0.25* |
| Cystatin C | −0.05 | 0.30* | 0.35* | 0.06 | 0.36* | 0.23* | 0.07 | 0.47*† | 0.20*§ | −0.006 | 0.38*† | 0.15 |
| Microalbumin (urine) | 0.01 | 0.16* | 0.13 | −0.09 | 0.12 | 0.09 | 0.05 | 0.20* | 0.13 | 0.08¶ | 0.1 | 0.04 |
| Microalbumin/ creatinine urine | 0.06 | 0.21* | 0.11 | −0.10 | 0.09 | 0.06 | 0.08 | 0.19* | 0.12 | 0.18 | 0.21‡ | 0.13 |
| C-reactive protein (mg/l) | −0.13¶ | 0.12¶ | 0.13‡ | −0.05 | 0.05 | 0.02 | −0.18‡ | 0.08 | −0.01 | −0.22‡ | −0.12 | −0.06 |
| Fibrinogen | −0.03 | 0.17* | 0.11 | −0.09 | 0.17† | −0.05 | −0.11 | 0.11 | −0.18* | −0.25* | 0.02 | −0.02 |
< 0.05. In analyses adjusted for age, gender, race, hypertension, diabetes, coronary artery disease, spironolactone, any diuretic, any ACE-inhibitor
p<0.0001
p=0.02
p =0.01
p=0.04
Inflammatory markers included C-reactive protein (CRP) and fibrinogen (Table 4). In the systolic HF group, PIP was inversely correlated with CRP and fibrinogen, whereas other correlations were non-significant. In diastolic HF, PIP was inversely associated with CRP and PIIINP with fibrinogen. In the control group with cardiovascular risk factors, CITP was associated with fibrinogen, whereas in healthy controls, an inverse association was found between PIP and CRP, whereas CITP was positively associated with CRP and fibrinogen and PIIINP with CRP.
NT-proBNP was measured in 484 participants and those with SHF had higher serum levels than those with DHF (p<0.0001). Pearson’s correlation coefficients between NT-proBNP and serum level of PIP, CITP and PIIINP were 0.11, 0.37 and 0.28, with p-values 0.02, and < 0.001 for the last 2 markers, respectively. The strongest correlations were found between NT-proBNP and CITP in DHF and SHF groups (r = 0.40, p< 0.001 and r = 0.41, p=0.001, respectively. The correlations remained significant when adjusting for covariates (age, sex, race, heart failure status, coronary heart disease, hypertension, diabetes, smoking, physical activity, and BMI).
Of all 3 markers evaluated, only CITP and PIIINP were associated with heart failure when adjusted for NT-proBNP (p=0.01 and 0.003, respectively).
There were significant differences in total bone mineral density between systolic HF and healthy controls (p = 0.0002) and between healthy control and heart failure free control groups (p<0.0001). However, total bone mineral density which was slightly increased in systolic HF group, was significantly correlated with CITP in these participants (r = 0.47, p= 0.007). No other significant associations between the total bone mineral density and fibrosis markers were found. Adjusting for bone mineral density did not affect the association of CITP and PIIINP with HF. Therefore osteoporosis was not a confounding variable of this relationship and did to contribute to the increase in CITP and PIIINP in HF patients.
DISCUSSION
The principal finding of this study is that serum markers of fibrosis, (CITP and PIIINP) are associated with prevalent systolic as well as diastolic HF in community-dwelling elderly individuals. These associations remained significant even after adjustment for demographic and clinical characteristics, as well as bone mineral density and NT-proBNP.
The present observations are consistent with the hypothesis that enhanced collagen type I degradation plays a role in elderly with HF. Collagen type I constitutes the majority of the cardiac interstitium, but PIP - the serum marker of collagen type I - was not associated with HF in the present investigation. While some studies have found associations between PIP and HF (5, 16–18) other studies have not (6, 19, 20). Cardiac aging is characterized by loss of myocytes which could explain the accumulation of collagen in the ventricular walls (21). The structure of collagen is also changed with aging. In a pathoanatomic study, the perimysial and endomysial collagen type I fibers was found to be increased in number and thickness in older hearts (22). The present investigation found that the ratio of PIP/CITP which reflects collagen type I turnover was lower in participants with HF than healthy controls, which was primarily due to higher levels of CITP in those with HF. An explanation for specific increase in CITP and PIIINP might be that in the early phase of ventricular remodeling, adequately cross-linked type I collagen is increasingly degraded subsequently and replaced by poorly cross-linked type III collagen in ventricular remodeling, thereby leading to left ventricular dilatation (19). Alternatively, our failure to find and association of PIP with HF, may reflect an elderly population where the degradation of collagen type I prevails over its synthesis. A shift from type I to type III collagen synthesis has been demonstrated in non-human primate models of arterial hypertension (3,4). It is also possible, that in our elderly population synthesis of type I collagen may have reached a plateau. In support of this is that PIP levels in our healthy control and control groups was much greater than that seen in normal individuals in other studies using the same method of measurement, and approximated that which was reported for diastolic HF including those with advanced diastolic dysfunction (18). Further research is needed into the dynamic changes of various components of the fibrosis process in progressive HF.
Systolic vs. Diastolic Heart Failure
In contrast to the serum levels of NT-proBNP which differed between SHF and DHF, collagen markers were similar in both types of HF. This finding was unanticipated because systolic HF is associated with a higher prevalence of coronary heart disease and prior myocardial revascularization than diastolic HF. Hence we anticipated that systolic HF would be associated with higher burden of fibrosis. The participants with diastolic HF had a higher prevalence of hypertension than those with systolic HF, and a significant albeit modest correlation was found between CITP and PIIINP with IVS diastolic thickness, implying that mechanisms related to the pathogenesis of arterial hypertension could lead to increased collagen synthesis and degradation. While we did not evaluate functional class of HF in the CHS, a previous study of HF with low ejection fraction did not find an association between markers of collagen turnover and NYHA functional class (23). Our findings support those of others (24) in showing absent or weak correlations between PIIINP and echocardiographic variables.
Inflammation, Renal Dysfunction, Total Bone Mineral Density and NT pro-BNP as Related to Fibrosis Markers
Inflammation is a plausible etiology mediating fibrosis (25). An inverse association between PIP with fibrinogen and CRP, and between PIIINP with fibrinogen were observed in the HF groups which has not been previously reported. The mechanisms of this finding require further investigation. Decreases in renal function may decrease the clearance of biomarkers and confound the association between fibrosis markers and heart failure. We found that cystatin C, a sensitive marker of renal dysfunction in the elderly (26) was associated only with CITP and PIIINP (Table 4) but not with PIP. Additionally, renal dysfunction could be mechanistically linked to increases in myocardial interstitial matrix formation, thereby promoting HF. However, in the present study, the relation of HF to fibrosis markers was independent of cystatin C.
No prior studies have addressed the potential confounding effect of bone tissue turnover on serum biomarkers level in HF in the elderly. Although the bones are one of the major contributors of collagen type I turnover, the only correlation was found between CITP and total bone mineral density, and that only in the participants with systolic HF. When total bone mineral density was included in a multivariate model next to age and gender (model 3, Table 2), it mildly diminished the strength of association strength between CITP with diastolic HF and between PIIINP with both types of HF, suggesting a minor impact of bone tissue turnover on serum degradation marker of collagen type I and synthesis of collagen type III. PIP levels were similar in the 4 study groups and did not enter in any model.
The association between NT-proBNP and fibrosis markers was significant even when adjusting for a large number of covariates including HF. This finding is not unexpected considering the relation between BNP and interstitial myocardial fibrosis (27–28). Importantly, in our study the association between CITP, PIIINP and HF remained significant even when adjusting for NT-proBNP which further supports the hypothesis that collagen turnover markers are relevant to the pathophysiology of HF.
Limitations
Serum markers of fibrosis are not specific to the myocardial muscle. Participants with liver dysfunction or severe pulmonary dysfunction were excluded and thus prominent causes of increased collagen synthesis and degradation were ruled out. Osteoporosis is another potential source of elevated collagen biomarker concentrations, particularly PIP. While this elderly cohort is subject to osteoporosis which can influence serum levels of fibrosis markers, adjustment for bone mineral density did not eliminate the associations of fibrosis markers with HF. However, bone mineral density measurements were available in a relatively small subset of the present cohort and therefore, the influence of total bone mineral density on fibrosis markers may have been underestimated.
Conclusions
This study shows that in an elderly community-dwelling population, increased collagen type I and III turnover, reflected in elevated serum level of CITP and PIIINP, is associated with both diastolic and systolic HF, independently of age, gender, race, total bone mineral density, NT-proBNP, and a large number of covariates. In contrast to NT-proBNP, none of the collagen turnover markers analyzed in this study was able to differentiate between diastolic and systolic HF.
Acknowledgments
Funding Sources:
The research reported in this article was supported by contracts N01-HC-85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC-85084, N01-HC-85085, N01-HC-85086, Georgetown Echo RC HL35129, JHU MRI RC HL15103, HL43201, and 1-T32-HL07902 from the National Heart Lung and Blood Institute, and by grant AG09556 from the National Institute of Aging. Also partially supported by grants 1RO3AG23291, and R37AG18915.
Footnotes
- - URL: http://www.chs-nhlbi.org
- - Clinical Trial Identifier: NCT00005133
Disclosures:
No conflicts to disclose.
REFERENCES
- 1.Weber KT, Sun Y, Tyagi SC, Cleutjens JPM. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:271–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 2.Brilla CG. The cardiac structure-function relationship and the renin-aldosterone system in hypertension and heart failure. Curr Opin Cardiol. 1994;9:S2–S10. doi: 10.1097/00001573-199407000-00002. 1. [DOI] [PubMed] [Google Scholar]
- 3.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure- overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 4.Pick R, Janicki JS, Weber KT. Myocardial fibrosis in nonhuman primate with pressure overload hypertrophy. Am J Pathol. 1989;135:771–781. [PMC free article] [PubMed] [Google Scholar]
- 5.Izawa H, Murohara12345 T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur J Heart Fail. 2002;4 doi: 10.1016/s1388-9842(02)00092-2. 439-4. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 8.Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, Frediani B, Rossini M. BONTURNO study group. Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int. 2008 May;82:341–347. doi: 10.1007/s00223-008-9126-5. [DOI] [PubMed] [Google Scholar]
- 9.Byrjalsen I, Leeming DJ, Qvist P, Christiansen C, Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: effects of antiresorptive therapies. Osteoporos Int. 2008;19:339–348. doi: 10.1007/s00198-007-0462-5. [DOI] [PubMed] [Google Scholar]
- 10.Eastell R, Hannon RA. Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 2008;67:157–162. doi: 10.1017/S002966510800699X. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB. Newman A for the CHS Collaborative Research group. The Cardiovascular Health Study; design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 13.Risteli J, Niemi S, Trivedi P, Maentausta O, Mowat AP, Risteli L. Rapid equilibrium radioimmunoassay for the amino-terminal propeptide of human type III procollagen. Clinical Chemistry. 1988;34:715–718. [PubMed] [Google Scholar]
- 14.Fried LF, Shlipak MG, Stehman-Breen C, Mittalhenkle A, Seliger S, Sarnak M, Robbins J, Siscovick D, Harris TB, Newman AB, Cauley JA. Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2006;61:743–748. doi: 10.1093/gerona/61.7.743. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 16.Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 17.López B, González A, Beaumont J, Querejeta R, Larman M, Díez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–867. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 18.Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 19.Timonen P, Magga J, Risteli J, Punnonen K, Vanninen E, Turpeinen A, Tuomainen P, Kuusisto J, Vuolteenaho O, Peuhkurinen K. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 21.de Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- 22.Gazoti Denessa CR, Mesiano Maifrino LB, de Souza RR. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 23.Cavallari LH, Groo VL, Momary KM, Stamos TD, Vaitkus PT. Markers of cardiac collagen turnover are similar in patients with mild and more severe symptoms of heart failure. Congest Heart Fail. 2007;13:275–279. doi: 10.1111/j.1527-5299.2007.07217.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Larson MG, Benjamin EJ, Siwik DA, Safa R, Guo CY, Corey D, Sundstrom J, Sawyer DB, Colucci WS, Vasan RS. Clinical and echocardiographic correlates of plasma procollagen type III amino-terminal peptide levels in the community. Am Heart J. 2007;154:291–297. doi: 10.1016/j.ahj.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber KT. From inflammation to fibrosis: a stiff stretch of highway. Hypertension. 2004;43:716–719. doi: 10.1161/01.HYP.0000118586.38323.5b. [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa Y, Tamura N, Chusho H, Nakao K. Brain natriuretic peptide appears to act locally as an antifibrotic factor in the heart. Can J Physiol Pharmacol. 2001;79:723–729. [PubMed] [Google Scholar]
- 28.Cao L, Garner DC. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension. 1995;25:227–234. doi: 10.1161/01.hyp.25.2.227. [DOI] [PubMed] [Google Scholar]